Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488On-line version ISSN 2665-4385
Rev. Colomb. Entomol. vol.36 no.1 Bogotá Jan./June 2010
Sección Agrícola
Induced defense in Eucalyptus trees increases with prolonged herbivory
Defensa inducida en plantas de Eucaliptus se incrementa con prolongada herbivoría
HAMILTON GOMES DE OLIVEIRA1, ADRIÁN JOSÉ MOLINA-RUGAMA2, MARCOS A. M. FADINI3, DANIELA REZENDE4, ALBERTO SOTO G.5, CLÉBER OLIVEIRA6 and ANGELO PALLINI7
1 Ph.D. Corporación Centro de Investigación en Palma de Aceite, Calle 20A, #43-50, Bogota. Coordinador de Investigación en Entomología. hgomes@cenipalma.org.co. Autor para correspondencia.
2 Ph.D. Universidade Federal de Campina Grande, Brasil ajmolina@ccta.ufcg.edu.br
3 Ph.D. Universidade Federal de Sao Joao Del Rei fadini@ufsj.dedu.br
4 M.Sc. Universidade Federal de Viçosa, Brasil daniagoufv@hotmail.com
5 Ph.D. Universidad de Caldas, Colombia asotog@hotmail.com
6 Estudiante de pregrado en Agronomía Induced defense in Eucalyptus trees increases with prolonged herbivory Defensa inducida en plantas de Eucaliptus se incrementa con prolongada herbivoría HAMILTON GOMES DE OLIVEIRA1, ADRIÁN JOSÉ MOLINA-RUGAMA2, MARCOS A. M. FADINI3, DANIELA REZENDE4, ALBERTO SOTO G.5, CLÉBER OLIVEIRA6 and ANGELO PALLINI7 1 Ph.D. Corporación Centro de Investigación en Palma de Aceite, Calle 20A, #43-50, Bogota. Coordinador de Investigación en Entomología. hgomes@cenipalma.org.co. Autor para correspondencia. 2 Ph.D. Universidade Federal de Campina Grande, Brasil ajmolina@ccta.ufcg.edu.br 3 Ph.D. Universidade Federal de Sao Joao Del Rei fadini@ufsj.dedu.br 4 M.Sc. Universidade Federal de Viçosa, Brasil daniagoufv@hotmail.com 5 Ph.D. Universidad de Caldas, Colombia asotog@hotmail.com 6 Estudiante de pregrado en Agronomia, Universidad de Caldas, Colombia clebim001@hotmail.com 7 Ph.D. Universidade Federal de Viçosa, Brasil pallini@ufv.br Recibido: 15-may-2009 - Aceptado: 30-ene-2010 Abstract: Mechanisms of defense in plants can be activated by external stimuli such as herbivory. It is well-known that such induced defense occurs after short periods of herbivory, but little is known about long-term induction. In this paper, we studied the effects of induced defenses of Eucalyptus trees on Thyrinteina arnobia (Lepidoptera: Geometridae) over four generations. The effects of induction of eucalypt plants seemed to increase gradually with prolonged periods of herbivory. To our knowledge it is the first demonstration that induced defense changes in a gradual way with long-term herbivory. This suggests that these trees, and possibly many other plants, gradually invest more in induced defense with prolonged herbivore attacks. Key words: Plant-herbivore interaction. Plant defense. Thyrinteina arnobia. Induction of defence. Herbivore fitness Resumen: Mecanismos de defensa en plantas pueden ser activados por estímulos externos como herbivoría. Es bien conocido que la defensa inducida ocurre después de cortos períodos de herbivoría, pero se conoce poco acerca de la inducción de larga duración. En este artículo se estudió los efectos de la defensa inducida en árboles de Eucalyptus sobre Thyrinteina arnobia (Lepidoptera: Geometridae) durante cuatro generaciones Los efectos de la inducción de plantas de Eucalipto parecieron aumentar gradualmente con períodos prolongados de herbivoría. Según nuestro conocimiento es la primera demostración que la defensa inducida cambia de modo gradual con la herbivoría a largo plazo. Eso sugiere que estos árboles, y posiblemente muchas otras plantas, gradualmente invierten más en defensa inducida con ataques prolongados de herbívoros. Palabras clave: Interacción planta-insecto. Defensas de plantas. Thyrinteina arnobia. Inducción de la defensa. Eficacia reproductiva del herbívoro. Introduction Plant defense mechanisms result from a coevolutionary process, where the attack of herbivores promotes an adaptation for defense in the plant whereas herbivores, in turn, develop strategies to overcome the defense of plant (Bernays and Chapman 2000; Vendramin and Castiglioni 2000). Physical or morphological plant defenses include trichomes, spines, waxes, and tough foliage, and chemical defenses include production of toxins, repellents, and digestibility reducers (Cortesero et al. 2000). The increased production of volatiles by plants that are attacked by herbivores is often also viewed as a defense mechanism, because the volatiles attract natural enemies of the herbivores (Turlings et al. 1995; De Moraes et al. 1998; Arimura et al. 2005). Plant defenses can be either constitutive, i.e. always expressed, or induced by herbivory (Karban et al. 1997; Agrawal 1998). Both mechanisms of defense have the potential to affect abundance, survival, and rate of herbivore feeding as well as the population dynamics of natural enemies (Cortesero et al. 2000). Several studies have shown that induced resistance has important consequences for arthropod populations (Dicke et al. 1990; Karban and Baldwin 1997; Agrawal 2005). The production of substances such as tannins, proteinase inhibitors, lectins and terpenoids can be increased or induced in response to herbivory (Krause and Raffa 1995; Underwood