Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488On-line version ISSN 2665-4385
Rev. Colomb. Entomol. vol.32 no.1 Bogotá Jan./June 2006
Caloric content of the sand fly Lutzomyia ovallesi (Diptera: Psychodidae) vector of Leishmania
Contenido calórico del flebotomíneo Lutzomyia ovallesi (Diptera: Psychodidae)vector de Leishmania.
PEDRO NOGUERA, MARITZA RONDON, ELSA NIEVES1
1 Laboratorio de Parasitología Experimental LAPEX, Departamento de Biología, Facultad de Ciencias. Universidad de Los Andes. La Hechicera, Mérida-EDO-Mérida, 5101. Venezuela. FAX: 00 58 02 74 2401286, e-mail:nevelsa@ula.ve
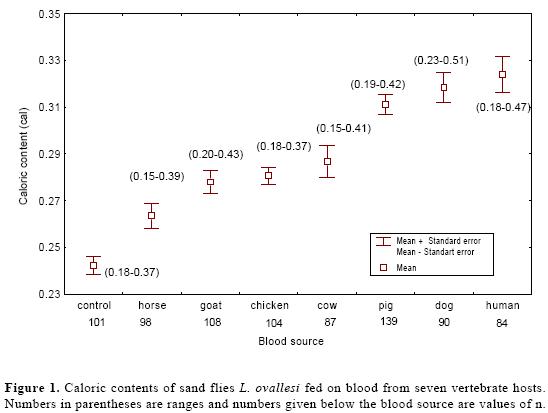
Abstract. Females of the sand fly Lutzomyia ovallesi (Ortiz) (Diptera: Psychodidae) were fed with blood from various species of vertebrates and analyzed to determine energy reserves under laboratory conditions. L. ovallesi specimens were allowed to artificially feed to satiation through chicken mem-branes on blood from horse, dog, cow, chicken, goat, pig or human. Caloric reserves were calculated spectrophotometrically after females were homogenized in a solution of sodium dichromate and sulfuric acid. The caloric content of L. ovallesi varied according to the type of vertebrate blood on which it had fed. The highest content (cal/insect) was found in females fed on human blood (0.33), followed in decreasing order by dog, pig, cow, chicken, goat and horse (0.26). Statistical analysis showed significant differences (P < 0.05) among sources. The results showed that human and dog blood meals were more nutritionally efficient. The most inefficient diet for L. ovallesi was horse blood manifested by its poor nutritional quality.
Key words: Sand flies, caloric reserves, biological potential, bloodmeal, insect vectors.
Resumen. Hembras del flebotomíneo Lutzomyia ovallesi (Ortiz) (Diptera: Psychodidae) fueron alimentadas con sangre proveniente de varias especies de vertebrados y analizadas para determinar las reservas energéticas en condiciones de laboratorio. Ejemplares de L. ovallesi se alimentaron artificialmente a repleción a través de membrana de pollo con sangre de caballo, perro, vaca, gallina, chivo, cerdo o humano. Las reservas calóricas se estimaron espectrofotométricamente, después de homogenizar las hembras en una solución de dicromato de sodio en ácido sulfúrico. El contenido calórico de L. ovallesi varió de acuerdo con el tipo de sangre con que se alimentaron. El mayor contenido calórico (cal/insect) fue encontrado en hembras alimentadas con sangre de humano (0,33), seguido en orden decreciente: perro, cerdo, vaca, pollo, chivo y caballo (0,26). El análisis estadístico mostró diferencias significativas (P<0.05) entre las fuentes. Los resultados mostraron que la sangre de humano y perro fueron más eficientes nutricionalmente. La dieta más ineficiente para L. ovallesi fue la sangre de caballo manifestada por su pobre calidad nutricional.
Palabras clave: Flebotomínos, contenido calórico, potencial biológico, fuentes sanguíneas, insectos vectores.
