Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488On-line version ISSN 2665-4385
Rev. Colomb. Entomol. vol.32 no.1 Bogotá Jan./June 2006
Effect of Jatropha gossypiifolia leaf extracts on three Lepidoptera species
Efecto de extractos de hojas de Jatropha gossypiifolia sobre tres especies de Lepidoptera
ARNUBIO VALENCIA J.1,4, BRIGITTE FRÉROT2, HERVE GUÉNEGO2, DIEGO F. MÚNERA3, MARIA FÁTIMA GROSSI DE SÁ4, PAUL-ANDRÉ CALATAYUD5*
1 Profesor Asociado. Dpto de Fitotecnia. Facultad de Ciencias Agropecuarias. Universidad de Caldas. Calle 65. Nº 26-10. Manizales-Colombia. Tel. (68) 861250. E-mail: arnubio@laciudad.com
2 Institut National de la Recherche Agronomique (INRA), Unité de Phytopharmacie et Médiateurs Chimiques, Route de St Cyr, 78026 Versailles, France. E-mail: frerot@versailles.inra.fr
3 International Center for Tropical Agriculture (CIAT), Cassava Entomology, CIAT AA 6713, Cali-Colombia. E-mail: difemusa@hotmail.com
4 EMBRAPA, Recursos Genéticos e Biotecnologia, Brasília, DF, Brazil.
5* Corresponding author: Institut de Recherche pour le Développement (IRD) c/o International Center for Insect. Physiology and Ecology (ICIPE), P.O. Box 30772, Nairobi-Kenya. E-mail: pcalatayud@icipe.org.
Abstract. Leaf extracts of Jatropha gossypiifolia L. (Euphorbiaceae) contain compounds that are toxic to insects. In this study, these extracts were tested against larvae of three lepidopteran species, Busseola fusca (Fuller) (Lepidoptera: Noctuidae), Ostrinia nubilalis Hubner (Lepidoptera: Pyralidae) and Sesamia nonagrioides Lef. (Lepidoptera: Noctuidae), which are important pests of maize in Africa, Europe and Mediterranean countries, respectively. Leaf extracts were shown to be highly toxic to neonate larvae of B. fusca and O. nubilalis quickly after they were ingested. In contrast, no effect was found on fourth instar O. nubilalis and a low level of toxicity was observed on neonates of S. nonagrioides. Given the toxicity of J. gossypiifolia to larval neonates of B. fusca and O. nubilalis, this extract can be used for the control of these species when they are colonizing the plant.
Key words: Euphorbiaceae. Noctuidae. Busseola fusca. Pyralidae. Ostrinia nubilalis. Sesamia nonagrioides. LC50
Resumen. Los extractos foliares de Jatropha gossypiifolia L. (Euphorbiaceae) contienen compuestos que son tóxicos a los insectos. En este estudio, se probaron estos extractos sobre las larvas de tres especies de lepidópteros, Busseola fusca (Fuller) (Lepidoptera: Noctuidae), Ostrinia nubilalis Hubner (Lepidoptera: Pyralidae) y Sesamia nonagrioides Lef. (Lepidoptera: Noctuidae); las cuales son plagas importantes del maíz en África, Europa y países mediterráneos respectivamente. Se encontró que los extractos foliares eran altamente tóxicos contra larvas neonatas de B. fusca y O. nubilalis rápidamente después de que fueron ingeridos. En contraste, no se encontró toxicidad frente a larvas en cuarto instar de O. nubilalis y un bajo nivel de toxicidad fue observado con neonatos de S. nonagrioides. Debido a la toxicidad de J. gossypiifolia hacia larvas neonatas de B. fusca y O. nubilalis, se puede usar este extracto para el control de estas especies cuando están colonizando plantas de maíz.
Palabras clave: Euphorbiaceae. Noctuidae. Busseola fusca. Pyralidae. Ostrinia nubilalis. Sesamia nonagrioides. LC50
Introduction
Maize and sorghum are two important food crops in Africa for commercial and resource-poor small-scale farmers (Kfir 1998; Seshu Reddy 1998); these crops are cultivated primarily for human con-sumption, and surpluses are used for feed-ing livestock (Sibanda 1985). In Africa, the productivity of these crops is very low partly due to the damage caused by lepidopteran stemborers (Haile and Hofsvang 2002). Among them, the stem borer Busseola fusca (Fuller) (Lepidop-tera: Noctuidae) is considered one of the major insect pests (Kfir et al. 2002).