et al. 2002). Generally, high levels of such secondary compounds render plants less attractive to herbivores (Rossi et al. 2004) or affect the life-history and consumption rate of herbivores (Lill and Marquis 2001; Kopper et al. 2002). The effects of induced defenses in different plant species affect the herbivores that caused the damage (Underwood 1999; Agrell et al. 2003; Foss y Rieske 2004; Agrawal 2005). The general idea seems to be that induction of plant resistance is either activated or not, and occurs quickly after the first damage was caused. Although this kind of rapid induced response is apparently very common among annual plants, there are indications that trees or perennial plants also possess delayed induction; early-season herbivory can alter host quality for later colonizers (Faeth 1986, 1992; Viswanathan et al. 2005). However, studies that have examined these interactions commonly use induction for a short time by herbivores or mechanical defoliation to mimic insect attack (Loughrin et al. 1994; Stevens and Lindroth 2005). To our knowledge, experiments that examine the effect of damage by herbivores for more that one generation of herbivores on defense of plants are particularly scarce. In this paper, we studied for four generations the effects of induced defences of Eucalyptus trees on Thyrinteina arnobia (Stoll, 1872) (Lepidoptera: Geometridae). The caterpillars T. arnobia (Lepidoptera: Geometridae) that lived on native Myrtaceae in Brazil turned voracious defoliators of various eucalypt species (Anjos et al. 1987; Zanuncio et al. 1994, 2000). Although eucalypt plants contain high concentrations of secondary compounds such as essential oils, tannins and phenols (Fox and Macauley 1977), these metabolites do not prevent T. arnobia from attacking eucalypt (Berti Filho et al. 1991). Little is known about the interactions between this defoliator and eucalypt trees, but it has been observed that 5th and 6th instars of T. arnobia change their feeding habits; they move from leaves to feed on the bark or on branches (Berti Filho and Wilcken 1993). In addition, this herbivore rarely attacks eucalypt plants that were previously damaged by conspecifics (J.C. Zanuncio, personal communication). Possibly, this feeding behavior is associated with the induced response after prolonged periods of damage. Here, we present results of attack in Eucalyptus trees for four generations on the fitness of T. arnobia. Materials and Methods Rearing of T. arnobia. Adults of T. arnobia were collected during an outbreak in eucalypt plantations in the municipality of Três Marias, state of Minas Gerais, Brazil. A culture was maintained at 25 ± 2ºC, 60 ± 10% of RH and 12 h photoperiod. The culture was initiated by placing pairs of males and females in plastic cups (500 ml). Each cup was provided with a strip of paper as oviposition substrate. Newly-hatched caterpillars were reared in insect cages (0.45 x 0.45 x 0.45 m) and could feed ad libitum on Eucalyptus spp. leaves until pupation (Holtz et al. 2003). Pupae of T. arnobia were removed from the cages, the sex was determined, and one male-female pair was put in clean plastic cups until adults emerged. After they had mated, females were transferred to oviposition cups as described above and eggs were collected and treated as above. Induced resistance on Eucalyptus plants. To determine how induced resistance affects the performance of T. arnobia, we compared its development and reproduction on two groups of eucalypt trees during four generations. A cohort of 20 plants of E.cloeziana (F. Muell.) (Myrtales: Myrtaceae), approximately 2 m tall and 1.5 years old was used. These plants were located outdoors near to the laboratory of Entomology of the Federal University of Viçosa, and were checked daily to remove and avoid attacks by other herbivores. The first group of trees consisted of plants without damage and each generation of caterpillars was allowed to feed and develop on different no induced trees. The second group consisted of plants that were previously injured by conspecifics. Thus, was hoped that damaged plants produced volatiles in response to herbivore damage, and these volatiles provide information about the presence of herbivores on these plants. Caterpillars were reared on the same group of trees for four generations; hence, the trees were induced for a prolonged period. The two groups of trees thus started differing in the period of induction after the first generation of caterpillars, because generation of Thyrinteina arnobia on damaged plants was longer that on the undamaged plants. Each treatment consisted of four replicates. Each replicate was represented by one tree containing 36 caterpillars. The eucalypt plants were infested according to the following procedure: Newly-hatched T. arnobia caterpillars were taken from the culture and divided into groups of six individuals and placed inside a mesh bag (0.25 x 0.20 m). Each host plant received randomly a total of six bags, enclosing part of a branch with intact foliage. The mesh bag served to confine the caterpillars on a part of the plant and to reduce the action of natural enemies. When the caterpillars had consumed