Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488On-line version ISSN 2665-4385
Rev. Colomb. Entomol. vol.35 no.2 Bogotá July/Dec. 2009
Multivariate morphometric differentiation between females of two cryptic species of Lutzomyia subgenus Helcocyrtomyia (Diptera: Psychodidae)
Diferenciación morfométrica multivariante entre hembras de dos especies crípticas de Lutzomyia subgénero Helcocyrtomyia (Diptera: Psychodidae)
DALMIRO CAZORLA P.1
1 Biologist, Dr. Sc. Laboratorio de Entomología, Parasitología y Medicina Tropical (L.E.P.A.M.E.T.), Centro de Investigaciones Biomédicas (C.I.B.), Universidad Nacional. Experimental “Francisco de Miranda” (UNEFM), Apdo. 7403, Coro 4101, Estado Falcón, Venezuela. Correo electrónico: lutzomyia@hotmail.com.
Recibido: 3-dic-2008 - Aceptado: 21-jul-2009
Abstract: The vectorial capacity of sibling species of the Neotropical genus Lutzomyia is likely to differ, thus a means of identifying the most important vector species is of critical importance to the epidemiology and control of the leishmanioses. Multivariate statistical procedures were employed to determine whether the females of two sibling sandfly species (genus Lutzomyia) of the subgenus Helcocyrtomyia, L. ceferinoi (N= 31) and L. erwindonaldoi (N= 32), can be discriminated on the basis of quantitative metric characters. Size independent discriminant analysis compared a set of three morphological characters of the wing (length of veins δ and α, and width of wing) measured from known specimens to detect differences between the two species. Morphometric discriminant analysis allowed differentiation of the females of both species with a high degree of accuracy (canonical correlation = 0.97; P << 0.01). The discriminant equations obtained may represent a useful and practical complementary taxonomic tool to distinguish accurately between previously unidentified female specimens of L. ceferinoi and L erwindonaldoi by measuring just three wing characters; these data can even be analyzed in the field for epidemiological in situ studies, aided by the widespread availability of laptops and statistical software.
Key words: Sandflies. Multivariate analysis. Leishmanioses. Sibling species.
Resumen: La capacidad vectorial de las especies crípticas que conforman al género Neotropical Lutzomyia puede diferir, por lo que la correcta identificación de las especies vectores más importantes es de suma relevancia para la epidemiología y control de las leishmaniosis. Se emplearon técnicas estadísticas de análisis multivariante para determinar si las hembras de dos especies crípticas de flebotominos (género Lutzomyia) del subgénero Helcocyrtomyia, L. ceferinoi (N=31) y L. erwindonaldoi (N=32), se pueden discriminar sobre la base de caracteres métricos cuantitativos. El análisis discriminante independiente de la talla comparó un grupo de tres caracteres morfológicos de las alas (longitudes de las venas alares δ y α y sus anchura), las cuales fueron medidas a partir de individuos conocidos para detectar diferencias entre las dos especies. El análisis discriminante morfométrico demostró la diferenciación de las hembras de ambas especies con un alto grado de exactitud (correlación canónica= 0.97; P<< 0.01). Las ecuaciones discriminantes obtenidas pueden representar una herramienta taxonómica complementaria y útil para distinguir con exactitud entre los especímenes hembra previamente desconocidos de L. ceferinoi y L. erwindonaldoi, con tan sólo medir tres caracteres alares; se podría analizar estos datos aun en campo para realizar estudios epidemiológicos in situ, apoyado por la amplia disponibilidad de computadores portátiles y software estadísticos.
Palabras clave: Flebotominos. Análisis multivariante. Leishmaniosis. Especies crípticas.
Introduction
One of the first steps in an epidemiological study on leishmanioses the correct identification of phlebotomine (Phlebotominae) vectors. Discriminating among isomorphic, cryptic species with close or related morphologies, or those presenting a wide range of clinal or teratological variability, is especially relevant due to differences in vector capacity or possible incidence of resistance or tolerance to chemicallyderived insecticides. This is an increasingly frequent problem in several countries (Lanzaro and Warburg 1995; Santamaría et al. 2002; Maroli and Khoury 2004; Watts et al. 2005).
