INTRODUCTION
Jatropha curcas is a perennial shrub of the Euphorbiaceae family that accumulates oil of adequate quality to be transformed into biofuels in its seeds, has a short development period, adapts to drought conditions, and helps soil conservation (Gangwar & Shankar, 2020). It is a plant native to Mesoamerica and is currently cultivated in various tropical and subtropical regions of the world (Adriano-Anaya et al., 2016; Kumar et al., 2017).
J. curcas is still in domestication, and many aspects of its biology must be studied in depth. In this context, little is known about water availability and its impact on plant development. For a given time and space, the amount of water a plant requires depends on the phenological stage (Santos et al., 2016). Therefore, understanding the relationship between growth, crop production, and soil moisture regimes is critical for planning comprehensive water use (Santillán-Fernández et al., 2016). In limited water environments, flowering and early fruit formation will be adversely affected (Kifle & Gebretsadikan, 2016). Some plants have strategies that allow them to tolerate different levels of water deficit, ranging from species that are slightly tolerant to water stress to those that survive in desert habitats (Craine et al., 2012). A water deficit occurs when there is little water in the environment. It is also caused by low temperatures and high soil salinity, which decreases water available from the cytoplasm (Lukowska & Józefaciuk, 2016).
It has recently been shown that moderate irrigation, also called deficit irrigation (DI), maintains grape production and helps save water (Martínez-Barba, 2015). DI during development and flowering of several plants improved fruit production (Quintal-Ortiz et al., 2012). Similar results were obtained by Li et al. (2010) in wheat plants (Triticum aestivum) and Diaz-Abril et al. (2016) in pear trees (Pyrus communis). However, for each cultivated plant species, there is a relationship between the degree of water stress and development, flowering, and production (Fernández et al., 2005). Cultivation of plants tolerant to water deficit is certainly an alternative for exploiting drought-prone areas with soils with marginal features (Ojeda-Silvera et al., 2013). J. curcas has become a study model because it tolerates low soil moisture (Silva et al., 2010; Yin et al., 2016).
Studies of J. curcas have focused on the vegetative growth of the plant in soil or substrates with different water potentials, in which the initial quantity of water may or may not be re-substituted (Abou & Atta, 2009; Arya et al., 2012; Fardim et al., 2015; Jayasundara et al., 2014; Yang et al., 2014). However, the constant presence of limited amounts of water and the impact on the development of J. curcas has scarcely been addressed. Therefore, this study aimed to analyze the development of J. curcas cultivated with limited amounts of a daily water supply.
MATERIAL AND METHODS
Biological material. Seeds (500) of J. curcas MAP-08 accession were collected from Jatropha Germplasm Bank in the Instituto de Biociencias from Universidad Autónoma de Chiapas, Mexico (14°49'45.45''N, 92°17'45.54''W, 58 masl). The work was carried out at the “Ayol'' Agroecological Ranch (14°49′48.56′′N, 92°17′46.98′′W, 76 masl) located in the Municipality of Tapachula, Chiapas, Mexico. The seeds were germinated in plastic trays, using peat moss (Premier®, Mexico) as substrate moistened to field capacity. After two weeks, 80 seedlings were selected, considering that all of them had the same characteristics (height of 15 cm and two cotyledonal leaves) and were planted in 20 kg capacity plastic pots (30cm diameter, 35cm depth) containing loamy soil (0.08% N) mixed with vermicompost (0.35% N) at a ratio of 19:1 w/w.
Irrigation scheduling. Considering the range of precipitation in which J. curcas develops (250 - 2250 mm; Noda-Leyva et al., 2015) and the average annual precipitation during the period 2012-2013 in the region (769,043 MMTP weather station), where the Germplasm Bank of J. curcas is located, a drip irrigation system was designed to simulate annual rainfall levels with five treatments: 250, 750, 1250, 1750 and 2250 mm per year. Based on the area of the pot (707 cm2), daily irrigation volumes for each treatment were calculated (48, 187, 242, 339 and 436 mL, respectively). The plants were bottom-watered with a drip irrigation system. Initially, treatments included 16 plants (each was considered a replicate). The treatments were established under a completely randomized design in a covered shade house so that the rains would not affect the plants. At the end of the study period (60 weeks after transplanting), the number of plants in the irrigation treatments of 250, 750, 1250, 1750 and 2250 mm per year was 12, 13, 15, 16, and 16, respectively. The death of the plants, derived from insects' destruction of the stem, was observed between week 14 and week 17 of cultivation, and the work period covered transplantation through flowering.