between 50 and 60% of the total leaf area inside the bag, they were switched to another branch. In this way, competition for food among caterpillars was avoided. The herbivores were removed upon reaching the pupal stage and transferred to plastic cups (500 ml), and incubated in the laboratory until adult emergence. Adult females from not induced and induced eucalypt plants were mated with males of the same origin and placed in oviposition cups. Newlyhatched caterpillars were again introduced in a bag on either an undamaged tree or the tree that was previously damaged by their parents. Newly, as in the first experiment and in the next generations on each plant where put 36 caterpillars per plant. This procedure was repeated for three subsequent generations. Larval and pupal development, larval and pupal survivorship, the total number of eggs, egg viability and longevity of males and females were measured during each generation on both groups of eucalypt plants. Statistical analysis. Data from life history parameters were subjected to analysis of variance to compare the effect of induction of host plants on performance of T. arnobia. All biological parameters are presented as means ± standard error. In order to estimate the effects of induction on the rate of increase of the population, the intrinsic rate of increase (rm) was estimated. The formula used was: where x = age; T = maximum age; lx = probability of surviving to age x; mx = number of female offspring/female of age x (Carey 1993). Results and Discussion Induced and not induced trees differently affected several life-history parameters of the T. arnobia (Table 1). It is known that plants produce chemical substances that can act as constitutive or induced defenses against herbivores (Underwood et al. 2002). Induction of plant defenses systems can affect the abundance, the attack rate, the survival and the development of herbivorous arthropods (Karban and Baldwin 1997; Rossi et al. 2004). The tree studied here, E. cloeziana, apparently does not possess an efficient system of constitutive defense against attacks of T. arnobia caterpillars, because the herbivore growth rate on plants without previous injury is high (Fig. 1). However, we found negative effects on the development and reproduction of T. arnobia on plants that were previously attacked by co-specifics (Table 1). We always offered ample undamaged leaves to the caterpillars, thus these effects cannot have been caused by lack of food. Hence, the quality of leaves was reduced on plants that were previously attacked, probably as a consequence of induced defense. Although we did not evaluate the phytochemistry of eucalypt leaves, it is known from other trees that early season herbivory results in changes in condensed tannins and proteins in damaged leaves (Faeth 1986). Although the duration of the larval and pupal stages were similar in both treatments, juvenile survival as well as the total number of eggs laid and the percentage of eggs hatched were lower on induced plants than on not induced trees after four generations (Table 1). Eucalypt plants are rich in essential oils and contain high concentration of tannins (Fox and Macauley 1977). Such secondary compounds have been reported to cause adverse effects on the growth and development of important pest insects (Faeth 1986; Coley and Barone 1996; Oliveira et al. 2004). Probably, the production of these and other secondary metabolites in eucalypt plants might change with herbivory, as it was found in other species of plants either in the laboratory or in the field (Schultz and Baldwin 1982; Underwood et al. 2002; Kranthi et al. 2003; Rossi et al. 2004). Although high densities of T. arnobia can defoliate eucalypt trees completely, our results show that undamaged eucalypt leaves of trees that have been attacked by herbivores for a long period are of inferior quality for the development of T. arnobia. This could explain why the geometrid does not feed on the same eucalypt trees in the field for several generations. In conclusion, the effects of induction of eucalypt plants seem to increase gradually with prolonged periods of herbivory. To our knowledge it is the first demonstration that induced defense changes in a gradual way with long-term herbivory. This suggests that these trees, and possibly many other plants, gradually invest more in induced defense with prolonged herbivore attacks. Acknowledgements We thank “Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)” and “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior” for financial support and also thanks Arne Jansen for your important comments. Literature cited AGRAWAL, A. A. 1998. Induced responses to herbivory and increased plant performance. Science 279: 1201-1202. AGRAWAL, A. A. 2005. Future directions in the study of induced plant responses to herbivory. Experimental and Applied Entomology 115: 97-105. AGRELL, J.; OLESZEK, W.; STOCHMAL, A.; OLSEN, M.; ANDERSON, P. 2003. Herbivore-induced responses in alfalfa (Medicado sativa). Journal of Chemical Ecology 29: 303-320. ANJOS, N.; SANTOS, G. P.; ZANUNCIO, J. C. 1987. Pragas do eucalipto e seu controle. Informe Agropecuario 12: 