The subgenus Helcocyrtomyia Barretto, 1962 belongs to the medically important phlebotomine sandfly genus Lutzomyia França, 1924, containing more than 30 Neotropical and Nearctic species, most of which have anthropophilic habits, and with at least three being suspected vectors of cutaneous leishmaniosis (Young and Duncan 1994). For the species identification within this subgenus, morphological characteristics of male genitalia, among others, have usually been used since females present very similar taxonomic characters, or even many of these are isomorphic or cryptic. Thus, morphological differentiation among females is undertaken principally by geographical association with the males (Galati and Cáceres 1994). This taxonomical problem is particularly difficult with respect to species in the Osornoi series (sensu Galati and Cáceres 1994) of this subgenus, three of which have been described for Venezuela, including Lutzomyia ceferinoi (Ortiz and Alvarez, 1963), Lutzomyia erwindonaldoi (Ortiz, 1978) (= Lutzomyia larensis Arredondo, 1987), and Lutzomyia strictivilla Young, 1979. The first two have been collected in sympatry in the Zulia state and the last two in Lara state; both places are in the western region of Venezuela (Feliciangeli 1988; Galati and Cáceres 1994; Young and Duncan 1994).
Recognizing that sibling species complexes occur among many medically important insects, new complementary tools were required to provide greater accuracy in taxonomic and epidemiological studies. Traditional multivariate morphometry has proven to be a useful additional method for recognition of specimens belonging to morphologically confusing taxa of several vector group species, including, among others, anophelines (Petrarca et al. 1998), simuliids (Krüger and Garms 1999) and triatomines (Costa et al. 2009). Concerning phlebotomine species, several methods have been used or proposed to solve the problem of differentiating those with closely-related morphologies, including morphometry (Gebre-Michael and Medhin 1997; Dujardin et al. 1999), gas chromatography analysis of cuticular hydrocarbon patterns (Ryan et al. 1986; Phillips et al. 1990; Mahamat and Hassanali 1998), cytogenetic karyotype studies (Kreutzer et al. 1988; Escovar et al. 2002), isoenzymatic profile analysis (Kreutzer et al. 1990; Márquez et al. 2001; Arrivillaga et al. 2003), and DNA patterns (Peixoto et al. 2001; Hodgkinson et al. 2003; Beati et al. 2004). Multivariate morphometric studies have been used successfully to solve taxonomic problems in the Phlebotominae subfamily, especially in cases involving cryptic species. Currently several research initiatives have been started to detect intraspecific geographical variation and their application to solve problems of morphological identification and stability of morphometric characters of taxonomic importance (Dujardin et al. 1999, 2003; Dujardin and Le Pont 2004).
In the course of an eco-epidemiological study on the transmission dynamics of tegumentary (TL) and visceral (VL) leishmanioses in endemic foci in Falcon state, in northwestern Venezuela, phlebotomine females belonging to the subgenus Helcocyrtomyia were initially collected without their respective males. By applying the typological taxonomic keys supplied by Young and Duncan (1994), in which we find ambiguous and subjective expressions such as “wing venation with beta longer than”, “individual sperm ducts wider than genital fork stem”, these specimens were erroneously identified as L. osornoi (Ristorcelli and Van Ty, 1941). Fortunately, in the last weeks of the study we were able to catch male specimens which, after the respective sex association and morphometric studies (Galati and Cáceres 1994), definitively corresponded to L. erwindonaldoi.
Accordingly, results are presented from a classical multivariate morphometric study that singled out discriminant functions that complement, even at field level, the traditional morphology for the accurate and specific identification of female specimens of L. erwindonaldoi and L. ceferinoi, by the measurement of just three morphological characters.
Materials and Methods
A total of 63 females were used in a classical morphometric analysis. The females were collected by a Shannon trap in several localities of Venezuela (Table 1). All specimens were cleared at room temperature in Nesbitt’ solution for 12-24 h, and later were mounted on microscope slides in Berlese’s medium. A detailed description of specimen preparation has been provided by Añez et al. (1988).
