Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488On-line version ISSN 2665-4385
Rev. Colomb. Entomol. vol.36 no.2 Bogotá July/Dec. 2010
Control of American cockroach (Periplaneta americana) and German cockroach (Blattella germanica) by entomopathogenic nematodes
Control de la cucaracha americana (Periplaneta americana) y de la cucaracha alemana (Blattella germanica) por nematodos entomopatógenos
MONCHAN MAKETON1, APINYA HOMINCHAN2 and DARARAT HOTAKA3
1 Ph.D., Zoology Department, Faculty of Science, Kasetsart University, Bangkok, Thailand. fscimcm@hotmail.com. Corresponding Author.
2 M. Sc. Departmentof Disease Control, Ministry of Public Health. Thailand.
3 M. Sc. Department of Land Development, Ministry of Agriculture and Cooperative. Thailand.
Recibido: 13-nov-2009 • Aceptado: 6-may-2010
Abstract: Two local and three imported entomopathogenic nematodes (EPNs) were tested for control of the American cockroach (Periplaneta americana) and the German cockroach (Blattella germanica). Only two Steinernematidae caused substantial cockroach mortality; one was a local strain of Steinernema sp. (strain T1), and the other was an imported strain of S. carpocapsae. A homemade bait that contained cat food and attapulgite clay at a ratio of 3:7 (W:W, 10 g total per bait) and 1x106 Steinernema sp. (T1) per bait resulted in 48.0 ± 4.7% mortality of the American cockroach and 57.7 ± 8.0% mortality of the German cockroach. A similar bait containing S. carpocapsae caused 40.0 ± 3.3% mortality of the American cockroach and 86.7 ± 4.7% mortality of the German cockroach. The optimal concentration of Steinernema sp. (T1) and S. carpocapsae to control the American and German cockroach was 1x106 EPNs and 5.4x104 EPNs per bait, respectively. The most susceptible stage of the American cockroach to both EPNs was the last instar, but susceptibility of the German cockroach to both EPNs did not differ among cockroach stages.
Key words: Cockroach bait. Steinernematidae. Steinernema carpocapsae.
Resumen: Se probaron dos nemátodos entomopatogenicos locales y tres importados (EPNs) para controlar la cucaracha americana (Periplaneta americana) y la cucaracha alemana (Blattella germanica). Solamente dos Steinernematidae causaron mortalidad sustancial de las cucarachas, una cepa local de Steinernema sp. (cepa T1), y la otra fue una cepa importada de S. carpocapsae. Un cebo casero que contiene arcilla atapulgita en una proporción 3:7 (W:W, 10 g total por cebo) y 1x106 Steinernema sp. (T1) resultó en una mortalidad de 48,0 ± 4,7% de la cucaracha americana y 57.7 ± 8.0% mortalidad de la cucaracha alemana. Un cebo similar que contenía S. carpocapsae causó una mortalidad de 40,0 ± 3,3% para la cucaracha americana y 86,7 ± 4,7% mortalidad para la cucaracha alemana. La concentración óptima de Steinernema sp. (T1) y S. carpocapsae para controlar la cucaracha americana y cucaracha alemana fue de 1x106 EPNs y 5,4x104 EPNs por cebo respectivamente. El último instar de la cucaracha americana fue la más susceptible al ataque de cualquier nematodo mientras que no hubo diferencias en la susceptibilidad entre estadios en la cucaracha alemana.
Palabras clave: cebos para cucarachas. Steinernematidae. Steinernema carpocapsae.
Introduction
American and German cockroaches (Dictyoptera: Blattidae and Blattellidae) are pests that can threat human health. The American cockroach, Periplaneta americana (Linnaeus, 1758) (Blattidae), is the largest of the house-infesting roaches, while the German cockroach, Blattella germanica (Linnaeus, 1767) (Blattellidae), is smaller. Both cockroaches have been spread throughout the world by commerce (Rust et al. 1991). Both cockroaches can contaminate food with bacterial diseases that result in food poisoning, dysentery, and diarrhea, and both can cause childhood asthma (Chanbang 1997). For the control of cockroaches, boric acid and chemical insecticides have been studied extensively (Appel and Benson 1995; Appel and Stanley 2000; Appel 2003; Wang and Bennett 2006). However, cockroach resistance has been reported to some compounds such as bendiocarb, cypermethrin, permethrin, propoxur, and chlorpyrifos (Valles and Yu 1996; Wei et al. 2001; Pridgeon et al. 2002).