50-58. ARIMURA, G.; KOST, C.; BOLAND, W. 2005. Herbivore-induced, indirect plant defenses. Acta Biochemica et Biophysica 1734: 91-111. BERNAYS, E. A.; CHAPMAN, R. F. 2000. Plant secondary compounds and grasshoppers: Beyond plant defenses. Journal of Chemical Ecology 26: 1773-1794. BERTI FILHO, E.; WILCKEN, C. F. 1993. Novo hábito alimentar de Thyrinteina arnobia (Lep.: Geometridae). Instituto de Pesquisa Estudos Florestais 46: 119-120. BERTI FILHO, E.; STAPE, J. L.; CERIGNONI, J. A. 1991. Surto de Thrynteina arnobia (Stoll, 1782) (Lepidoptera, Geometridae) em Eucalyptus citriodora Hook (Myrtaceae) no Estado de São Paulo. Revista de Agricultura 66: 46-46. CAREY, J. R. 1993. Applied Demography for Biologists with Special Emphasis on Insects. Oxford University Press, New York, USA. 206 p. COLEY, P. D.; BARONE, J. A. 1996. Herbivory and plant defenses in tropical forests. Annual Review of Ecology and Systematics 27: 305-335. CORTESERO, A.M.; STAPEL, J. O.; LEWIS, W. J. 2000. Understanding and manipulating plant attributes to enhance biological control. Biological Control 17: 35-49. DE MORAES, C. M.; LEWIS, W. J.; PARÉ, P. W.; ALBORN, H. T.; TUMLINSON, J. H. 1998. Herbivore-infested plants selectively attract parasitoids. Nature 393: 570-573. DICKE, M.; SABELIS, M.; TAKABAYASHI, J.; BRUIN, J.; POSTHUMUS, M. A. 1990. Plant strategies of manipulating predator-prey interactions through allelochemicals: prospects for application in pest control. Journal of Chemical Ecology 16: 3091-3118. FAETH. S. H. 1986. Indirect interactions between temporally separated herbivores mediated by the host plant. Ecology 67: 479- 494. FAETH. S. H. 1992. Interspecific and intraspecific interactions via plant responses to folivory: an experimental field test. Ecology 73: 1802-1813. FOSS, L. K.; RIESKE, L. K. 2004. Stem galls affect oak foliage with potential consequences for herbivory. Ecological Entomology 29: 273-280. FOX, L. R.; MACAULEY, B. J. 1977. Insect grazing on Eucalyptus in response to variation in leaf tannins and nitrogen. Oecologia 29: 145-162. HOLTZ, A. M.; OLIVEIRA, H. G.; PALLINI, A.; VENZON, M.; ZANUNCIO, J. C.; OLIVEIRA, C. L.; MARINHO, J. S.; ROSADO, M. C.. 2003. Desempenho de Thyrinteina arnobia Stoll (Lepidoptera: Geometridae) em eucalipto e goiaba: o hospedeiro nativo não é um bom hospedeiro? Neotropical Entomology 3: 427-431. KARBAN, R.; BALDWIN, I. T. 1997. Induced Responses to Herbivory. University of Chicago Press, Chicago, IL. 330 p. KARBAN, R.; AGRAWAL, A. A.; MANGEL, M. 1997. The benefits of induced defenses against herbivores. Ecology 78: 1351- 1355. KOPPER, B. J.; JAKOBI, V. N.; OSIER, T. L.; LINDROTH, R. L. 2002. Effects of paper birch condensed tannin on whitemarked tussock moth (Lepidoptera: Lymantriidae) performance. Environmental Entomology 31: 10-14. KRANTHI, S.; KRANTHI, K. R.; WANJARI, R. R. 2003. Influence of semilooper damage on cotton host-plant resistance to Helicoverpa armigera (Hub). Plant Science 164: 157-163. KRAUSE, S. C.; RAFFA, R. F. 1995. Defoliation intensity and larval age interact to affect sawfly performance on previously injured Pinus resinosa Oecologia 102: 24-30. LILL, J. T.; MARQUIS, R. J. 2001. The effects of leaf quality on herbivore performance and attack from natural enemies. Oecologia 126: 418-428. LOUGHRIN, J. H.; MANUKIAN, A.; HEATH, R. R.; TURLINGS, T. C. J.. 1994. Diurnal cycle of emission of induced volatile terpenoids by herbivore-injured cotton plants. Proceedings of the National Academy of Sciences USA 91: 11836-11840. OLIVEIRA, H. G.; LACERDA, F. G.; MARINHO, C. G. S.; DELLA LUCIA, T. M. C. 2004. Atratividade de Atta sexdens rubropilosa por plantas de eucalipto atacadas previamente ou não por Thyrinteina arnobia. Pesquisa Agropecuária Brasileira 39: 285-287. ROSSI, A. M.; STILING, P.; MOON, D. C.; CATTELL, M. V.; DRAKE, B. G. 2004. Induced defensive response of myrtle oak to foliar insect herbivory in ambient and elevated CO2. Journal of Chemical Ecology 30: 1143-1152. SCHULTZ, J. C.; BALDWIN, I. T. 1982. Oak leaf quality declines in response to defoliation by gypsy moth larvae. Science 217: 149-151. STEVENS M. T.; LINDROTH, R. L. 2005. Induced resistance in the indeterminate growth of aspen (Populus tremuloides). Oecologia 145: 298-306. , Universidad de Caldas, Colombia clebim001@hotmail.com [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ]
7 Ph.D. Universidade Federal de Viçosa, Brasil pallini@ufv.br
Recibido: 15-may-2009 - Aceptado: 30-ene-2010
Abstract: Mechanisms of defense in plants can be activated by external stimuli such as herbivory. It is well-known that such induced defense occurs after short periods of herbivory, but little is known about long-term induction. In this paper, we studied the effects of induced defenses of Eucalyptus trees on Thyrinteina arnobia (Lepidoptera: Geometridae) over four generations. The effects of induction of eucalypt plants seemed to increase gradually with prolonged periods of herbivory. To our knowledge it is the first demonstration that induced defense changes in a gradual way with long-term herbivory. This suggests that these trees, and possibly many other plants, gradually invest more in induced defense with prolonged herbivore attacks.