Three wing characters (Table 2) were measured using a light microscope fitted with an ocular micrometer. Morphological terminology is in accordance with Young and Duncan (1994).
Multivariate statistics were used to compare all morphological characters. Measurements were first transformed into natural logarithms (Jolicoeur 1963). A covariate matrix based on principal component analysis (PCA) was used to summarize total variation, without the necessity of assigning individuals to taxa. The first principal component (PC1) is generally considered a multivariate size vector, as verified by the positive and close correlation of each variable with PC1 (Dos Reis et al. 1990). Thus, the effect of size variation among wing characters was removed by regressing each character on PC1, and then applying size free discriminant function analysis (DA) to residues obtained from the regressions to assess the degree of distinctiveness of both sandfly taxa without allometric trends (Strauss 1985; Dos Reis et al. 1990). Therefore, the multivariate discriminant functions obtained could be used to discriminate between unknown females of both Helcocyrtomyia species on the basis of size independent shape differences rather than those caused by ecophysiographic dependent factors (Dujardin et al. 1999; Dujardin and Le Pont 2004). The Kappa index was used for verifying conflicting classifications (Landis and Koch 1977).
The discriminating utility of the three wing characters was evaluated in a blind test on a mixture of 11 L. erwindonaldoi and 10 L. ceferinoi. Additionally, a cluster analysis was performed based on Manhattan distance matrices, which were used to construct a dendrogram using the unweighted pairs group method analysis (UPGMA). Data were analyzed using PAST version 1.29 (Hammer and Harper 1999-2004), STATGRAPHICS Plus for Windows 20 packages (Statistical Graphic Corp., 1994-1996) and Web pages for statistical calculations (StatPages.net, members.aol.com/john71/javastat. html).
Results
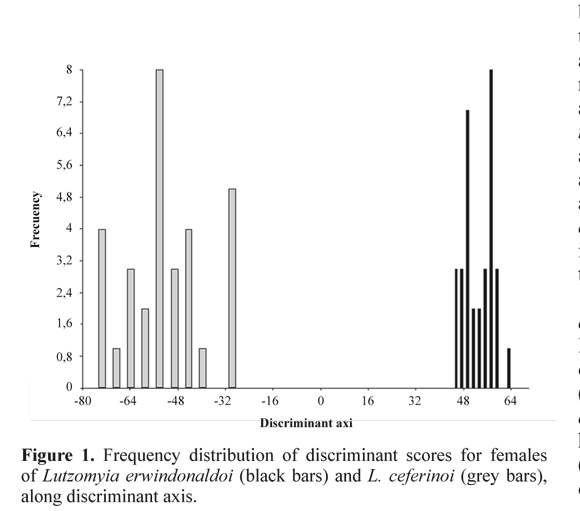
The first pool within group PC1 represented 99.85% of the total variation, and showed a highly positive correlation with each variable (Ww: 0.99; δ: 0.99; α: 0.99), thus representing a good size factor. DA produced two well defined and non overlapping phenetic groupings that corresponded to the two Helcocyrtomyia species (Fig. 1). These characters provided a highly significant canonical correlation (0.97; P << 0.01) for the derived discriminant function and allowed a perfect identification of individuals (100%; kappa = 1.0; Wilks lambda = 0.055; P < 0.0001) greater than would be expected by chance (50.27%). Therefore, an unknown sandfly could be identified accurately as L. erwindonaldoi or L. ceferinoi by multiplying the measured variables (in micrometers) with their respective classification coefficients. Thus, the classification function for L. erwindonaldoi was: y= -1.09010 x 1010 – 677.610 (Ww) + 4.90323 x 109 (α) – 2.00612 x 109 (δ). For L. ceferinoi it was: y= -1.09015 x 1010 – 677.639 (Ww) + 4.9035 x 109 (α) – 2.00615 x 109 (δ). In the blind test, 100% of specimens from two species populations were placed into the correct species groups. As shown in the dendrogram (Fig. 2), the geographic groups of both species had a wide phenetic separation. Nevertheless, even these species groups showed a visible intraspecific differentiation.