Introduction
The ability of any hematophagous insect to survive and transmit pathogens de-pends principally on its caloric reserves (Van Handel 1972; Magnarelli and Modi 1988; Briegel et al. 2001). The energy requirements of female phlebotomine sand flies (Diptera: Psychodidae) are sup-plied by three sources: caloric reserves built up during the larval stage and sugar and vertebrate blood ingested as an adult (Van Handel 1972, 1984; Magnarelli and Burger 1984; Magnarelli and Modi 1988; Mostowy and Foster 2004).
Fecundity variations in sand flies accord-ing to bloodmeal source may be attrib-uted to significant differences in the caloric content of carbohydrates, lipids, and proteins from the ingestion and metabolization of blood. Large caloric reserves could provide greater potential
energy for egg production, oviposition survival, and flight capacity (Magna-relli and Modi 1988; Harre et al. 2001), increasing the biological potential of a specific sand fly population, resulting in increased transmission of Leishmania (Kinetoplastida) (Schlein et al. 1983; Daba et al. 1997; Schlein and Jacobson 1998; Hurd 2003). When available en-ergy reserves in both sexes of the sand flies Lutzomyia longipalpis (Lutz & Neiva) and Phlebotomus papatasi (Sco-poli) were quantified, those with access to fructose or sucrose solutions in the laboratory had higher levels than those supplied with labeled glucose. Caloric assays can be used to evaluate larval and adult diets (Magnarelli and Modi 1988).
The sand fly L. ovallesi (Ortiz) is the principal vector of Leishmania braziliensis in western and central Venezuela (Bon-fante-Garrido et al. 1991a; 1991b;Feliciangeli 1991) and one of the most important vectors in the Venezuelan Andes (Añez et al. 1988). The purpose of the present study was to determine ca-loric contents of L. ovallesi fed with ver-tebrate blood from different sources, under laboratory conditions.
Materials and methods
Sand flies. Sand flies of the species L. ovallesi were reared in a closed laboratory colony and only females were used in the experiments. The colony originated from specimens collected at 1360 masl at El Arenal (8 º 35" N, 71º 9' W), Ejido, in the Venezuelan state of Mérida. The colony was maintained in an incubator at 25°C ± 1° and RH 80% ± 10% and provided with saccharose solution ad libitum, in the Experimental Parasitology Laboratory of the University of Los Andes, Mérida, using the methods of Killick-Kendrick et al. (1977).
Bloodmeal sources. Blood was collected in heparinized tubes from humans and healthy animals horse Equus caballus, chicken Gallus domesticus, pig Sus scrofa domestica, cow Bos taurus, goat Capra hircus and dog Canis familiaris. It was used when fresh and at least six replicate samples were taken from each species.
Artificial feeding. Two day-old females of L. ovallesi (n = 811) were allowed to take blood from an artificial feeding ap-paratus across a chick-skin membrane, with water circulating at a temperature of 39 ºC. Females were separated into batches of 100 in plastic containers (5.5 cm. per 2.0 cm.) and fed on blood from different vertebrate sources. The flies were allowed to feed through a chick-skin membrane fitted to a glass feeding apparatus with a well into which blood was introduced. Only fully engorged fe-males were used in the analyses. These insects were maintained individually in glass tubes within an incubator at 25 ± 1°C, RH 80 ± 10% and 12:12 light/dark cycle. As a dietary supplement they were provided with saccharose solution ad li-bitum, which was renewed daily. The control group was fed with saccharose solution alone.