Key words: Plant-herbivore interaction. Plant defense. Thyrinteina arnobia. Induction of defence. Herbivore fitness
Resumen: Mecanismos de defensa en plantas pueden ser activados por estímulos externos como herbivoría. Es bien conocido que la defensa inducida ocurre después de cortos períodos de herbivoría, pero se conoce poco acerca de la inducción de larga duración. En este artículo se estudió los efectos de la defensa inducida en árboles de Eucalyptus sobre Thyrinteina arnobia (Lepidoptera: Geometridae) durante cuatro generaciones Los efectos de la inducción de plantas de Eucalipto parecieron aumentar gradualmente con períodos prolongados de herbivoría. Según nuestro conocimiento es la primera demostración que la defensa inducida cambia de modo gradual con la herbivoría a largo plazo. Eso sugiere que estos árboles, y posiblemente muchas otras plantas, gradualmente invierten más en defensa inducida con ataques prolongados de herbívoros.
Palabras clave: Interacción planta-insecto. Defensas de plantas. Thyrinteina arnobia. Inducción de la defensa. Eficacia reproductiva del herbívoro.
Introduction
Plant defense mechanisms result from a coevolutionary process, where the attack of herbivores promotes an adaptation for defense in the plant whereas herbivores, in turn, develop strategies to overcome the defense of plant (Bernays and Chapman 2000; Vendramin and Castiglioni 2000). Physical or morphological plant defenses include trichomes, spines, waxes, and tough foliage, and chemical defenses include production of toxins, repellents, and digestibility reducers (Cortesero et al. 2000). The increased production of volatiles by plants that are attacked by herbivores is often also viewed as a defense mechanism, because the volatiles attract natural enemies of the herbivores (Turlings et al. 1995; De Moraes et al. 1998; Arimura et al. 2005). Plant defenses can be either constitutive, i.e. always expressed, or induced by herbivory (Karban et al. 1997; Agrawal 1998). Both mechanisms of defense have the potential to affect abundance, survival, and rate of herbivore feeding as well as the population dynamics of natural enemies (Cortesero et al. 2000).
Several studies have shown that induced resistance has important consequences for arthropod populations (Dicke et al. 1990; Karban and Baldwin 1997; Agrawal 2005). The production of substances such as tannins, proteinase inhibitors, lectins and terpenoids can be increased or induced in response to herbivory (Krause and Raffa 1995; Underwood et al. 2002). Generally, high levels of such secondary compounds render plants less attractive to herbivores (Rossi et al. 2004) or affect the life-history and consumption rate of herbivores (Lill and Marquis 2001; Kopper et al. 2002). The effects of induced defenses in different plant species affect the herbivores that caused the damage (Underwood 1999; Agrell et al. 2003; Foss y Rieske 2004; Agrawal 2005). The general idea seems to be that induction of plant resistance is either activated or not, and occurs quickly after the first damage was caused. Although this kind of rapid induced response is apparently very common among annual plants, there are indications that trees or perennial plants also possess delayed induction; early-season herbivory can alter host quality for later colonizers (Faeth 1986, 1992; Viswanathan et al. 2005). However, studies that have examined these interactions commonly use induction for a short time by herbivores or mechanical defoliation to mimic insect attack (Loughrin et al. 1994; Stevens and Lindroth 2005). To our knowledge, experiments that examine the effect of damage by herbivores for more that one generation of herbivores on defense of plants are particularly scarce. In this paper, we studied for four generations the effects of induced defences of Eucalyptus trees on Thyrinteina arnobia (Stoll, 1872) (Lepidoptera: Geometridae).
The caterpillars T. arnobia (Lepidoptera: Geometridae) that lived on native Myrtaceae in Brazil turned voracious defoliators of various eucalypt species (Anjos et al. 1987; Zanuncio et al. 1994, 2000). Although eucalypt plants contain high concentrations of secondary compounds such as essential oils, tannins and phenols (Fox and Macauley 1977), these metabolites do not prevent T. arnobia from attacking eucalypt (Berti Filho et al. 1991). Little is known about the interactions between this defoliator and eucalypt trees, but it has been observed that 5th and 6th instars of T. arnobia change their feeding habits; they move from leaves to feed on the bark or on branches (Berti Filho and Wilcken 1993). In addition, this herbivore rarely attacks eucalypt plants that were previously damaged by conspecifics (J.C. Zanuncio, personal communication). Possibly, this feeding behavior is associated with the induced response after prolonged periods of damage. Here, we present results of attack in Eucalyptus trees for four generations on the fitness of T. arnobia.
