Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488On-line version ISSN 2665-4385
Rev. Colomb. Entomol. vol.36 no.2 Bogotá July/Dec. 2010
Selectivity of insecticides used in corn crops to adult Trichogrammaatopovirilia (Hymenoptera: Trichogrammatidae)
Selectividad de insecticidas usados en maíz a los adultos Trichogrammaatopovirilia (Hymenoptera: Trichogrammatidae)
JADER BRAGA MAIA1, GERALDO ANDRADE CARVALHO2, MARIA ISABELLA SANTOS LEITE3, RODRIGO LOPES DE OLIVEIRA4 and LETÍCIA MAKYAMA5
1 Ph.D. candidate in Entomology at the Department of Entomology. Federal University of Lavras (UFLA). Cx. Postal: 3037, CEP: 37200-000, Lavras. Minas Gerais, Brazil. maiajader@yahoo.com.br Corresponding author.
2 Dr. Prof. gacarval@den.ufla.br.
3 M.Sc. maryisabella@yahoo.com.br.
4 Undergraduate student of Agronomy. rodrigo_lopes_oliveira@yahoo.com.br.
5 Undergraduate student of Zootechny. lemakiyama@gmail.com.
Recibido: 21-nov-2009 • Aceptado: 1-sep-2010
Abstract: The aim of this work was to evaluate the toxicity of the insecticides imidacloprid/β-cyfluthrin (Connect 100/12.5 SC), chlorfenapyr (Pirate 240 SC), chlorpyrifos (Astro 450 EW), novaluron (Rimon 100 CE), spinosad (Tracer 480 SC) and triflumuron (Certero 480 SC), as used in corn crops ( Zea mays ), to parent generation females and to F1 and F2 generation specimens of Trichogrammaatopovirilia. Eggs of Anagasta kuehniella were glued to cardstock strips and placed under a germicidal lamp to kill embryos. These were then sprayed with the chemical products using a Potter tower and exposed to parasitism 24, 48, and 96 hours after application of the compounds for a span of 24 hours. We evaluated the number of dead specimens and the number of eggs parasitized by parent generation females, as well as the percent emergence and parasitic capacity of the F1 and F2 generations. Chlorfenapyr, spinosad, chlorpyrifos, and imidacloprid/β-cyfluthrin were moderately harmful to adult T. atopovirilia, while novaluron was slightly harmful. Triflumuron was harmless and could be used in integrated pest management programs intended to preserve adult T. atopovirilia in corn crops.
Key words: Pesticides. Parasitoids. Zea mays . Toxicity. Anagasta kuehniella.
Resumen: Se evaluó la toxicidad de los insecticidas imidacloprid/ β-ciflutrina (Connect 100/12,5 SC), clorfenapir (Pirate 240 SC), clorpirifos (Astro 450 EW), novalurom (Rimon 100 CE), spinosade (Tracer 480 SC) y triflumurom (Certero 480 SC), utilizados en el cultivo de maíz (Zea mays ), para adultos de la generación maternal de Trichogramma atopovirila y para especímenes de las generaciones F1 y F2. Huevos de Anagasta kuehniella fueron adheridos a cartulinas e irradiados con una lámpara antigermicida para matar a los embriones. Luego, se les aplicó mediante una torre de Potter los productos químicos y se expusieron durante 24 h a parasitismo a las 24, 48 y 96 h después de la aplicación de los compuestos. Se evaluó el número de especímenes muertos y el número de huevos parasitados por hembra de la generación maternal, también el porcentaje de emergencia y la capacidad de parasitismo de las generaciones F1 y F2. Clorfenapis, espinosade, clorpirifós e imidacloprid/β-ciflutrina fueron moderadamente perjudiciales para los adultos de T. atopovirila, mientras novalurom fue levemente perjudicial. Triflumurom fue inocuo y puede ser utilizado en programas de manejo integrado de plagas donde se busque preservar adultos de T. atopovirila en el cultivo de maíz.
Palabras clave: Pesticidas. Parasitoides. Zea mays . Toxicidad. Anagasta kuehniella.