Using Galati and Cáceres’s cladistic criterion, L. erwindonaldoi (= L. larensis), L. ceferinoi, and L. strictivilla are classified in the series Osornoi, while L. scorzai (Ortiz, 1965) belongs to the series Sanguinaria. It is significant that in these four species, as in several species groups and subgenera of Lutzomyia (sensu Young and Duncan 1994), females are difficult to identify morphologically unless they are associated with their respective male counterparts; this situation often leads to identification problems (Galati and Cáceres 1994; Young and Duncan 1994). For example, the female individuals of L. ceferinoi used in this study were referred to initially as L. sp. of the vexator series, and could not be correctly identified until male specimens from recent colonization were obtained (Cazorla and Añez 1988). Similarly, the females of L. erwindonaldoi also studied here were first described as L. sp. of Helcocyrtomyia, or erroneously as “L. osornoi”, in accordance with the type keys of Young and Duncan (1994), which included females of L. erwindonaldoi and “L. larensis”, and in which subjective, vague or inaccurate expressions, such as “longer or shorter than” or “subequal to” were used. Eventually, their correct morphological identification was possible only after their respective males had been collected for a comparative morphological identification, and complemented by multivariate morphometric discrimination with L. ceferinoi females, as this paper shows.
Despite the above, confirming the taxonomic status of L. ceferinoi and L. erwindonaldoi has had, and still presents, several difficulties. In fact, L. ceferinoi was described by Ortiz and Álvarez (1963) from a male specimen collected in Biscucuy, Portuguesa state, in the western region of Venezuela. Later on, Ortiz (1978) described L. erwindonaldoi and widened the variation of L. ceferinoi from males (one for each sp.) collected under sympatric conditions from tree holes in Caja Seca, Zulia state, western Venezuela. It should be noted that in that publication (Ortiz 1978), the pictures of the external genitalia from both species were swapped, probably by an involuntary editorial or printing error, and this may easily be verified when measurements of both species are compared with both the original description for L. ceferinoi and the taxonomic keys presented by Ortiz (1978) (Galati and Cáceres 1994). This error might have led induced Young and Morales (1987), as Galati and Cáceres (1994) suggested, and with whom I agree, to the erroneous identification of L. erwindonaldoi in Colombia, and to the subsequent erroneous identification of the female. Thus, everything suggests that these authors really studied L. ceferinoi (Galati and Cáceres 1994). As mentioned above, the female and the male of L. ceferinoi were already described on the basis of material collected from populations in Mérida state, in the Andean region of Venezuela (Cazorla and Añez 1988). Young and Duncan (1994) considered this description and redescription of L. ceferinoi as “not valid”, because the entomological material had not been collected in the “type” locality of Caja Seca (Zulia state), and further because “there is no convincing evidence that it is conspecific with L. ceferinoi”. However, as stated previously, the above authors did not detect the “printing error” in Ortiz (1978), which might explain their confusion about the taxonomic status of L. ceferinoi. Additionally, they also did not consider that the type material of this species was virtually missing.
Concerning L. erwindonaldoi, the “printing/editorial error” in the original description by Ortiz (1978) may also lead to confusion if not taken in account. Additionally, there is complete agreement with the analysis of Galati and Cáceres (1994), which concluded that the L. larensis described by Arredondo (1987) was in fact a synonymy of L. erwindonaldoi, as the measurements of the two species did not distinguish between them, and that further comparing the external genitalia of a specimen from the type locality of L. larensis with those of specimens of L. erwindonaldoi, they concluded they were similar. Arredondo (1987), as Galati and Cáceres (1994) also point out, made no comparisons between the two species in spite of their clear morphological and morphometric affinities. Given that females of species of the Osornoi series (Galati and Cáceres 1994) are hard to identify morphologically if not associated with their respective males, and that L. ceferinoi and L. erwindonaldoi may coexist sympatrically (Ortiz 1978), the discriminating functions obtained in this study can be used as a practical complementary tool for the accurate identification of specimens by virtue of the measurements of just three wing characters. This may be done with the use of laptop computers and statistical software, even in the field for epidemiological in situ studies. This becomes especially relevant when dichotomous keys, such as those of Galati and Cáceres (1994, 2003), which contain subjective or vague expressions such as “delta ca. ½ of alpha”, “ flagellomere III longer than labrum-epipharynx”, are used for species identification in the Osornoi series.