Materials and Methods
Rearing of T. arnobia. Adults of T. arnobia were collected during an outbreak in eucalypt plantations in the municipality of Três Marias, state of Minas Gerais, Brazil. A culture was maintained at 25 ± 2ºC, 60 ± 10% of RH and 12 h photoperiod. The culture was initiated by placing pairs of males and females in plastic cups (500 ml). Each cup was provided with a strip of paper as oviposition substrate. Newly-hatched caterpillars were reared in insect cages (0.45 x 0.45 x 0.45 m) and could feed ad libitum on Eucalyptus spp. leaves until pupation (Holtz et al. 2003). Pupae of T. arnobia were removed from the cages, the sex was determined, and one male-female pair was put in clean plastic cups until adults emerged. After they had mated, females were transferred to oviposition cups as described above and eggs were collected and treated as above.
Induced resistance on Eucalyptus plants. To determine how induced resistance affects the performance of T. arnobia, we compared its development and reproduction on two groups of eucalypt trees during four generations. A cohort of 20 plants of E.cloeziana (F. Muell.) (Myrtales: Myrtaceae), approximately 2 m tall and 1.5 years old was used. These plants were located outdoors near to the laboratory of Entomology of the Federal University of Viçosa, and were checked daily to remove and avoid attacks by other herbivores. The first group of trees consisted of plants without damage and each generation of caterpillars was allowed to feed and develop on different no induced trees. The second group consisted of plants that were previously injured by conspecifics. Thus, was hoped that damaged plants produced volatiles in response to herbivore damage, and these volatiles provide information about the presence of herbivores on these plants. Caterpillars were reared on the same group of trees for four generations; hence, the trees were induced for a prolonged period. The two groups of trees thus started differing in the period of induction after the first generation of caterpillars, because generation of Thyrinteina arnobia on damaged plants was longer that on the undamaged plants. Each treatment consisted of four replicates. Each replicate was represented by one tree containing 36 caterpillars.
The eucalypt plants were infested according to the following procedure: Newly-hatched T. arnobia caterpillars were taken from the culture and divided into groups of six individuals and placed inside a mesh bag (0.25 x 0.20 m). Each host plant received randomly a total of six bags, enclosing part of a branch with intact foliage. The mesh bag served to confine the caterpillars on a part of the plant and to reduce the action of natural enemies. When the caterpillars had consumed between 50 and 60% of the total leaf area inside the bag, they were switched to another branch. In this way, competition for food among caterpillars was avoided. The herbivores were removed upon reaching the pupal stage and transferred to plastic cups (500 ml), and incubated in the laboratory until adult emergence. Adult females from not induced and induced eucalypt plants were mated with males of the same origin and placed in oviposition cups. Newlyhatched caterpillars were again introduced in a bag on either an undamaged tree or the tree that was previously damaged by their parents. Newly, as in the first experiment and in the next generations on each plant where put 36 caterpillars per plant. This procedure was repeated for three subsequent generations. Larval and pupal development, larval and pupal survivorship, the total number of eggs, egg viability and longevity of males and females were measured during each generation on both groups of eucalypt plants.
Statistical analysis. Data from life history parameters were subjected to analysis of variance to compare the effect of induction of host plants on performance of T. arnobia. All biological parameters are presented as means ± standard error. In order to estimate the effects of induction on the rate of increase of the population, the intrinsic rate of increase (rm) was estimated. The formula used was:
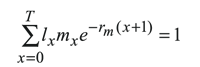
where x = age; T = maximum age; lx = probability of surviving to age x; mx = number of female offspring/female of age x (Carey 1993).
Results and Discussion
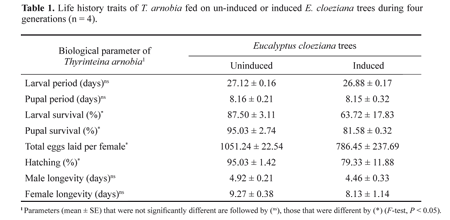
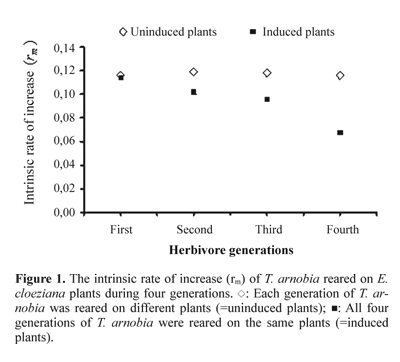
Induced and not induced trees differently affected several life-history parameters of the T. arnobia (Table 1). It is known that plants produce chemical substances that can act as constitutive or induced defenses against herbivores (Underwood et al. 2002). Induction of plant defenses systems can affect the abundance, the attack rate, the survival and the development of herbivorous arthropods (Karban and Baldwin 1997; Rossi et al. 2004). The tree studied here, E. cloeziana, apparently does not possess an efficient system of constitutive defense against attacks of T. arnobia caterpillars, because the herbivore growth rate on plants without previous injury is high (Fig. 1). However, we found negative effects on the development and reproduction of T. arnobia on plants that were previously attacked by co-specifics (Table 1). We always offered ample undamaged leaves to the caterpillars, thus these effects cannot have been caused by lack of food. Hence, the quality of leaves was reduced on plants that were previously attacked, probably as a consequence of induced defense. Although we did not evaluate the phytochemistry of eucalypt leaves, it is known from other trees that early season herbivory results in changes in condensed tannins and proteins in damaged leaves (Faeth 1986).