Response variables. After transplanting, the variables survival rate (%), plant height (cm), stem diameter at 2 cm from the ground (cm), number of leaves, and number of branches were evaluated every seven days for 60 weeks. In four well-developed leaves (Phase IV, data not shown) of each plant, the chlorophyll content (SPAD units) was quantified every two weeks. Plants with inflorescences were counted starting at week 52 after transplanting.
Statistical analysis. The values of all variables, height, stem diameter, total leaves, branches, and SPAD, were subjected to analysis of variance after checking for normality (Shapiro-Wilk) and homoscedasticity. The Duncan method (α = 0.05) for all variables was used where differences were found. Software InfoStat Professional ver. 2011 was used. In order to show the differences in the development of the plants of the different irrigation treatments, the average values of height and diameter were subjected to linear regression analysis (Microsoft Excel). With this, the range of weeks of the development stages was established, such as the weekly gain in height and diameter. The flourished plants were counted as a percentage of the population from each treatment.
Additional calculations. Soil matric potential (Ψm), for the different treatments of this study, was calculated based on the equation derived from the data of Matsumoto et al. (2014): y = 3 E-05 x2 + 0.0365x-13.431; R2= 0.99905, where y = Ψm (kPa) and x = volume of water added to each treatment. Average evapotranspiration was calculated using the formula ET0=(0.0023*RS)* √(TPMMx - TPMMi)*(17.8+ TMM), where RS=external radiation; TPMMx = monthly average maximum temperature; TPMMi = monthly average minimum temperature, and TMM = monthly average temperature (Hargreaves & Samani, 1985). The historical climatological data for the Tapachula area was taken from Serrano et al. (2006) and Escalante-Sandoval & Amores-Rovelo (2014).
RESULTS AND DISCUSSION
Plant height dynamics of J. curcas cultivated for 60 weeks using different levels of daily irrigation volumes are shown in Figure 1. Plants grown with an irrigation amount of 2.250 mm per year had the most height gain. The plants of the 750 mm and 250 mm treatments had the lowest height gain, and there was no statistical difference between them (P>0.05). Plants irrigated with 2250 mm per year were 1.95, 1.51 and 1.28 times higher than those that received irrigation of 250 or 750 mm per year, 1.250 mm per year, and 1.750 mm per year, respectively.
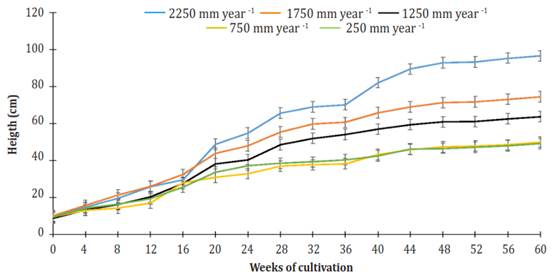
Figure 1 Effect of the different levels of irrigation in Jatropha curcas MAP08 plants height. The vertical bars represent the standard error value (2.87).
In general, three phases or stages (S) of development were observed in all treatments: From week 0 to week 20 (S1), from week 20 to week 36 (S2), and from week 36 to week 60 (S3). All stages showed linear behavior (R² ranging from 0.80645 for S3 of the irrigation treatment of 250 mm per year to 0.97394 for S1 of the irrigation treatment of 1750 mm per year). For all treatments, the most significant gain in height (GH = cm per week) was found in S1 (Figure 2). At that stage, plants irrigated using 2250 mm per year obtained 1.10, 1.27, 1.59, and 1.56 times more GH than plants watered 1750, 1250, 750 and 250 mm per year.
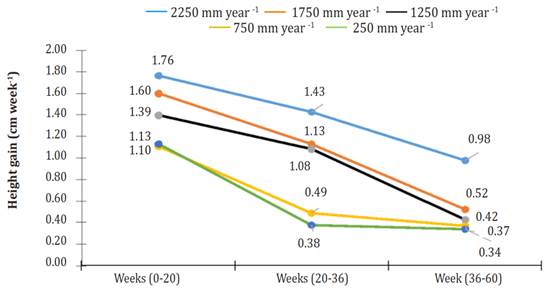
Figure 2 Height gain (cm week-1) of Jatropha curcas MAP08 plants cultivated using different irrigation levels.
In S2, GH growth decreased by 19.0, 29.4, 22.4, 55.9 and 66.6% relative to the first stage, under irrigation treatments of 2250, 1750, 1250, 750 and 250 mm per year, respectively. For the same treatment arrangement, at S3, GH growth reduction relative to S1 was 44.5, 67.2, 69.7, 66.4 and 69.8%. Until week 20 of cultivation, the height differences between plants getting different treatments were minimal. However, from week 20 of cultivation up to week 60, height differences among all treatments were highly significant (P<0.0001).