The use of insecticides to control stem borers has been proven inefficient due to the cryptic habit of the larvae, which pro-tects them against insecticide sprays. In addition, insecticides are generally not favorable in durable pest management systems due to eco-toxicity and are not affordable to African peasant farmers. Botanicals are one of the alternatives considered environmentally friendly.
This method does not only reduce appli-cation of synthetic insecticides, but also reduce the cost with pest management, which is an important factor for farmers in developing countries. The efficacy of botanicals are largely demonstrated in grain storage insects (Huang et al. 1997, 2000; Liu and Ho 1999; Dal Bello et al. 2001; Taponjou et al. 2002). Further-more, extracts from the Indian neem tree, Azadirachta indica A. Juss. (Meliaceae), are widely used to control various insect species (Saxena 1989; Schmutterer 1990).
The bellyache bush (Jatropha gossy-piifolia L. [Euphorbiaceae]), native of tropical America is now widespread in the tropics. It is used for medicinal purposes in Africa, Thailand and tropical America and is cultivated as an ornamental plant in Florida. Few insects have been ob-served to be associated with this plant species apart of a single whitefly species (Sauvion N., pers. observ.) and occasional infestations by thrips and a polyphagous mealybug species (Calatayud P.-A., pers. observ.). Leaf extracts of the plant were shown to be toxic to Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) and Phenacoccus herreni William & Cock (Sternorrhyncha: Pseudococcidae) (Dev and Koul 1997; CIAT 2001). To our knowledge, nothing have been reported showing toxicity of J. gossypiifolia leaves to Lepidotera insects.
The purpose of this work was to evaluate the toxicity of J. gossypiifolia leaf ex-tracts towards B. fusca, to Ostrinia nubilalis Hübner (Lepidoptera: Pyrali-dae), and to Sesamia nonagrioides Lef. (Lepidoptera: Noctuidae), which are im-portant pests in many countries in Africa and Europe respectively.
Materials and Methods
Insects and plant material. Busseola fusca (Fuller) was provided by the ICIPE mass-rearing unit (Nairobi, Kenya) and was reared on an artificial diet under labo-ratory conditions (Onyango and Ochieng-Odero 1994). To regenerate the colony, new insects collected from the field were added three times per year. Ostrinia nubilalis and Sesamia nona-grioides. larvae were collected from the South part of France and then reared for more than two generations on an artificial diet at the Institut National de Re-cherche Agronomique (INRA, Versailles, France) (Poitout and Bues 1974). The in-sects were maintained in a controlled chamber under the following conditions: 25.3 ± 0.9 °C, 68.6 ± 12.8 % r.h. (means ± SE) and L12:D12 reversed photoperiod. Both neonate larvae (0 to 24 h old) and fourth instar larvae (about 20-25 days old) were used in these experiments. All experiments were carried out in France at the INRA, Versailles.
Two-month old J. gossypiifolia L. plants were used. Cuttings were planted in individual cylindrical plastic pots (ID = 30 cm; height = 22 cm) containing peat and sand, and kept in a glasshouse at 28-35 °C and L12:D12 photoperiod. Only ma-ture leaves were selected for active material extractions (CIAT 2001).
Leaf sample collection and crude ex-traction method. Leaves were harvested and frozen in liquid nitrogen. Thirty minutes later, extraction, based on the method described by Valencia-Jiménez et al. (2000) for plant proteins, was per-formed. One gram of fresh leaves was powdered in a mortar containing liquid nitrogen. Thereafter, 4 ml of 0.1 M so-dium chloride solution were added. The mixture was stirred for 6h at 4 °C. The slurry was filtered and centrifuged at 8,000 rpm at 4°C during 20 min. The supernatant was centrifuged one more time until no pellet was obtained. Then, the supernatant was dialyzed against water with a MWCO 3.5 kDa cellulose membrane at 2 - 4°C for 3 days and freeze-dried. The resulting powder was stored at –20°C and used for toxicity experiments.