Some parasitoids have been tested for the biological control of gravid stages of cockroaches. These parasitoids include Aprostocetus hagenowii (Ratzeburg, 1852); (Hymenoptera: Eulophidae), Anastatus tenuipes (Bolivar y Pieltain, 1925); (Hymenoptera: Eupelmidae), Comperia merceti (Compere, 1938); (Hymenoptera: Encyrtidae) (Lebeck 1991), Aprostocetus asthenogmus (Waterston, 1915); (Hymenoptera: Eulophidae) (Shamim et al. 2001), and Evania appendigaster (Linnaeus, 1758); (Hymenoptera: Evaniidae) (Hwang and Chen 2004). Potential microbial biological control agents include fungi belonging to the genera Metarhizium, Paecilomyces, Verticillium, and Aspergillus (Pathak and Kulshrestha 1998). An isolate of the bacterium Bacillus thuringiensis Berliner, 1915 was also shown to induce cockroach mortality (Payne et al. 1994). The virus Periplaneta fuliginosa densovirus has been proposed for the control of the smoky-brown cockroach, P. fuliginosa (Serville, 1839) (Jiang et al. 2008).
Entomopathogenic nematodes (Nematoda: Steinernematidae and Heterorhabditidae) (EPNs) are commonly used as biological control agents of insects in cryptic habitats (Somsook 1991; Ramos-Rodríguez et al. 2006). Kochler et al. (1992) determined that, among five cockroach species, the American cockroach was the least susceptible to infection by Steinernema carpocapsae (Weiser, 1955) whether the nematode was applied directly or in baits; in particular, no mortality occurred with bait stations. Appel et al. (1993) evaluated the efficacy of EPNs in the Steinernematidae for controlling the German cockroach. Nguyen and Smart Jr. (1996) identified the Steinernematidae and Heterorhabditidae for control of the German cockroach. However, both EPNs were effective in controlling the German cockroach but were ineffective in controlling the American cockroach.
The current research evaluated the potential of five EPNs for the control of both the American and German cockroach. The EPNs were tested in baits containing cat food and attapulgite clay because such baits would be relatively inexpensive to produce and easy to use.
Materials and Methods
Two EPNs native to Thailand were used: Steinernema sp. strain T1 and Heterorhabditis indica (Kaya, 1990) strain T2 were originally isolated from Kanchanaburi Province in western Thailand (14°0’15”N 993257”E) and have been maintained in our laboratory. Three imported EPNs were also used: S. carpocapsae, S. glaseri (Steiner, 1932), and H. bacteriophora Poinar, 1975 were obtained from the Thai Department of Agriculture. All EPNs were raised in wax moth larvae (Galleria mellonella L., 1758) in our laboratory. Specimens of the American and German cockroach were obtained from the Thai Department of Health Science.
Efficacy test. One Petri dish bottom (9 cm diameter) filled with cotton wool was placed in a sterile plastic box (18.0 x 12.5 x 7.0 cm). EPNs were added to each dish in 6 ml of distilled water that contained 0.0, 1.7x103, 8.3x103, 1.7x104, 8.3x104, or 1.7x105 EPNs per ml. Dry cat food (Purina Corp., MO, USA) was then added to each dish (1 g/dish). Ten male American cockroaches were then released into each box because they have low body weight distribution (Appel et al. 1993). There were three replicate boxes for each combination of EPN species and concentration. The boxes were maintained at 25°C for seven days. The number of dead roaches was checked daily. All dead cockroaches were removed from the boxes and were examined to determine whether mortality was caused by EPN followed the method of White (1927). The same procedure was followed with the German cockroach but the concentrations of the EPNs were lower because German cockroaches are much smaller than American cockroaches. The concentrations were 0.0, 1x103, 3x103, 6x103, 9x103, and 1.2x104 EPNs per ml of distilled water.
Bait compositions. Dry cat food (Purina Corp., MO, USA) and crackers (Nabisco Corp., NY, USA) mixed with attapulgite clay (AGSORB-325 LVM-GA, Agrisorbents, IL, USA) were tested as baits. Attapulgite clay was included because it might make the bait environment resemble the soil environment, which is the natural habitat for EPNs.
Five kinds of baits, each with 10 g total contents, were prepared: 1) ground cracker: attapulgite clay 1:1 (W:W); 2) ground cracker: attapulgite clay 3:7; 3) ground cat food: attapulgite clay 1:1; 4) ground cat food: attapulgite clay 3:7; and 5) attapulgite clay alone. These components were mixed together before EPNs were added. The moisture content was 50% for each bait at the time of preparation.