Although the duration of the larval and pupal stages were similar in both treatments, juvenile survival as well as the total number of eggs laid and the percentage of eggs hatched were lower on induced plants than on not induced trees after four generations (Table 1). Eucalypt plants are rich in essential oils and contain high concentration of tannins (Fox and Macauley 1977). Such secondary compounds have been reported to cause adverse effects on the growth and development of important pest insects (Faeth 1986; Coley and Barone 1996; Oliveira et al. 2004). Probably, the production of these and other secondary metabolites in eucalypt plants might change with herbivory, as it was found in other species of plants either in the laboratory or in the field (Schultz and Baldwin 1982; Underwood et al. 2002; Kranthi et al. 2003; Rossi et al. 2004). Although high densities of T. arnobia can defoliate eucalypt trees completely, our results show that undamaged eucalypt leaves of trees that have been attacked by herbivores for a long period are of inferior quality for the development of T. arnobia. This could explain why the geometrid does not feed on the same eucalypt trees in the field for several generations.
In conclusion, the effects of induction of eucalypt plants seem to increase gradually with prolonged periods of herbivory. To our knowledge it is the first demonstration that induced defense changes in a gradual way with long-term herbivory. This suggests that these trees, and possibly many other plants, gradually invest more in induced defense with prolonged herbivore attacks.
Acknowledgements
We thank “Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)” and “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior” for financial support and also thanks Arne Jansen for your important comments.
Literature cited
AGRAWAL, A. A. 1998. Induced responses to herbivory and increased plant performance. Science 279: 1201-1202.
AGRAWAL, A. A. 2005. Future directions in the study of induced plant responses to herbivory. Experimental and Applied Entomology 115: 97-105.
AGRELL, J.; OLESZEK, W.; STOCHMAL, A.; OLSEN, M.; ANDERSON, P. 2003. Herbivore-induced responses in alfalfa (Medicado sativa). Journal of Chemical Ecology 29: 303-320.
ANJOS, N.; SANTOS, G. P.; ZANUNCIO, J. C. 1987. Pragas do eucalipto e seu controle. Informe Agropecuario 12: 50-58.
ARIMURA, G.; KOST, C.; BOLAND, W. 2005. Herbivore-induced, indirect plant defenses. Acta Biochemica et Biophysica 1734: 91-111.
BERNAYS, E. A.; CHAPMAN, R. F. 2000. Plant secondary compounds and grasshoppers: Beyond plant defenses. Journal of Chemical Ecology 26: 1773-1794.
BERTI FILHO, E.; WILCKEN, C. F. 1993. Novo hábito alimentar de Thyrinteina arnobia (Lep.: Geometridae). Instituto de Pesquisa Estudos Florestais 46: 119-120.
BERTI FILHO, E.; STAPE, J. L.; CERIGNONI, J. A. 1991. Surto de Thrynteina arnobia (Stoll, 1782) (Lepidoptera, Geometridae) em Eucalyptus citriodora Hook (Myrtaceae) no Estado de São Paulo. Revista de Agricultura 66: 46-46.
CAREY, J. R. 1993. Applied Demography for Biologists with Special Emphasis on Insects. Oxford University Press, New York, USA. 206 p.
COLEY, P. D.; BARONE, J. A. 1996. Herbivory and plant defenses in tropical forests. Annual Review of Ecology and Systematics 27: 305-335.
CORTESERO, A.M.; STAPEL, J. O.; LEWIS, W. J. 2000. Understanding and manipulating plant attributes to enhance biological control. Biological Control 17: 35-49.
DE MORAES, C. M.; LEWIS, W. J.; PARÉ, P. W.; ALBORN, H. T.; TUMLINSON, J. H. 1998. Herbivore-infested plants selectively attract parasitoids. Nature 393: 570-573.
DICKE, M.; SABELIS, M.; TAKABAYASHI, J.; BRUIN, J.; POSTHUMUS, M. A. 1990. Plant strategies of manipulating predator-prey interactions through allelochemicals: prospects for application in pest control. Journal of Chemical Ecology 16: 3091-3118.
FAETH. S. H. 1986. Indirect interactions between temporally separated herbivores mediated by the host plant. Ecology 67: 479- 494.
FAETH. S. H. 1992. Interspecific and intraspecific interactions via plant responses to folivory: an experimental field test. Ecology 73: 1802-1813.
FOSS, L. K.; RIESKE, L. K. 2004. Stem galls affect oak foliage with potential consequences for herbivory. Ecological Entomology 29: 273-280.