Introduction
Fall armyworm Spodoptera frugiperda (J.E. Smith, 1797) (Lepidoptera: Noctuidae) is the most common pest to affect corn crops, being present every crop year and causing increasingly extensive damage to plantations, potentially affecting as much as 37% of the crop (Mendes et al. 2008). That and other pests of corn crops are mainly controlled by chemical products, which in turn have been causing emergence of secondary pests, resurgence and selection of resistant populations, in addition to causing a negative impact on the environment (Economic and Social Research Council 2009). Bearing that in mind, the use of alternative pest control methods to protect corn crops is a matter of the greatest importance as an effort to minimize these damaging effects, including biological control using parasitoids and predators.
Among natural enemies that help control fall armyworm in corn crops are parasitoids of the genus Trichogramma, drawing worldwide attention for being egg parasites and killing their hosts before emergence and attack to the plant (Lundgren et al. 2002).
Some authors noted the presence of Trichogrammaatopovirilia Oatman & Platner,1983 (Hymenoptera: Trichogrammatidae) parasitizing eggs of S. frugiperda in field conditions, demonstrating their potential as pest control agents in corn crops (Alvarez and Roa 1995; Zucchi and Monteiro 1997). Beserra and Parra (2004) evaluated the parasitic capacity and number of adults emerging per egg of S. frugiperda in a laboratory and observed that mean values were higher for T. atopovirilia than for Trichogramma pretiosum Riley, 1879 (Hymenoptera: Trichogrammatidae). They reported that T. atopovirilia has stronger chances of increasing its population in a shorter time than T. pretiosum, thus being apparently more suitable for biological control of S. frugiperda.
The effectiveness of these parasitoids in integrated pest control management programs, however, is conditional on the use of chemical products that will not affect the parasitism and development of parasite populations, in other words, it is conditional to the application of selective compounds (Carvalho et al. 2002; Degrande et al. 2002; Foerster 2002).
In studying the effect of 40 agrochemicals in various commercial formulations on adult individuals of T. atopovirilia, Manzoni et al. (2007) verified that 45% (18) were found to be harmless, 15% (6) were slightly harmful, 12.5% (5) were moderately harmful and 27.50% (11) were harmful. The variations in how this parasitoid species responded to pesticides demonstrate the need for studies in search of new molecules to find compatibility between chemical and biological methods for this particular parasitoid species.
Given the above, the objective of this work is to evaluate the effect of new insecticides recommended for corn crops on T. atopovirilia in order to gather information that will help decision making as to which pesticides to use in pest management programs involving corn crops, ultimately seeking to preserve this parasitoid species.
Material and Methods
The following insecticides were used in this study (technical and commercial name, formulation, dosage and chemical group are listed for each one): imidacloprid/β-cyfluthrin (Connect 100/12.5 SC - 0.33/0.04 g a.i. L-1, Neonicotinoid/ Pyrethroid), chlorfenapyr (Pirate 240 SC - 0.6 g a.i. L-1, Pyrazole Derivative), chlorpyrifos (Astro 450 EW - 0.75 g a.i. L-1, Organophosphate), novaluron (Rimon 100 CE - 0.05 g a.i. L-1, Benzoylurea), spinosad (Tracer 480 SC - 0.16 g a.i. L-1, Spinosyn) and triflumuron (Certero 480 SC - 0.048 g a.i. L-1, Benzoylurea). Water was used as control treatment.
Twenty females up to 24 hours old per treatment were collected from a laboratory nursery and were placed separately in 8 cm x 2.5 cm glass tubes, fed with honey droplets smeared on the inside of the tubes which were then sealed with PVC film. Approximately 125 eggs of Anagasta kuehniella (Zeller, 1879) (Lepidoptera: Pyralidae) up to 24 hours old were glued to 5 cm x 0.5 cm strips of blue cardstock and placed under a germicidal lamp to kill embryos according to Parra (1997), then pulverized with chemical compounds using a Potter spray tower regulated at a pressure of 15 lb/in2 and applied volume of 1.5 ± 0.5 μL/cm2.
The treated eggs were placed in an environmental chamber at 24±2oC, RH 70±10%, with 14 hours of photophase, being then offered to female individuals of T. atopovirilia 24, 48, and 96 hours after application of the insecticides, for 24 hours. The female individuals were then kept in the same tubes in order to assess their mortality throughout, while the cards containing the supposedly parasitized eggs were transferred into new tubes and stored in an environmental chamber until emergence of F1 generation parasitoids. Items being assessed included daily mortality over eight days, parasitic capacity, and percentage of insect emergence.