The two most effective EPNs in controlling the American cockroach from the efficacy tested (Steinernema sp. strain T1 and the imported S. carpocapsae) were selected and prepared at the concentrations of 0.0 (the control) and 1.7x105 EPNs per ml of a 0.1% formalin solution. Six milliliters of the EPNs or the control (0.1% formalin without EPNs) were then mixed with each bait in a clean plastic bag; at this point, the moisture content of the baits had dropped to 40-42%. Every bait was then placed in a Petri dish bottom and the dish was placed in a plastic box as described above. Ten male American cockroaches were released into each box, and boxes were kept at 25°C for seven days. There were five replicate boxes for each combination of bait, EPN strain, and EPN concentration. Cockroach mortality was recorded daily.
The same procedure was followed with the German cockroach. In addition, the survival of the EPNs in the baits was determined. Baits were placed in Petri dishes and boxes without cockroaches at 25°C. EPN survival was quantified followed the method of White (1927).
Optimal EPN concentration. Bait formulation four (ground cat food: attapulgite clay 3:7) was used for this experiment. Two EPNs that were effective in killing the American cockroach (Steinernema sp. strain T1 and the imported S. carpocapsae) were prepared at seven concentrations: 0.0, 1.7x103, 8.3x103, 1.7x104, 8.3x104, 1.7x105, and 8.3x105 EPNs per ml of 0.1% formalin solution. Mixing and testing procedures were performed as described in the previous experiment. Five replications were used. The experiment was also performed with the German cockroach but the EPN concentrations were 0.0, 1x103, 3x103, 6x103, 9x103, and 1.2x104 per ml.
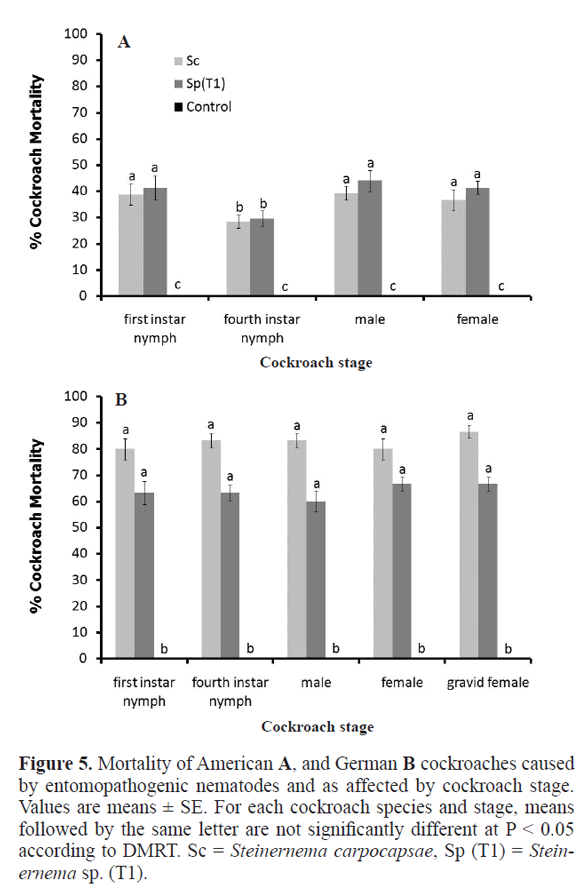
Susceptibility of American cockroach and German cockroach stages. Bait number four, the two most effective EPNs (Steinernema sp. strain T1 and the imported S. carpocapsae), and their optimal concentrations obtained from the test (1.7x105 EPNs per ml for the American cockroach and 9x103 EPNs per ml for the German cockroach) were used to test the susceptibility of each stage of development of the cockroach. three stages of the American cockroach were used: first instar nymph, fourth instar nymph, adult male, and adult female. Ten specimens of each type were released into a plastic box containing a Petri dish with bait number four and EPN (or bait without EPN) as described earlier. There were five replicate boxes for each combination of EPN, cockroach stage, and EPN level (plus or minus); boxes were kept at 25°C for seven days. Dead cockroaches were counted daily for seven days. The experiment was also performed with the German cockroach but gravid females were included.