FOX, L. R.; MACAULEY, B. J. 1977. Insect grazing on Eucalyptus in response to variation in leaf tannins and nitrogen. Oecologia 29: 145-162.
HOLTZ, A. M.; OLIVEIRA, H. G.; PALLINI, A.; VENZON, M.; ZANUNCIO, J. C.; OLIVEIRA, C. L.; MARINHO, J. S.; ROSADO, M. C.. 2003. Desempenho de Thyrinteina arnobia Stoll (Lepidoptera: Geometridae) em eucalipto e goiaba: o hospedeiro nativo não é um bom hospedeiro? Neotropical Entomology 3: 427-431.
KARBAN, R.; BALDWIN, I. T. 1997. Induced Responses to Herbivory. University of Chicago Press, Chicago, IL. 330 p.
KARBAN, R.; AGRAWAL, A. A.; MANGEL, M. 1997. The benefits of induced defenses against herbivores. Ecology 78: 1351- 1355.
KOPPER, B. J.; JAKOBI, V. N.; OSIER, T. L.; LINDROTH, R. L. 2002. Effects of paper birch condensed tannin on whitemarked tussock moth (Lepidoptera: Lymantriidae) performance. Environmental Entomology 31: 10-14.
KRANTHI, S.; KRANTHI, K. R.; WANJARI, R. R. 2003. Influence of semilooper damage on cotton host-plant resistance to Helicoverpa armigera (Hub). Plant Science 164: 157-163.
KRAUSE, S. C.; RAFFA, R. F. 1995. Defoliation intensity and larval age interact to affect sawfly performance on previously injured Pinus resinosa Oecologia 102: 24-30.
LILL, J. T.; MARQUIS, R. J. 2001. The effects of leaf quality on herbivore performance and attack from natural enemies. Oecologia 126: 418-428.
LOUGHRIN, J. H.; MANUKIAN, A.; HEATH, R. R.; TURLINGS, T. C. J.. 1994. Diurnal cycle of emission of induced volatile terpenoids by herbivore-injured cotton plants. Proceedings of the National Academy of Sciences USA 91: 11836-11840.
OLIVEIRA, H. G.; LACERDA, F. G.; MARINHO, C. G. S.; DELLA LUCIA, T. M. C. 2004. Atratividade de Atta sexdens rubropilosa por plantas de eucalipto atacadas previamente ou não por Thyrinteina arnobia. Pesquisa Agropecuária Brasileira 39: 285-287.
ROSSI, A. M.; STILING, P.; MOON, D. C.; CATTELL, M. V.; DRAKE, B. G. 2004. Induced defensive response of myrtle oak to foliar insect herbivory in ambient and elevated CO2. Journal of Chemical Ecology 30: 1143-1152.
SCHULTZ, J. C.; BALDWIN, I. T. 1982. Oak leaf quality declines in response to defoliation by gypsy moth larvae. Science 217: 149-151.
STEVENS M. T.; LINDROTH, R. L. 2005. Induced resistance in the indeterminate growth of aspen (Populus tremuloides). Oecologia 145: 298-306.
TURLINGS, T. C. J.; LOUGHRIN, J. H.; MCCALL, P. J.; RÖSE, U. S. R.; LEWIS, W. J.; TUMLINSON, J. H. 1995. How caterpillar- damaged plants protect themselves by attracting parasitic wasps. Proceedings of the National Academy of Sciences USA 92: 4169-4174. [ Links ]
UNDERWOOD, N. 1999. The influence of plant and herbivore characteristics on the interactions between induced resistance and herbivore population dynamics. American Naturalist 153: 282-294. [ Links ]
UNDERWOOD, N.; RAUSHER, M.; COOK, W. 2002. Bioassay versus chemical assay: measuring the impact of induced and constitutive resistance on herbivores in the field. Oecologia 131: 211-219. [ Links ]
VENDRAMIM, J. D.; CASTIGLIONI, E. 2000. Aleloquímicos, resistência de plantas e plantas inseticidas. In: Guedes, J.C.; Costa, I. D.; Castiglioni, E. (Eds.). Bases e Técnicas do Manejo de Insetos. Santa Maria: UFSM/CCR/DFS, Brazil, pp. 113-128. [ Links ]
VISWANATHAN, D. V.; NARWANI, A. J. T.; THALER, J. S. 2005. Specificity in induced plant responses shapes patterns of herbivore occurrence on Solanum dulcamara. Ecology 86: 886-896. [ Links ]
ZANUNCIO, J. C.; NASCIMENTO, E. C.; ZANUNCIO, T. V. 1994. Major lepidopterous defoliators of eucalypt in southeast Brazil. Forest Ecology and Management 65: 53-63. [ Links ]
ZANUNCIO, J. C.; ZANUNCIO, T. V.; LOPES, E. T.; RAMALHO, F. S. 2000. Temporal variations of Lepidoptera collected in a Eucalyptus plantation in the State of Goiás, Brazil. Netherlands Journal of Zoology 50: 435-443. [ Links ]