To evaluate the effects of pesticides on the newly emerged F1 generation adults originating from the eggs of A. kuehniella previously treated and then exposed to parasitism 24, 48, and 96 hours after application of the products, twenty female individuals of T. atopovirilia per treatment were placed in separate glass tubes, each receiving 125 untreated eggs of A. kuehniella up to 24 hours old, glued to card strips and placed under a germicidal lamp to kill embryos, as mentioned previously. The parasitic period was 24 hours, at the end of which the females were discarded and the card strips containing the supposedly parasitized eggs were kept in an environmental chamber under the above mentioned conditions, until full development and emergence of F2 generation parasitoids was reached. Items assessed included parasitic rate of F1 generation females and emergence percentage of F2 generation specimens.
Each treatment consisted of five replicates and each experimental portion comprised four card strips with eggs previously offered to the wasps for parazitation. The bioassays used a completely randomized design in a three exposure times x seven compounds factorial arrangement, totaling 21 treatments.
The data were submitted to analysis of variance and the mean values were compared using the Scott-Knott cluster analysis at the 5% significance level (Scott and Knott 1974). The evaluated pesticides were further grouped into the following toxicity categories as a function of the reduction in parasitoid survival rate according to IOBC recommendations: class 1 = harmless (<30% reduction), class 2 = slightly harmful (30% to 79% reduction), class 3 = moderately harmful (80% to 99% reduction) and class 4 = harmful (>99% reduction) (Sterk et al. 1999, Van de Veire et al. 2002). The control treatment was used as reference parameter. The mean percentage of reduction in parasitoid survival was obtained using the following equation: % of reduction = 100 – [(% general mean of treatment with pesticide / % general mean of control treatment) x 100].
Results and Discussion
The parasitic capacity of female individuals of T. atopovirilia that had come into contact with host eggs 24, 48, and 96 hours after their contamination was reduced by virtually every compound, although triflumuron and novaluron allowed 100% parasitism and thus these were classified as harmless (class 1); the remaining products were considered moderately harmful (class 3) (Table 1). These results agree with Carvalho et al. (2001) and Parreira (2007), who failed to identify negative effects of triflumuron and novaluron respectively on the parasitic capacity of T. pretiosum when exposed to eggs of A. kuehniella 24 and 48 hours after contamination with the product.
A substantial decrease in the number of eggs parasitized by parent generation females of T. atopovirilia when exposed to eggs treated with chlorpyrifos 24, 48 and 96 hours after contamination was also verified by Moscardini et al. (2008). These authors evaluated fenitrothion and methidathion, organophosphate products, and concluded that the reduced parasitism probably resulted from the mortality inflicted on female individuals, whether by direct contact with residues or by ingestion of such products during the parasitic process.
As for emergence of F1 generation parasitoids, chlorfenapyr was noted to be slightly harmful to females exposed to host eggs 24, 48 and 96 hours after application of the compound. Insecticide novaluron was slightly harmful, noting that 96 hours after application it presented one of the lowest mean values of emergence, approximately 31.3%. The remaining compounds were rated as harmless (class 1) (Table 2).
Parreira (2007) verified that novaluron caused a reduction in the emergence percentage of F1 generation individuals of T. pretiosum when exposed to treated eggs of alternative host A. kuehniella, similarly to the result found in this work, where novaluron was rated as slightly harmful (class 2) (Table 2).
The parasitic capacity of F1 generation females of T. atopovirilia could not be evaluated in chlorfenapyr and chlorpyrifos treatments when the parent generation females were exposed to contaminated eggs 24, 48 and 96 hours after contamination due the high mortality inflicted by these products soon after insect emergence (Table 3).
None of the insecticides were noted to affect the number of eggs parasitized by the F1 generation of T. atopovirilia 24 hours after application of the compounds, with novaluron causing a decreasing reduction in the number of parasitized eggs throughout the evaluations. When parasitoids came into contact with host eggs 48 and 96 hours after contamination with triflumuron and novaluron, no reduction occurred in the parasitic rate, with mean values 46,9 and 43,9, and 21,1 and 22,4 for parasitized eggs / female respectively (Table 3).
Similar results for triflumuron and novaluron were found by Carvalho et al. (2001) and Stefanello Jr et al. (2008), who, in evaluating the effects of these products on the parasitic capacity of T. pretiosum on treated eggs of A. kuehniella, observed that they were slightly harmful.
As for the emergence of F2 generation of T. atopovirilia originating from F1 generation females that had come into contact with eggs of A. kuehniella 24 hours after their contamination, it was noted that parasitoid emergence was not affected, with mean values ranging from 96.8% to 99.2% (Table 4).