The initial stem diameter of the plants for all treatments was 5.35 ± 0.51 mm. After 60 weeks of culture, plants using an irrigation of 2250 mm per year grew 15.6, 22.2, 41.9 and 47.7% larger stem diameter than plants using treatments of 1750, 1250, 750, and 250 mm per year. Furthermore, at the end of the study period, there was no difference in the stem diameter of plants using treatments of 750 and 250 mm per year. However, there were highly significant differences (P<0.0001) between these treatments and the rest.
Over time, the increase in diameter of the stem showed two phases of increase and two phases of "rest" (Figure 3). The latter occurred from weeks 16 to 28 and 44 to 56. Both stages of diameter increase showed linear behavior relative to cultivation time. R2 at phase 1 (week 0 to week 16) ranged from 0.920, under irrigation of 750 mm per year, to 0994, using an irrigation of 1 750 mm per year. R2 at phase 2 (week 28 to week 40) ranged from 0.975, using an irrigation of 250 mm per year, to 0988, using an irrigation of 750 mm per year. The most significant gain in diameter (GD = mm per week) was in plants that were watered 2250 mm per year (Figure 4).
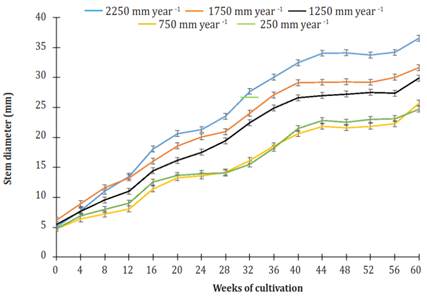
Figure 3 Effect of different levels of irrigation in the stem diameter of Jatropha curcas MAP08 plants. The vertical bars represent the standard error value (0.49).
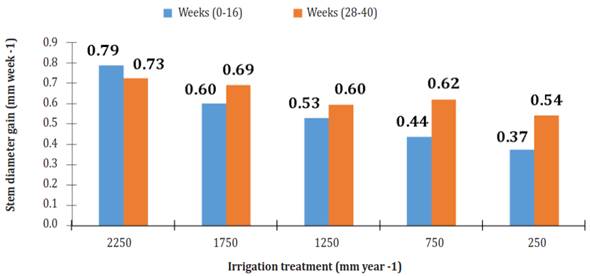
Figure 4 Diameter gain (mm week-1) of Jatropha curcas MAP08 plants grown using different irrigation levels.
In the first phase of diameter increase, plants irrigated with 2250 mm per year had 23.7, 32.9, 44.5 and 52.6% more GD than those with irrigation of 1750, 1250, 750 and 250 mm per year. In the second phase, the diameter of the plants with irrigation of 1750, 1250, 750 and 250 mm per year had 4.8, 17.9, 14.4 and 25.2 lower GD than those with irrigation of 2250 mm per year.
An important point is that in phase 2, the increase in diameter of the plants using irrigation of 1750, 1250, 750 and 250 mm per year GD was 14.9, 12.7, 42.1 and 45.2% larger, respectively, compared to phase 1; whereas plants watered 2250 mm per year showed a 7.9% GD decrease.
During the first 12 weeks of cultivation, the number of leaves in the plants for all treatments increased, and no statistical differences were found among them. From the 16th week of cultivation up to week 28, there was no increase in the number of leaves for plants given irrigation treatments of 750 and 250 mm per year (25.7 ± 2.1 leaves per plant on average). In the previously mentioned period, the number of leaves on plants using treatments of 2250 and 1750 mm per year continued to increase to 61.1 ± 1.3 leaves per plant for the former treatment and 57.9 ± 1.6 leaves per plant for the latter, whereas the plants using 1250 mm per year, irrigation treatment did not develop more leaves, keeping the same number (36.2 ± 1.0 leaves per plant) from week 20 through week 28 (Figure 5).
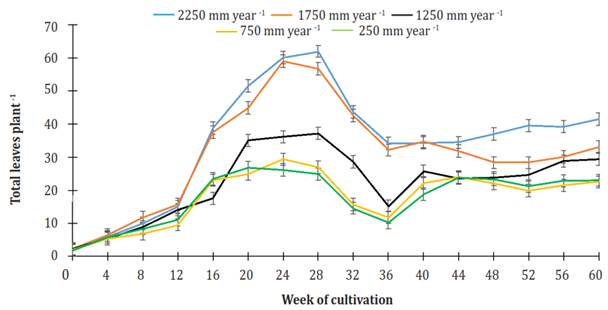
Figure 5 Effect of the different levels of irrigation on the number of leaves in Jatropha curcas MAP08 plants. The vertical bars represent the standard error value (1.86).