Caloric content. Caloric contents were calculated for all groups of females fed on different sources of vertebrate blood under laboratory conditions. Results are presented as calories per female, the mean being calculated using between 84 (human) and 108 (goat) blood-fed females. Values were calculated after bloodmeals had been fully digested, based on micro-scopic examination of sand fly guts. A solution of sodium dichromate in sulfuric acid was used to determine caloric reserves in individual insects, as described by Van Handel (1972). This involved ho-mogenizing each female in 1.2 ml sodium dichromate solution in sulfuric acid within a glass test tube and boiling for 20 min. After heating, 1.8 ml of distilled water was added to each preparation. Color changes in the test solutions were then measured using a Milton Roy Spectronic 20D spectrophotometer. Op-tical density (OD) values of test solutions were compared with a standard curve for densities of various saccharose concen-trations to convert readings into calories. All assays included saccharose standards as references, the color produced by 1 mg of saccharose (0.1 ml of 1% solution) with an optical density of 0.38 being equivalent to 4 cal. One cal is equivalent to an optical density of 0.095.
Statistical analysis. The data from opti-cal density value of the sand flies were analysed by means of one-way ANOVA and statistical analyses for significance were based on the Tukey´s test for differ-ent values of n. All statistical analyses were carried out using the MINITAB com-puter program (version 10) and the pro-gram Statistics version 6.0.
Results
Caloric contents of L. ovallesi fed on blood from each of seven vertebrate spe-cies are shown in Figure 1. The highest caloric content (cal/female) was ob-tained from insects fed on human (x = 0.33; range 0.18-0.47), and the lowest from those fed on horse blood (x = 0.26; 0.15-0.39). In decreasing order of mag-nitude the caloric content for L. ovallesi fed on different types of blood was as follows: control < horse < goat < chicken < cow < pig < dog < human. Significant differences (P < 0.05) were seen for the following comparisons: cow vs dog, human vs control; pig vs horse, goat, chicken and control; dog vs cow, horse, goat, chicken blood and control; horse vs pig, dog, human and control; and human vs cow, horse, goat, chicken, and control (Table 1).
Discussion and Conclusions
The enormous reproductive potential of hematophagous insects is largely due to the females success in locating a host, approaching it to feed, utilizing the blood to mature an optimal number of eggs and then finding a suitable site for oviposi-tion. This pattern of behavior also means that females can transmit pathogens be-tween hosts (Briegel 1990).
Ingestion of blood swells the epithelial cells, causing reversible phenomena such as secretion of the peritrophic matrix and liberation of proteolytic enzymes. The main products of blood digestion are amino acids. When blood is digested, the final product is excreted as ammonium urate (Rudin and Hecker 1982; Magna-relli and Burger 1984).
Dichromate solution oxidizes the in-sect completely, with proteins, carbo-hydrates, lipids and chitin being converted into carbon dioxide (Van Handel 1984; Magnarelli and Modi 1988). This technique is both rapid and sensitive and can be used to determine the nutritional state of females in a labo-ratory colony or assess the value of blood from different vertebrate hosts. Based on our results, all the blood sources provided energy for L. ovallesi although the caloric value of sand flies fed on horse blood was not signifi-cantly greater than that of unfed flies. These values obtained do not necessar-ily reflect the total caloric reserves available to the females, since they were gravid; a large proportion of the caloric reserves is used in egg production, with very little used for female nutri-tion (Rudin and Hecker 1982; Magnarelli and Burger 1984).
Nasci (1986) reported that large females of the mosquito species Aedes aegypti possess large energy reserves at eclo-sion, providing them with great flight potential and the ability to contact more hosts and transmit pathogens. However, Landry et al. (1988) found that signifi-cant seasonal differences in the body size of Ae. triseriatus had no effect on flight potential or life-span. Harre et al. (2001) found that P. papatasi fed on blood from eight species of mammals and detected no appreciable difference between these hosts with respect to sand fly mortality rates after 24h, number of eggs laid per blood-fed female or egg viability. Labo-ratory-reared males and females of both L. longipalpis and P. papatasi which had access to fructose or sucrose solutions had greater mean available energy reserves (x = 1.3 cal/insect) than individu-als provided with glucose solution (x= 0.55). Available caloric reserves were low in natural populations of P. papatasi and these insects probably must feed re-peatedly on vertebrate hosts and sugar sources to obtain sufficient nutrients for survival and reproduction (Magnarelli and Modi 1988).