It is worth mentioning that the traditional multivariate morphometry has demonstrated its usefulness in separating accurately isomorphic species in several Lutzomyia subgenus or species groups, including verrucarum (Añez et al. 1997) and aragoi (Dujardin et al., 2005) groups, and the subgenus Micropygomyia (Cazorla and Acosta 2003). Nevertheless, in those studies more than three morphological characters were required.
Acknowledgements
I am indebted to P. Morales for his assistance in the collection of sandfly specimens. Financial support was given by Fundacite Falcon (Grant Nº S197-012) and Decanato de Investigaciones, Universidad Nacional Experimental “Francisco de Miranda”, Coro, Falcon State, Venezuela.
Literature cited
Añez , N.; Cazorla , D.; Nieves , E.; Chataing , B.; Castro , M.; De Yarbuh , A. 1988. Epidemiología de la leishmaniasis tegumentaria en Mérida, Venezuela. I.- Diversidad y dispersión de especies flebotominas en tres pisos altitudinales y su posible role en la transmisión de la enfermedad. Memorias do Instituto Oswaldo Cruz 83 (4): 455-463. [ Links ]
Añez , N.; Valenta , D.; Cazorla , D.; Quicke , D.; Feliciangeli , M. 1997. Multivariate analysis to discriminate species of phlebotomine sand flies (Diptera: Psychodidae): Lutzomyia townsendi, L. spinicrassa, and L. youngi. Journal of Medical Entomology 34 (3): 312-316. [ Links ]
ARREDONDO, C. 1987. Descripción del macho y de la hembra de Lutzomyia larensis n. sp. (Diptera,Psychodidae, Phlebotominae) del Estado Lara, Venezuela. Memorias do Instituto Oswaldo Cruz 82 (3): 395-398. [ Links ]
Arrivilaga , J.; Mutebi , J.; Piñango , H.; Noris , D.; Ale xander , B.; Feliciangeli , M.; Lanzaro , G. 2003. The taxonomic status of genetically divergent populations of Lutzomyia longipalpis (Diptera: Psychodidae) based on the distribution of mitochondrial and isozyme variation. Journal of Medical Entomology 40 (5): 615-627. [ Links ]
Beati , L.; Cáceres , A.; Lee , J.; MUNSTERMANN, L. 2004. Systematic relationships among Lutzomyia sand flies (Diptera: Psychodidae) of Peru and Colombia based on the analysis of 12S and 28S ribosomal DNA sequences. International Journal of Parasitology 34 (2): 225-234. [ Links ]
Cazorla , D.; Añez , N. 1988. Descripción de la hembra de Lutzomyia ceferinoi (Diptera: Psychodidae) y redescripción del macho. Memorias do Instituto Oswaldo Cruz 83 (3): 313-322. [ Links ]
Cazorla , D.; Acosta , M. 2003. Multivariate morphometric discrimination among three species of Lutzomyia subgenus Micropygomyia (Diptera: Psychodidae). Journal of Medical Entomology 40 (6): 750-754. [ Links ]
Costa , J.; Peterson , A.; Dujardin , J. 2009. Morphological evidence suggests homoploid hybridization as a possible mode of speciation in the Triatominae (Hemiptera, Heteroptera, Reduviidae). Infection, Genetics and Evolution 9 (2): 263-270. [ Links ]
Dos Reis , S.; Pesoa , L.; Straus , R. 1990. Application of size-free canonical discriminant analysis to studies of geographic differentiation. Brazilian Journal of Genetics 13 (3): 509-520. [ Links ]
Dujardin , J.; Le Pont , F. 2004. Geographic variation of metric properties within the neotropical sandflies. Infection, Genetics and Evolution 4 (4): 353- 359. [ Links ]