Toxicity tests. Leaf extract was added to the artificial diet of Onyango and Ochieng-Odero (1994) at six concentra-tions ranging from 0.01 to 100 mg/ml. Its toxic effect was tested on B. fusca lar-vae. The highest concentration (100 mg/ ml) was also tested on larvae of O. nubilalis and S. nonagrioides by mix-ing the extract with the artificial diet of Poitout and Bues (1974). In another ex-periment, the leaf extract was boiled for 10 min before adding it to the artificial diet, and then the toxicity was tested at 100 mg/ml for each Lepidoptera species.
Five individual insects were introduced into a Petri dish (Ø 35 mm) containing 1g of diet. Mortalities were recorded 24 and 48 hours later. Each test was repli-cated four times. To verify that the larvae fed normally, a mixture containing diet and 1% (w/w) of bromocresol purple pH indicator as described by Sinha (1959) was prepared and used in the same condition.
All experiments were conducted at 25.3 ± 0.9 °C, 68.6 ± 12.8 % r.h. (means ± SE) and L12:D12 reversed photoperiod. For each experiment, the controls corresponded to the meridic diets without leaf extract.
Data analysis. Statistical analyses were performed with Statview version 5.0 (© 1998, SAS Institute Inc., Abacus Concept, USA). When possible to calculate the variance, homogeneity of variance and data normality were examined by F-test and Kolmogorov-Smirnov methods re-spectively, before running the ANOVAs. All proportions were transformed to arcsin before being subjected to ANOVA. Fishers PLSD (Protected Least Signifi-cant Difference) test was used for mean separation. For B. fusca neonates, the four replicates used for each leaf extract con-centration yielded four mortality percent-ages, allowing toxicity indices to be calculated. The Log (C) and probit (%) transformations were used to calculate LC50 and LC90, and their confidence in-tervals at 5% level (Bliss 1935). A pro-gram allowing the easy calculation and statistical analyses of these indices is freely available from the authors (Febvay and Rahbé 1991).
Results and discussion
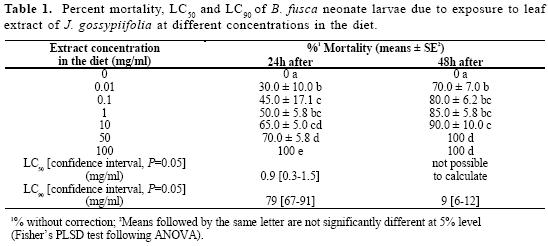
The effect on the mortality of B. fusca neonate larvaes was analyzed using six leaf extract concentrations. The results are presented in Table 1. With the increase in the concentration, a significantly level of larval mortality were found at both, after 24 and 48 hours of feeding, indicat-ing that J. gossypiifolia leaf extracts are highly toxic to the insect. The LC50 and LC90 after 24 hours were 0.9 and 79 mg/ ml, respectively. After 48 hours feeding, only the LC90 could be calculated for the lower concentration (9 mg/ml). A high mortality at 70% was still obtained with the lowest concentration tested.
The highest mortality of B. fusca after 24 hours was found at 100 mg/ml. Based on this data, the toxicity of J. gossypiifolia leaf extracts to both O. nubilalis and S. nonagrioides was determined using this concentration.
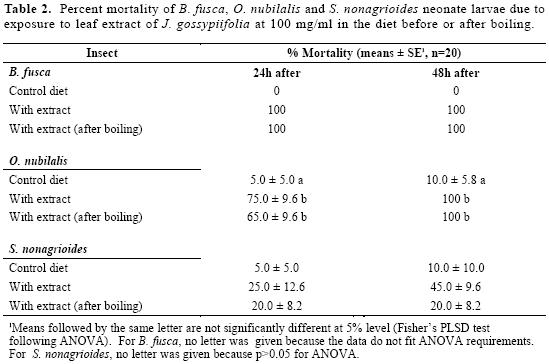
The table 2 shows that 100 mg/ml of ex-tract in the artificial diet induced 75% and 100% of mortality of neonates of O.nubilalis after 24 hour and 48 hour of feeding period, respectively. In contrast, no effects were found when the same extract was evaluated with S. nonagrioides larvae.