Although, a high number of sand fly spe-cies have been successfully colonized during the last decade, the factors limit-ing their productivity and fecundity in the laboratory are unknown (Montoya et al. 1998; Luitgards-Moura et al. 2000). Knowledge about the physiological events taking place in the vector is im-portant in understanding vector-parasite interactions necessary for disease trans-mission. Nutritional quality of blood varies between host species and may influence egg productivity, reduces de-velopment rates, longevity, and fecun-dity of the insects (Alexander et al. 2002). For an understanding the role of blood meal sources on sandfly biology, physi-ology, and Leishmania transmission both more field observations and laboratory studies comparing egg productivity of sandflies fed on different hosts, are nec-essary (Alexander et al. 2002; Hurd 2003).
The compatibility of the sand fly and its specific Leishmania parasite depends on the choice of host animals available, it could be an important factor in the dis-tribution of leishmaniasis (Schlein et al. 1983). The proteins from the blood meal are digested by the sand fly gut. It ap-pears that the enzymatic processes in the sand fly gut, functions differently when triggered by different types of meals, and the blood meal from distinct animal sources can be lethal to Leishmania (Adler 1964). L. tropica infection was inhibited in P. papatasi fed on turkey blood be-cause a relatively high DNAase activity level was induced in the sand fly gut by nucleated erythrocytes (Schlein et al. 1983). However, the blood meals from different species of vertebrates have no deleterious effect on the development of either L. braziliensis and L. amazonensis in the gut of L. migonei; also, parasite development was compatible with diges-tion, independent of the blood meal source (Nieves and Pimenta 2002). The development of L. infantum infection was associated with suppression of blood pro-tein digestion by sand flies fed on human or dog blood (Schlein et al. 1983; Daba et al. 1997). It also was demon-strated that the rate of blood meal diges-tion in P. langeroni varied according to the source of the vertebrate blood and Leishmania species involved (Daba et al. 1997).
Very little is known about how these nu-trients are used during adulthood. Sand fly reproduction depends on the avail-ability of blood meal sources such as domestic animals and synanthropic spe-cies. In endemic areas where some spe-cies of domestic animals are sources of blood meals, a higher number of sand fly vectors with more parasites occur. This fact provides a selective advantage to the vector competence in transmitting Leish-mania to vertebrates. This was possible due to the relatively high isoleucine con-tent in rodent blood, as opposed to its role as a limiting factor for oogenesis with human blood. Important role of isoleu-cine explained the results of several pre-vious reports that showed variable mosquito fecundity with different host (Briegel 1990). Similar physiological mechanisms may play a role in the sand flies. Although, feeding on blood from rodents was superior to that from humans with respect to fecundity in Ae. aegypti, it may be sub-optimal energetically (Briegel 1990). L. braziliensis has been found in domestic animals as dogs and equines as well as in wild mammals such as rodents, edentata and opossums (Aguilar et al. 1984; Grimaldi and Tesh 1993). L. ovallesi feeds upon a variety of vertebrate hosts, and could be considered as an opportunistic species (Añez et al. 1988; Nieves et al. 2004). Based on the results of the present study, there are sig-nificant differences in the caloric con-tents of female L. ovallesi fed on blood from different sources, with human, dog, and pig blood providing most energy. It might therefore, benefit females of this species to feed preferentially on these hosts. Values for females fed on horse blood were as low as those in the control group, which had been fed only in sugar. Further studies are required to determine how certain dietary factors affect vector potential and their consequences for Leishmania transmission. This informa-tion may enable health authorities to adopt policies concerning the presence of domestic animals in endemic areas and may comprise factor risk for Leishmania transmission.
Acknowledgments
We thank Carlos Araque for assistance with the laboratory colony; Luis Chavez for his invaluable help and cooperation; Efrain Entralgo, Guillermo Bianchi and Paolo Ramoni for guidance in statistical analysis; Leyda Quintero for help in pro-viding blood samples; and the CDCHT-ULA (C-1278-04-03-B) and CONICIT (S1-2000000818) for providing financial support for this work.