Dujardin , J.; Le Pont , F.; Martínez , E. 1999. Quantitative morphological evidence for incipient species within Lutzomyia quinquefer (Diptera: Psychodidae). Memorias do Instituto Oswaldo Cruz 94 (6): 829- 836. [ Links ]
Dujardin , J.; Le Pont , F.; Baylac , M. 2003. Geographic versus interspecific differentiation of sandflies: a landmark data analysis. Bulletin of Entomological Research 93 (1): 87- 90. [ Links ]
Dujardin , J.; Le Pont , F.; Matias , A.; De la Riva , J. 2005. Morphometrical evidence for speciation within Bolivian Lutzomyia aragaoi (Diptera: Psychodidae). Infection, Genetics and Evolution 5 (4): 362-365. [ Links ]
Escovar , J.; Fero , C.; Cárdenas , E; Belo , F. 2002. Comparación cariotípica de cinco especies de Lutzomyia (Diptera: Psychodidae) de la serie townsendi grupo verrucarum en Colombia. Biomédica 22 (4): 499- 509. [ Links ]
Feliciangeli , M. 1988. La fauna flebotómica (Diptera: Psychodidae) en Venezuela. I.- Taxonomía y distribución geográfica. Boletín de la Dirección de Malariología y Saneamiento Ambiental 28 (3-4): 99-113. [ Links ]
Galati , E.; Cáceres , A. 1994. Descrição de Lutzomyia pallidithorax, sp. n. e de Lutzomyia castanea, sp. n. do Peru e análise cladística das séries do subgênero Helcocyrtomyia Barretto (Diptera, Psychodidae). Revista Brasileira de Entomologia 38 (2): 471-488. [ Links ]
Galati , E.; Cáceres , A. 2003. Description of Lutzomyia ( Helcocyrtomyia) herreri sp. nov. (Diptera, Psychodidae, Phlebotominae) from the South Peruvian Andes. Revista Brasileira de Entomología 47 (4): 607-613. [ Links ]
Gebre -Michael , T.; Medhin , G. 1997. Morphometric separation of females of Phlebotomus ( Phlebotomus) dubocqui and P. (P.) bergeroti (Diptera: Psychodidae). Journal of Medical Entomology 34 (4): 383-386. [ Links ]
Hodgkinson , V.; Birungi , J.; Quintana , M.; Dietze , R.; Munsterman , L. 2003. Mitochondrial cytochrome b variation in populations of the visceral leishmaniasis vector Lutzomyia longipalpis across eastern Brazil. American Journal of Tropical Medicine and Hygiene 69 (4): 386-392. [ Links ]
Jolicoeur , P. 1963. The multivariate generalization of the allometry equation. Biometrics 19 (3): 497-499. [ Links ]
Kreutzer , R.; Morales , A.; Cura , E.; Fero , C.; Young , D. 1988. Brain cell karyotypes of six new world sand flies (Diptera: Psychodidae). Journal of the American Mosquito Control Association 4 (4): 453-455. [ Links ]
Kreutzer , R.; Palau , M.; Morales , A.; Fero , C.; Feliciangeli , D.; Young , D. 1990. Genetic relationships among phlebotomine sand flies (Diptera: Psychodidae) in the verrucarum species group. Journal of Medical Entomology 27 (1): 1-8. [ Links ]
Krüger , A.; Garms , R. 1999. Morphometric characterization of members of the Simulium damnosum Theobald complex (Diptera: Simuliidae) from East and West Africa. Annals of Tropical Medicine and Parasitology 93 (7):753-761. [ Links ]
Landis , J.; Koch , G. 1977. The measurement of observer agreement for categorical data. Biometrics 33 (2): 159-174. [ Links ]
Lanzaro , G.; Wasburg , A. 1995. Genetic variability in phlebotomine sandflies: implications for leishmaniasis epidemiology. Parasitology Today 11 (4): 151- 154. [ Links ]