Chlorfenapyr and chlorpyrifos caused 100% of mortality in F1 generation insects immediately after their emergence (Tables 3 and 4), preventing evaluation of the emergence percentage of F2 generation specimens. Similar results were found by Moscardini et al. (2008), who, in evaluating the effect of fenitrothion and methidathion on T. pretiosum, failed to obtain the emergence percentage of F2 generation specimens due to 100% of mortality in F1 generation parasitoids.
Spinosad, triflumuron and novaluron did not affect the emergence of F2 generation females throughout the evaluation period, being rated as harmless (class 1). The evaluation at 96 hours revealed that spinosad and imidacloprid reduced the percentage of parasitoid emergence, with mean values 76.7% and 58.9% respectively (Table 4).
Parreira (2007) evaluated the action of triflumuron, imidacloprid and novaluron on the emergence of F2 generation specimens of T. pretiosum originating from F1 generation females that had come into contact with eggs of A. kuehniella 24 and 48 hours after contamination and noted that these compounds did not cause reduction in the emergence percentage, being thus rated as harmless. Carvalho et al. (2003) studied the effect of triflumuron on T. pretiosum and also observed innocuousness when this parasitoid was exposed to eggs of alternative host A. kuehniella 24 and 48 hours after application of the product, causing no reduction in the emergence of F2 generation insects.
As a function of the reduction in emergence percentage of F2 generation females as caused by spinosad, triflumuron, imidacloprid/β-cyfluthrin and novaluron, these products were rated as class 1 = harmless (Table 4).
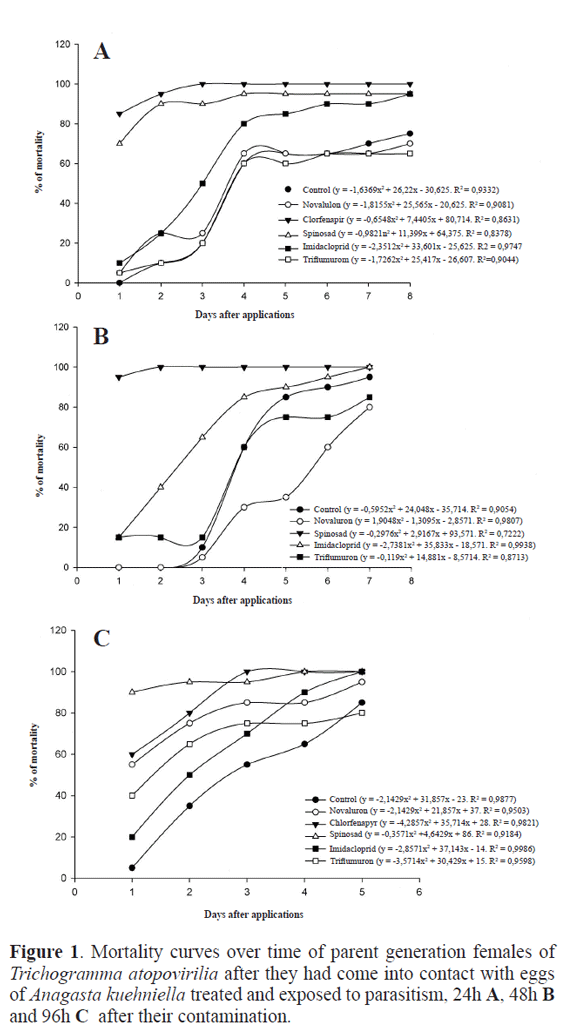
Spinosad and chlorpyrifos caused 95% and 100% of mortality in the fourth evaluation (4 days after application) and on the first evaluation (24 hours after application) respectively, while triflumuron caused 65.0% of mortality eight days after application. Imidacloprid/β-cyfluthrin only caused 10.0% of mortality 24 hours after application, increasing throughout the evaluation period to 95.0% of mortality on day eight. Novaluron revealed a similar pattern to the control treatment, presenting a peak mortality of 70,0% (Fig. 1A).
Chlorfenapyr and chlorpyrifos caused 100% of mortality in insects right in the first evaluation, while spinosad caused 100% of mortality in the second evaluation. Triflumuron and novaluron revealed a similar pattern to the control treatment, with mean values of mortality at around 95.0% and 80.0% on the last evaluation day respectively (Fig. 1B).