For each insect species, the bromocresol-containing diet induced a color change of the larvae intestinal duct, after 60 min of feeding. This confirms that larvae fed normally on the modified diet and indi-cate that the mortality was linked to diet toxicity. Moreover, its toxicity to B. fusca and O. nubilalis was not affected after boiling the leaf extracts (Table 2), indi-cating that the toxicity could be due to thermo-stable compound(s). For the fourth instar larvae of B. fusca, no mor-tality was recorded after 24 hours at 100 mg/ml; however 70% of mortality was obtained after 48 hours. Thus, the toxicity of J. gossypiifolia leaf extract for B. fusca decreases with the age of the lar-vae. In the case of O. nubilalis and S. nonagrioides, no mortality was recorded at 100 mg/ml in the diet after 48 hours or after five days. All species larvae showed a colored intestinal tract when these had been fed on the diet containing pH indi-cator, thus, the low toxicity on old larvae was not related with starvation but more probably to an increased tolerance of the larvae to the toxin.
In conclusion, J. gossypiifolia leaf ex-tracts demonstrated to be highly toxic to both B. fusca and O. nubilalis. Neonate larvae revealed to be more sensitive than older larval stage. Other reports demon-strated the presence of several secondary compounds from J. gossypiifolia leaves and its implication in the toxicity, including flavonoids (e.g. apigenin, isovitexin, vitexin) and diterpenoids (e.g. jatro-phone) (Kupchan et al. 1970; Subra-manian et al. 1971). However, these compounds are generally not water soluble, and thus could not have been ex-tracted from the leaves in our study. In addition, these molecules possess a molecular weight lower than 3.5 kDa and would have been removed during the di-alysis. Only molecules with molecular weight greater than 3.5 kDa could there-fore be involved in the toxicity. Also, Euphobiaceae plants are known to pos-sess polyisoprenes with high molecular weights, in the form of latex (Archer 1980). Such compounds are mostly soluble in organic solvents such as benzene and chloroform. Their presence in the leaf extract described here can be ruled out. Therefore, compounds having molecular weights of over 3.5 kDa and a thermo-stable characteristic appeared as the most plausible chemical involved in the tox-icity for moth neonates.
Plant extracts have been proven success-ful for the control of grain storage insects in the form of essential oil from plant leaves (Taponjou et al. 2002), specially using neem seed oil (Schmutterer 1990). To control maize stemborers, the treat-ments should be aimed at the first instar, when these migrate from the oviposition site to the whorl, where the larval feeding causes conspicuous leaf damage. How-ever, such extract will probably not control all Lepidoptera species to the same extent, as a quasi-absence of toxicity was found in S. nonagrioides. Additionally, a formulation and an easier process to ex-tract the leaves should be developed.
Acknowledgements
The work was supported by grants from COLCIENCIAS (Colombia) and the Institut National de la Recherche Agronomique (INRA–France) for A.V.J. We are pleased to thank Dr. Marie-Louise Milat and Dr. Fritz Schulthess for their valuable critical readings of the manu-script and for their English corrections.
Cited literature
ARCHER, B. L. 1980. Polyisoprene. In: E. A. Bell and B. V. Charlwood (eds), Encyclo-pedia of Plant Physiology. New Series. Volume 8. Secondary Plant Products. Springer-Verlag, Berlin, Heidelberg, New York, pp. 310-327. [ Links ]
BLISS, C. I. 1935. The calculation of the dos-age mortality curve. Annals of Applied Biology 22: 134-167. [ Links ]
CIAT (Centro Internacional de Agricultura Tropical) 2001. Annual Report IPM Project, Cali, Colombia: Centro Internacional de Agricultura Tropical 211 pp. [ Links ]
DAL BELLO, G.; S. PADIN; C. LOPEZ-LASTRA; FABRIZIO, M. 2001. Labora-tory evaluation of Chemical-biological control of the rice weevil (Sitophilus oryzae L.) in stored grains. Journal of Stored Prod-ucts Research 37: 77-84. [ Links ]