Mahamat , H.; Hasanali , A. 1998. Cuticular hydrocarbon composition analysis for taxonomic differentiation of phlebotomine sandfly species (Diptera: Psychodidae) in Kenya. Journal of Medical Entomology 35 (5):778-781. [ Links ]
Maroli , M.; Khoury , C. 2004. Prevention and control of leishmaniasis vectors: current approaches. Parasitologia 46 (1-2): 211- 215. [ Links ]
Márquez , L.; Lampo , M.; Rinaldi , M.; Lau , P. 2001. Gene flow between natural and domestic populations of Lutzomyia longipalpis (Diptera: Psychodidae) in a restricted focus of American visceral leishmaniasis in Venezuela. Journal of Medical Entomology 38 (1): 12-16. [ Links ]
Ortiz , I.; Álvarez , A. 1963. Sobre un nuevo Phlebotomus (P. ceferinoi nov. Sp.) de Venezuela (Insecta: Diptera, Psychodidae). Salud Pública 5 (23): 285- 288. [ Links ]
Ortiz , I. 1978. Phlebotomus erwindonaldoi sp. n. del grupo Peruensis (Diptera: Psychodidae) de Trujillo, Venezuela. Boletín de la Dirección de Malariología y Saneamiento Ambiental 18 (3): 205- 210. [ Links ]
Peixoto , A.; Gomes C de Amorety , P.; Lins , R.; Meire - les -Filho , A.; de Souza , N.; Kyriacou , C. 2001. New molecular markers for phlebotomine sand flies. International Journal of Parasitology 31 (5-6): 635-639. [ Links ]
Petrarca , V.; Sabatineli , G.; Touré, Y.; Di Deco , M.1998. Morphometric multivariate analysis of field samples of adult Anopheles arabiensis and An. gambiae s.s. (Diptera: Culicidae). Journal of Medical Entomology 35 (1):16-25. [ Links ]
Philips , A.; Le Pont , F.; Desjeux, P.; Bromfield , G.; Molyneux, D. 1990. Separation of Psychodopygus carrerai carrerai and P. yucumensis (Diptera: Psychodidae) by gas chromatography of cuticular hydrocarbons. Acta Tropica 47 (3): 145-149. [ Links ]
Ryan , L.; Philips , A.; Miligan , P.; Lainson , R.; Molyneux, D.; Shaw , J. 1986. Separation of female Psychodopygus wellcomei and P. complexus (Diptera: Psychodidae) by cuticular hydrocarbon analysis. Acta Tropica 43 (1): 85-89. [ Links ]
Santamaría, E.; Munsterman , L.; Fero , C. 2002. Estandarización del método propuesto por la Organización Mundial de la Salud para determinar los niveles de susceptibilidad de los vectores de leishmaniasis a insecticidas. Biomédica 22 (2): 211-218. [ Links ]
Straus , R. 1985. Evolutionary allometry and variation in body form in the South American catfish genus Corydoras (Callichthyidae). Systematic Zoology 34 (4): 381-396. [ Links ]
Wats , P.; Hamilton , J.; Ward , R.; Noves , H.; Souza , N.; Kemp , S.; Feliciangeli , M.; Brazil , R.; Maignon , R. 2005. Male sex pheromones and the phylogeographic structure of the Lutzomyia longipalpis species complex (Diptera: Psychodidae) from Brazil and Venezuela. American Journal of Tropical Medicine and Hygiene 73 (4): 734-743. [ Links ]
Young , D.; Duncan , M. 1994. Guide to the identification and geographic distribution of Lutzomyia sandflies in Mexico, the West Indies, Central and South America (Diptera: Psychodidae). Memories of the American Entomological Institute, Number 54. Associated Publishers, Gainesville, Florida, USA. 881 p. [ Links ]
Young , D.; Morales , A. 1987. New species and records of Phlebotomine sand flies from Colombia (Diptera: Psychodidae). Journal of Medical Entomology 24 (6): 651-665. [ Links ]