Chlorpyrifos caused 100% of mortality 24 hours after being applied, while chlorfenapyr caused 60.0% of mortality in the first evaluation, reaching 100% on day three. Spinosad presented 90.0% of mortality right on day one and 100% on day four. Triflumuron, imidacloprid/β-cyfluthrin and novaluron caused 40.0%, 20.0% and 55.0% of mortality respectively in the first evaluation, increasing to around 95.0% on the last day. The control treatment presented 5.0% of mortality in the first evaluation, increasing to around 85.0% on the last day (Fig. 1C).
The results found in this work can contribute to a better understanding of the potentialities of combined use of parasitoids T. atopovirilia and selective compounds, ultimately seeking to control fall armyworm infestation in corn crops. However, additional studies are required using natural hosts, preferably under field conditions, to finally validate or refute the toxicity of compounds to this beneficial species.
Acknowledgments
Many thanks to the Research Aid Foundation of Minas Gerais State (FAPEMIG) and CNPq for providing the necessary financial support to accomplish this work.
Cited literature
ÁLVAREZ, L. R.; ROA, F. G. 1995. Comportamento parasítico de Telenomus sp. em Spodoptera frugiperda. Revista Colombiana de Entomología 21(2): 191-196. [ Links ]
BESERRA. E. B., PARRA J. R. P. 2004. Biologia e parasitismo de Trichogramma atopovirilia Oatman & Platner e Trichogramma pretiosum Riley (Hymenoptera, Trichogrammatidae) em ovos de Spodoptera frugiperda (J. E. Smith) (Lepidoptera, Noctuidae). Revista Brasileira de Entomologia 48(1): 119-126. [ Links ]
CARVALHO, G. A.; PARRA, J. R. P.; BAPTISTA, G. C. 2001. Impacto de produtos fitossanitários utilizados na cultura do tomateiro na fase adulta de duas linhagens de Trichogramma pretiosum Riley, 1879 (Hymenoptera: Trichogrammatidae). Ciência e Agrotecnologia 25 (3): 560-568. [ Links ]
CARVALHO, G. A.; REIS, P. R.; MORAES, J. C.; FUINI, L. C.; ROCHA, L. C. D.; GOUSSAIN, M. M. 2002. Efeitos de alguns inseticidas utilizados na cultura do tomateiro (Lycopersicon esculentum Mill.) a Trichogramma pretiosum Riley, 1879 (Hymenoptera: Trichogrammatidae). Ciência e Agrotecnologia 26 (6): 1160-1166. [ Links ]
CARVALHO, G. A.; PARRA J. R. P.; BATISTA, G. C. 2003. Efeito de produtos fitossanitários utilizados na cultura do tomateiro (Lycopersicon esculentum Mill.) sobre Trichogramma pretiosum Riley, 1879 nas gerações F1 e F2 em ovos de Anagasta kuehniella (Zeller, 1879). Ciência e Agrotecnologia 27(2): 295-304. [ Links ]
DEGRANDE, E. P.; REIS, P. R.; CARVALHO, G. A.; BELARMINO, L. C. 2002. Metodologia para Avaliar o impacto de pesticidas sobre inimigos naturais. p.71- 93. In PARRA, J. R. P.; BOTELHO. P. S. M.; CORRÊA-FERREIRA. B. S.; SIMÕES BENTO. J. M. (Ed). Controle Biológico no Brasil: parasitóides e predadores. São Paulo: Manole. 609p. [ Links ]
ECONOMIC AND SOCIAL RESEARCH COUNCIL (2008 October 10). 2009. Biological Alternatives to Chemical Pesticides. Science Daily. In http://www.sciencedaily.com/releases/2008/10/081008065841.htm. [Date of revision: 14 april 2009]. [ Links ]
FOERSTER, L. A. 2002. Seletividade de inseticidas a predadores e parasitóides. p.95-114. In PARRA, J. R. P.; BOTELHO. P. S. M.; CORRÊA-FERREIRA. B. S.; SIMÕES BENTO. J. M. Controle Biológico no Brasil: parasitóides e predadores. São Paulo: Manole. 609p [ Links ]