Statistical analysis. An analysis of variance (ANOVA) was used to compare the mortality of cockroaches and efficacy among EPN and baits. Mortality data were normalized by log transformation. Duncan’s new multiple range test (DMRT) were used if there is any significant difference among treatments. Lethal times were analyzed by Probit analysis with 95% confidence intervals, using SAS version 9.1.3.
Results and Discussion
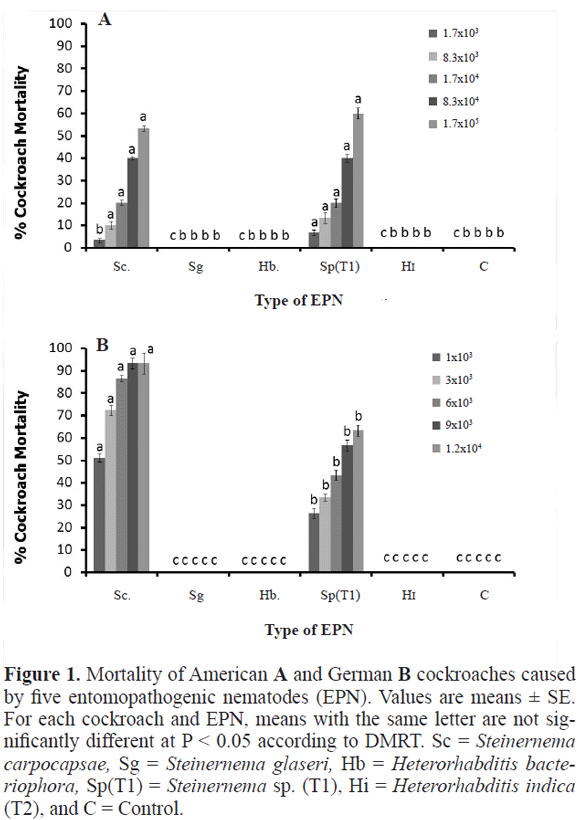
Efficacy test. At 1.7x105 EPNs per ml, Steinernema sp. (T1) caused the highest mortality (60.0 ± 2.4%) with a lethal time (LT50) of 4.7 days (Fig. 1A). The second most effective EPN was S. carpocapsae, which caused 53.3 ± 2.1% mortality and had an LT50 of 5.5 days. American cockroach mortalitycaused by Steinernema sp. (T1) was significantly greater (P < 0.05, F = 132, df = 5) than that caused by S. carpocapsae. S. glaseri , H. bacteriophora, and H. indica (T2) caused no mortality of the American cockroach (Fig. 1A).
Whereas Steinernema sp. (T1) caused higher mortality of the American cockroach than S. carpocapsae, the opposite was true with the German cockroach (F = 850, P < 0.05) (Fig. 1B). S. carpocapsae caused 93.3 ± 4.7% mortality and had an LT50 of 2.8 days while Steinernema sp. (T1) caused only 63.3 ± 2.4% mortality and had an LT50 of 4.5 days. As with the American cockroach, S. glaseri , H. bacteriophora, and H. indica (T2) caused no mortality of the German cockroach. The subsequent experiments therefore used only Steinernema sp. (T1) and S. carpocapsae.