References cited
ADLER, S. 1964. Leishmania. Advances in Parasitology 2: 35-96. [ Links ]
ALEXANDER, B.; CARVALHO, R.L; MCCALLUM, H.; PEREIRA, M.H. 2002. Role of the domestic chicken (Gallus gallus) in the epidemiology of urban visceral leishmaniasis in Brazil. Emerging Infectious Diseases 8 (12) :1480-1485. [ Links ]
AGUILAR, C.M.; FERNANDEZ, E.; DE FERNANDEZ, R.; DEANE, L.M. 1984 Study of an outbreak of cutaneous leish-maniasis in Venezuela. The role of domestic animals. Memórias do Instituto Oswaldo Cruz 79 (2): 185-195. [ Links ]
AÑEZ, N.; CAZORLA, D.; NIEVES, E.; CHATAING, B.; CASTRO, M.; DE YARBUH, A. L. 1988. Epidemiología de la leishmaniasis tegumentaria en Mérida, Venezuela. I. Diversidad y dispersión de especies flebotominas en tres pisos altitu-dinales y su posible role en la transmisión de la enfermedad. Memórias do Instituto Oswaldo Cruz 83 (4): 455-463. [ Links ]
BONFANTE-GARRIDO, R.; URDANETA, R.; URDANETA, I.; ALVARADO J. 1991a. Natural infection of Lutzomyia ovallesi (Diptera: Psychodidae) with leish-maniasis in Duaca, Lara State, Venezuela. Transactions of the Royal Society of Tropical Medicine and Hygiene 85 (1): 61. [ Links ]
BONFANTE-GARRIDO, R.; SPINETTI, H.; CUPILLO, E.; MOMEN, H.; GRIMAL-DI, G.1991b. Lutzomyia ovallesi (Diptera: Psychodidae) as a vector of cutaneous leish-maniasis in Venezuela. Parasitologia 33 (suppl): 99-104. [ Links ]
BRIEGEL, H. 1990 Metabolic relationship between female body size, reserves, and fecundity of Aedes aegypti. Journal of In-sect Physiology. 36 (3):165-172. [ Links ]
BRIEGEL, H.; WALTERT, A.; KUHN A, R. 2001. Reproductive physiology of Aedes (Aedimorphus) vexans (Diptera: Culicidae) in relation to flight potential. Journal of Medical Entomology 38 (4): 557-565. [ Links ]
DABA, S.; MANSOUR, N. S.; YOUSSEF, F. G.; SHANBAKY, N. M.; SHEHATA, M. G.; SAWAF, B. M. 1997. Vector-host-parasite inter-relationships in leishmania-sis. III. Impact of blood meal from natural vertebrate host on survival and the devel-opment of Leishmania infantum and L. major in Phlebotomus langeroni (Diptera: Psychodidae). Journal of Egypt Society of Parasitology. 27(3):781-794 [ Links ]
FELICIANGELI, M. D. 1991.Vector of leish-maniases in Venezuela. Parasitologia 33 (suppl): 229-236. [ Links ]
GRIMALDI, G.; TESH, R. B. 1993. Leishma-niasis of the New World: current concepts and implications for future research. Clini-cal Microbiology Reviews 6 (3): 230-250 [ Links ]
HARRE, J. G.; DORSEY, K. M.; ARMS-TRONG, K. L.; BURGE, J. R,; KINNA-MON, K. E. 2001. Comparative fecundity and survival rates of Phlebotomus papatasi sandflies membrane fed on blood from eight mammal species. Medical and Vet-erinary Entomology 15 (2): 189-196. [ Links ]
HURD, H. 2003. Manipulation of medically important insect vectors by their parasites. Annual Review of Entomology. 48 (4): 141-161. [ Links ]