From week 28 of cultivation to week 36, there was a decrease in the number of plant leaves for all treatments. The reduction showed a linear behavior over time (R2 ranging from 0.928, using irrigation treatment of 750 mm per year, to 0992, using irrigation of 1750 mm per year). Values were -3.43, -3.08, -2.74, -1.89, and -1.82 -1 leaves per week using irrigation treatments of 2250, 1750, 1250, 750, and 250 mm per year, respectively.
From week 40 up to the end of the study, the number of leaves in plants for all treatments, 2250, 1750, 1250, 750 and 250 mm per year, maintained the average values of 37.7 ± 2.9, 31.1 ± 2.6, 26.0 ± 2.5, 22.0 ± 1.3, and 22.2 ± 1.8 leaves per plant, respectively. At week 60, the number of leaves in plants irrigated 2250 mm per year was significantly different (P<0.05) from plants watered 1,750 or 1,250 mm per year (without significant difference between them) and from plants watered 750 or 250 mm per year (without significant difference between them). Typical drought symptoms were not observed in the leaves.
Figure 6 shows the dynamics of branching in plants under various irrigation treatments. Branching started between weeks 12 and 16 in plants irrigated at 2250, 1750, 1250, and 750 mm per year. Meanwhile, plants that watered 250 mm per year started branching from the 16th to the 20th week of cultivation.
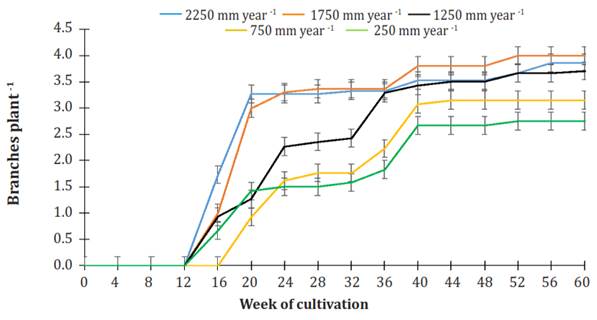
Figure 6 Effect of various levels of irrigation on the number of branches in Jatropha curcas MAP08 plants. The vertical bars represent the standard error value (0.17).
In week 20 of cultivation, in the irrigation treatments of 2250 and 1750 mm per year, the number of branches per plant was 3.14 ± 0.19, and in the irrigation treatments with 1250, 750, and 250 mm per year, it was 1.20 ± 1.20. In both cases, no significant difference was found (P>0.05). From the week of cultivation mentioned and on, there was a steady increase in the number of branches on the plants following a particular dynamic. At the end of the study period, plants watered 2250, 1750, or 1250 mm per year had, on average, 3.86 ± 0.15 branches per plant without significantly difference (P>0.05) among them, whereas plants using irrigation of 750 or 250 mm per year produced, on average, 2.90 ± 0.28 branches per plant without significant difference between them (P>0.05).
This study showed that under the constant presence of water, J. curcas does not transition to leaf loss. However, morphological and reproductive characteristics were adversely affected when grown with 22, 44, 67, and 89% less water than the annual maximum registered in the work area (Figures 1, 3, 5 and 6). The negative effect of the decrease in available water in the soil on the morphological characteristics of the plant was similar to that reported by Abou & Atta (2009), Arya et al. (2012), Fardim et al. (2015), Jayasundara et al. (2014) and Yang et al. (2014).
Figure 7 shows the chlorophyll content in the leaves of J. curcas MAP08 grown using different irrigation regimes for 60 weeks. For all irrigation treatments, the chlorophyll content was within the range of 32.3 to 44.2 SPAD. From week 24 to week 36, the leaves had the highest chlorophyll content. The data variance analysis showed that the average chlorophyll content was significantly different among treatments (P=0.0008). The average values of chlorophyll (38.01 ± 0.23, 38.29 ± 0.22, and 37.9 ± 0.21 SPAD) in the leaves using irrigation treatments of 250, 750, and 1250 mm per year belong to the same statistical group (b). The leaves of plants using irrigation treatments of 1750 and 2250 mm per year had, on average, 38.90 ± 0.20 and 38.32 ± 0.21 SPAD and were combined into the same statistical group (a).