DEV, S.; O. KOUL. 1997. Insecticides of natural origin. Taylor and Francis, New York. 352 pp. [ Links ]
FEBVAY, G.; Y. RAHBÉ. 1991. Toxicology, un programme pour lanalyse des courbes de mortalité par la méthode des probits sur MacIntosh. Cahiers Techniques INRA 27: 77-78. [ Links ]
HAILE, A.; T. HOFSVANG. 2002. Host plant preference of the stem borer Busseola fusca (Fuller) (Lepidoptera: Noctuidae). Crop Protection 21: 227-233. [ Links ]
HUANG, Y.; J. M. W. TAN; R. M. KINI; HO, H. S. 1997. Toxic and antifeedant ac-tion of Nutmeg oil against Tribolium cataneum (Herbst) and Sitophilus zeamais Motschulky. Journal of Stored Products Research 33: 289-298. [ Links ]
HUANG, Y.; S. L. LAM; HO, S. H. 2000. Bioactivities of essential oil from Elletaria cardamomum (L.) Maton. to Sitophilus zeamais Motschulky and Tribolium castaneum (Herbst). Journal of Stored Products Research 36: 11-17. [ Links ]
KFIR, R. 1998. Maize and grain sorghum: south-ern Africa. In: A. Polaszek (ed), African Cereal Stem Borers: Economic Importance, Taxonomy, Natural enemies and Control. CABI, Wallingford, UK, pp. 29-37. [ Links ]
KFIR, R.; W. A. OVERHOLT; Z. R. KHAN; POLASZEK, A. 2002. Biology and man-agement of economically important lepi-dopteran cereal stem borers in Africa. Annual Review of Entomology 47: 701-731. [ Links ]
KUPCHAN, S. M.; C. R. WANGER; R. F. BRYAN. 1970. Jatrophone, a novel macrocyclic diterpenoid tumor inhibitor from Jatropha gossypiifolia. Journal of American Chemical Society 92: 4476-4477. [ Links ]
LIU, Z. L.; S. H. HO. 1999. Bioactivity of the essential oil extracted from Evodia rutae-carpa Hook f. & Thomas against the grain storage insects, Sitophilus zeamais Motschulky and Tribolium cataneum (Herbst). Journal of Stored Products Re-search 35: 317-328. [ Links ]
ONYANGO, F. O.; J. P. R. OCHIENG-ODERO. 1994. Continuous rearing of the maize stem borer Busseola fusca on an artificial diet. Entomologia Experimentalis et Applicata 73: 139-144. [ Links ]
POITOUT, S.; R. BUES. 1974. Elevage de 28 espèces de lépidoptères Noctuidae et de 2 espèces dArctiidae sur milieu artificiel simplifié. Particularités selon les espèces. Annales de Zoologie et dEcologie Animale 6 : 431-441. [ Links ]
SAXENA, R. C. 1989. Insecticides from neem. In: J. T. Arnason, B. J. R. Philogene and P. Morand (eds), Insecticides of plant origin. ACS Symposium Series No.387. Ameri-can Chemical Society, Washington, DC, pp. 110-135. [ Links ]
SESHU REDDY, K. V. 1998. Integrated pest management. In: A. Polaszek (ed), African Cereal Stem Borers: Economic Importance, Taxonomy, Natural enemies and Control. CABI, Wallingford, UK, pp. 39-45. [ Links ]
SCHMUTTERER, H. 1990. Properties and potentials of natural pesticides from the neem tree, Azadirachta indica. Annual Review of Entomology 35: 271-297. [ Links ]
SIBANDA, S. 1985. The use of sorghum and millets for feeding livestock. Proceedings of the Regional Workshop of Sorghum Millets for South Africa, Gaborone, Botswana, pp 228-247. [ Links ]
SINHA, R. N. 1959. The hydrogenion concentration in the alimentary canal of bee-tles infesting stored grain and grain products. Annals of the Entomological Society of America 52: 763-765. [ Links ]
SUBRAMANIAN, S. S.; NAGARAJAN S.; SULOCHANA N. 1971. Flavonoids of the leaves of Jatropha gossypiifolia. Phy-tochemistry 10: 1690. [ Links ]
TAPONJOU, L. A.; C. ADLER; H. BOUDA; FONTEM D. A. 2002. Efficacy of pow-der and essential oil from Chenopodium ambrosioides leaves as post-harvest grain protectants against six-stored product bee-tles. Journal of Stored Products Research 38: 395-402. [ Links ]
VALENCIA-JIMENEZ, A.; BUSTILLO A. E. ; OSSA G. A. ; CHRISPEELS M. J. 2000. a-amylases of the coffee berry borer (Hypothenemus hampei) and their inhibi-tion by two plant amylase inhibitors. Insect Biochemistry and Molecular Biology 30: 207-213. [ Links ]
Recibido: 10-nov-04 Aceptado: 10-ene-06