KILLICK-KENDRICK, R.; LEANEY, A. J.; READY, P. D. 1977. The establishment, maintenance and productivity of a labora-tory colony of Lutzomyia longipalpis (Diptera: Psychodidae). Journal of Medical Entomology. 13 (4-5): 429-440. [ Links ]
LANDRY, S. V.; DEFOLIART, G. R.; HOGG, D. B. 1988. Adult body size and survivor-ship in a field population of Aedes triseriatus. Journal of the American Mosquito Control Association. 4 (2): 121-128. [ Links ]
LUITGARDS-MOURA, J.F.; CASTELLON, E.G.; ROSA-FREITAS, M.G. 2000. Aspects related to productivity for four generations of a Lutzomyia longipalpis labo-ratory colony. Memórias do Instituto Oswaldo Cruz 95 (2):251-257. [ Links ]
MAGNARELLI, L. A.; BURGER, J. F. 1984. Caloric reserves in natural populations of blackfly, Simulius decorum (Diptera: Simuliidae), and deerfly, Chrysops ater (Diptera: Tabanidae). Canadian Journal of Zoology. 62 (12): 2589-2593. [ Links ]
MAGNARELLI, L. A.; MODI, G. B. 1988. Caloric determinations of phlebotomine sand flies (Diptera: Psychodidae). Journal of Medical Entomology. 25(2): 127-130. [ Links ]
MONTOYA, J.; CADENA , H.; JARAMILLO, C. 1998. Rearing and colonization of Lutzomyia evansi (Diptera: Psychodidae), a vector of visceral leishmaniasis in Colombia. Memórias do Instituto Oswaldo Cruz 93 (2): 263-268. [ Links ]
MOSTOWY, W. M.; FOSTER, W. A. 2004. Antagonistic effects of energy status on meal size and egg-batch size of Aedes aegypti (Diptera: Culicidae). Journal of Vector Ecology 29 (1): 89-93. [ Links ]
NASCI, R.S. 1986. The relationship between adult mosquito body size and parity in field populations. Environmental Entomology 15 (4): 874-876. [ Links ]
NIEVES, E.; PIMENTA, P. F. 2002. Influ-ence of vertebrate blood meals on the de-velopment of Leishmania (Viannia) braziliensis and Leishmania ( Leishmania) amazonensis in the sand fly Lutzomyia migonei (Diptera: Psychodidae). American Journal Tropical Medicine and Hygiene 67 (6): 640-647. [ Links ]
NIEVES, E.; DÁVILA-VERA, D.; PALA-CIOS-PRÜ, E. 2004. Daño ultraestructural del intestino medio abdominal de Lutzomyia ovallesi (Ortiz) (Diptera: Psychodidae) ocasionado por Leishmania (Leishmania) amazonensis. Parasitologia Latinoamericana 59 (3-4): 115-122. [ Links ]
RUDIN, W.; HECKER, H. 1982. Functional morphology of the midgut of sandfly as compared to other hematophagous nema-tocera. Tissue & Cell 14 (4): 751-758. [ Links ]
SCHLEIN, Y.; WARBURG, A.; SCHNUR, L.F.; SHLOMAI, J. 1983. Vector compat-ibility of Phlebotomus papatasi depend-ent on differentially induced digestion. Acta Tropica 40 (1): 65-70. [ Links ]
SCHLEIN, Y.; JACOBSON, R. L. 1998. Re-sistance of Phlebotomus papatasi to infec-tion with Leishmania donovani is modulated by components of the infective bloodmeal. Parasitology. 117 (5): 467-473. [ Links ]
VAN HANDEL, E. 1972. Simple biological and chemical methods to determine the ca-loric reserves of mosquitoes. Mosquito News. 32 (4): 589-591. [ Links ]
VAN HANDEL, E. 1984. Metabolism nutri-ents in the adult mosquito. Mosquito News. 44 (4): 573-579. [ Links ]
Recibido: 12-oct-05 Aceptado: 26-dic-05