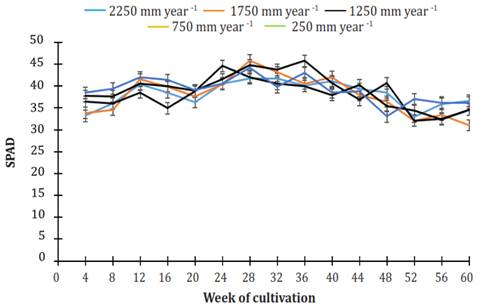
Figure 7 Behavior of chlorophyll (SPAD) in Jatropha. curcas MAP08 plants grown using different levels of irrigation. The vertical bars represent the standard error value (1.30).
The decrease in morphological parameters, and possibly in the biomass produced, is related to water balance and availability (Matsumoto et al., 2014). In this sense, the water potential of the soil where plants were grown, determined as matric potential (Ym), was in the range of -3.1 to -11.7 kPa, and average evapotranspiration was 27.72 ± 2.73 mL per pot per day. The treatment of 2250 mm per year had Ym> -3.4 kPa, considered optimal for biomass generation in J. curcas by Matsumoto et al. (2014). However, the value of Ym was 2.17 to 2.87 times higher than that reported by De Santana et al. (2015); these authors reported Ym ranging from -9.8 to -7.4 kPa for irrigation treatment and Ym = -98.6 to -33.5 kPa for treatments with water deficit. Despite the above, the negative impact on the morphological variables of J. curcas was similar to the findings of this study.
Jatropha curcasis classified as a light wood tree (deciduous) that stores a large amount of water in the stem or as a succulent deciduous tree in which the water of the sap plays a role in regulating YL and serves as a buffer against low Ys (Chapotinet al., 2006a). In this sense, Maeset al. (2009) reported that the YL ofJ. curcaswas unaffected by Ys reduction. This may explain why plants with a limited amount of water, including the ones using the treatment with Ym = -11.7 kPa, did not show the classic symptoms of drought, that is, accelerated senescence and the loss of leaves, since the leaves with high water content, high specific area (SLF) and minor adaptations to drought typically fell off at shallow values of DY, soon after or even before the start of the dry season (Chapotinet al., 2006b).
Of all the treatments, only plants grown with an irrigation of 1750 and 2250 mm per year developed inflorescences. The onset of flowering in plants that got more water was at week 52 of cultivation, and for plants that watered 1750 mm per year, it was at week 58. Of the latter, only 25% of the plants had inflorescence, whereas 69% of the former developed inflorescence.
In treatments using limited water, a decrease in the number of leaves (Figure 5) translated to a decrease in leaf area (A), together with a reduction in hydraulic conductance (KL) and transpiration (E) (De Santanaet al., 2015), and a decrease in stomatal conductance (gs) due to stomatal closure (Arcoverdeet al., 2011), limits in the capture of light energy, resulting in a low photosynthesis rate (A). Therefore, the significant effect of reducing water availability, even if the stress is moderate, is that the reduction in carbon fixation affects the growth of the plant, as proposed by Chaves & Oliveira (2004). Given the above, the observed reduction in height of plants grown under water stress, also reported by Achtenet al. (2010), was a result of the decrease in the speed of photosynthesis rather than a decrease in the chlorophyll content of the leaves. So, the chlorophyll content (SPAD) differences found in the leaves of plants (Figure 7) under the different treatments of this study probably had no impact on carbon fixation.
Since SPAD correlates chlorophyll concentration with nitrogen content (Mielkeet al., 2010), the SPAD values obtained in this study signal that the fertility of the substrate used was not limited to the development of the plant. Such values are within the previously reported range, from 24.4 SPAD (Putrantoet al., 2014) to 45.0 SPAD (Ferreiraet al., 2013).
Furthermore, the delay in the appearance of inflorescences in plants that use irrigation treatments of 2250 and 1750 mm per year [The presence of inflorescences in J. curcas has been reported between week 20 after sowing (SDS) (Machado & Suárez, 2009) at 50 SDS (Guerrero et al., 2011), depending on the accession and the edaphoclimatic conditions of the workplace] may indicate the need for a period of drought, as has been predicted for this plant. Likewise, the absence of inflorescences in plants under irrigation treatments of 250, 750 mm and 1250 mm per year could indicate the existence of other limitations by photosynthates in addition to the previous restriction.
The data obtained in this work support the concept that J. curcas is tolerant to water deficit; however, this information showed that by reducing water availability, its development is negatively altered, impacting the flowering process, which is essential for seed production.
CONCLUSIONS
Daily water supply in amounts more minor than the annual average rainfall in the region of origin adversely affects the development and flourishing ofJ.curcasMAP-08.Morphological and reproductive characteristics were adversely affected when grown with 22, 44, 67, and 89% less water than the annual maximum registered in the work area (2250 mm).