LUNDGREN, J. G.; HEIMPEL, G. E.; BOMGREN A.S. 2002. Comparison of Trichogramma brassicae (Hymenoptera: Trichogrammatidae) augmentation with organic and synthetic pesticides for control of cruciferous Lepidoptera. Environmental Entomology 31(6):1231-1239. [ Links ]
MANZONI, C.G.; GRÜTZMACHER, A.D.; GIOLO, F. P.; HÄRTER, W, R.; CASTILHOS, R.V.; PASCHOAL, M. D. F. 2007. Seletividade de Agroquímicos utilizados na produção integrada de maçã aos parasitóides Trichogramma pretiosum Riley e Trichogramma atopovirilia Oatman & Platner (Hymenoptera: Trichogrammatidae). BioAssay 2 (1): 11p. Available at: http:// www.bioassay.org.br/articles/2.1/BA2.1.pdf. [Date of revision: 27 july 2008]. [ Links ]
MENDES, S. M.; MARUCCI, R. C.; MOREIRA, S. G.; WAQUIL, J. M. 2008. Milho Bt: avaliação preliminar da resistência de híbridos comerciais à lagarta-do-cartucho, Spodoptera frugiperda (J. E. Smith, 1797). Comunicado técnico 157, 8p. Sete Lagoas, MG. [ Links ]
MOSCARDINI, V. F.; MOURA, A.P.; CARVALHO, G. A. LASMAR, O. 2008. Efeito residual de inseticidas sintéticos sobre Trichogramma pretiosum Riley (Hym., Trichogrammatidae) em diferentes gerações. Revista Acta Scientiarum 30(2): 177-182. [ Links ]
PARRA, J. R. P. 1997. Técnicas de criação de Anagasta kuehniella, hospedeiro alternativo para produção de Trichogramma, p.121- 150. In J. R. P. Parra & R. A. Zucchi (eds.), Trichogramma e o controle biológico aplicado. Piracicaba, Fealq. 324p. [ Links ]
PARREIRA, D. S. 2007. Seletividade de inseticidas reguladores de crescimento de neonicotinóides a Trichogramma pretiosum Riley, 1879 (Hymenoptera: Trichogrammatidae). 52p. (Masters dissertation in Agronomy/Entomology) Universidade Federal de Lavras, Lavras. [ Links ]
SCOTT, A. J.; KNOTT, M. A. 1974. A cluster analysis method for grouping means in the analysis of variance. Biometrics 30(3): 507-512. [ Links ]
STERK, G.; HASSAN, S. A.; BAILLOD, M.; BAKKER, F.; BIGLER, F.; BLÜMEL, S.; BOGENSCHÜTZ, H.; BOLLER, E.; BROMAND, B.; BRUN, J.; CALIS, J.N.M.; COREMANSPELSENEER, J.; DUSO, C.; GARRIDO, A.; GROVE, A.; HEIMBACH, U.; HOKKANEN, H.; JACAS, J.; LEWIS, G.; MORETH, L.; POLGAR, L.; ROVERSTI, L.; SAMSØE-PETERSEN, L.; SAUPHANOR, B.; SCHAUB, L.; STÄUBLI, A.; TUSET, J.J.; VAINIO, A.; VAN DE VEIRE, M.; VIGGIANI, G.; VIÑUELA, E.; VOGT, H. 1999. Results of the seventh joint pesticide testing programme carried out by the IOBC/WPRSWorking Group ´Pesticides and Beneficial Organisms´. BioControl 44 (1): 99-117. [ Links ]
STEFANELLO J.R., G.J., GRÜTZMACHER, A.D.; GRÜTZMACHER, D.D.; DALMAZO, G.O.; PASCHOAL, M. D. F.; HÄRTER, W.R. 2008. Efeito de inseticidas usados em cultura de milho sobre a capacidade de parasitismo de Trichogramma pretiosum Riley, 1879 (Hymenoptera: Trichogrammatidae). Arquivos do Instituto Biológico 75(2): 187-193. [ Links ]
VAN DE VEIRE, M.; STERK, G.; VAN DER STAAIJ, M.; RAMAKERS, P. M. J.; TIRRY, L. 2002. Sequential testing scheme for the assessment of the side-effects of plant protection products on the predatory bug Orius laevigatus. BioControl 47(1): 101-113. [ Links ]
ZUCCHI, R. A.; MONTEIRO, R. C. 1997. O gênero Trichogramma na América do Sul. p. 41-66. In: PARRA, J.R.P.; ZUCCHI, R.A. (Ed.) Trichogramma e o controle biológico aplicado. Piracicaba: FEALQ. 324p. [ Links ]