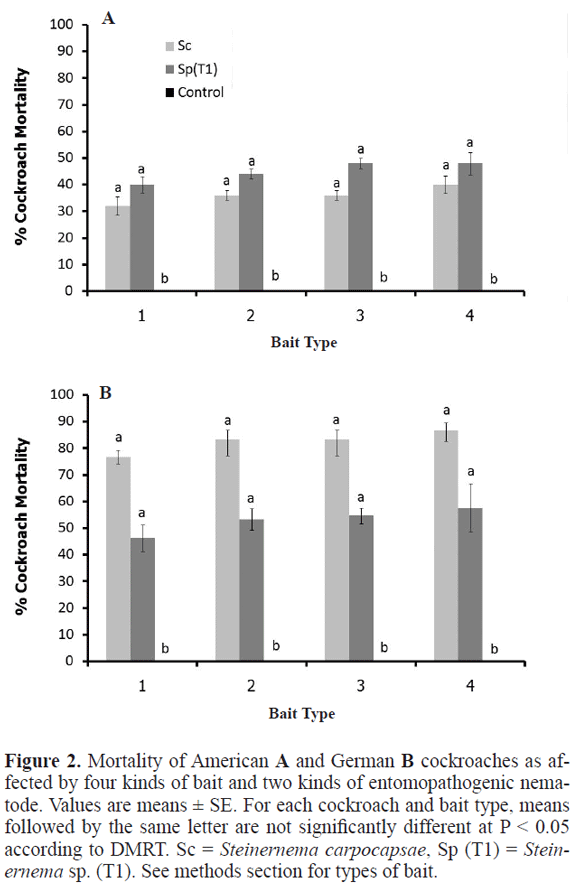
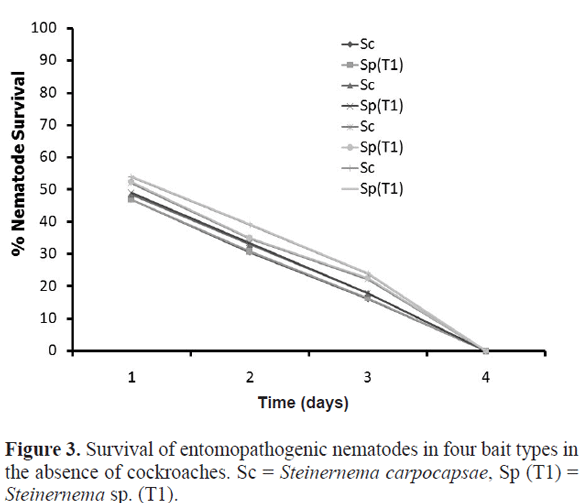
Bait compositions. Bait number four supported the best candidate in mortality of the American cockroach, and mortality was similar (F = 0.2 and 0.3 P > 0.05), with either S. carpocapsae or Steinernema sp. (T1) (Fig. 2A). Mortality of the German cockroach was greater with S. carpocapsae than with Steinernema sp. (T1) in all four baits. Mortality tended to be highest with bait number four but German cockroach mortality did not statistically differ among the baits (F = 0.3 and 0.2, P > 0.05) (Fig. 2B). Survival of EPN in the four kinds of baits in the absence of cockroaches declined rapidly down to 0% after four days (Fig. 3).
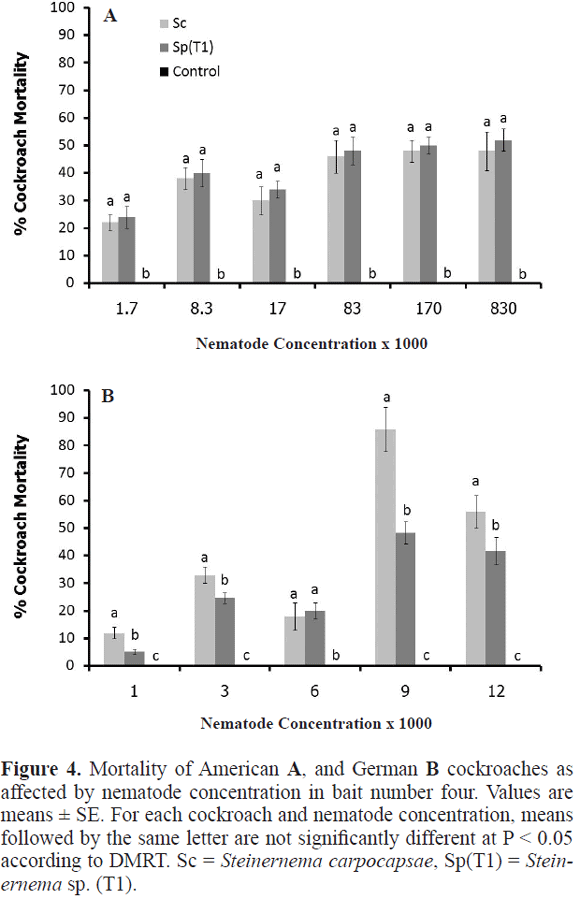
Optimal EPN concentration. For both EPN, the optimal concentration to kill the American cockroach was 1.7x105 EPNs per ml. There was no statistical difference in the mortality caused by the two EPNs. Both caused it around 52.0 ± 2.0% but while Steinermema sp. had an LT50 of 4.8 days, S. carpocapsae had an LT50 of 5.5 days. As expected, mortality was greater with the EPNs than in the control (F = 3309, P < 0.05) (Fig. 4A).
The optimal concentration for controlling the German cockroach was 9x103 EPNs per ml (Fig. 4B). However, there was a significant difference between the mortality caused by S. carpocapsae and Steinernema sp. (T1) (F = 261, P < 0.05). S. carpocapsae caused 89.1 ± 1.4% mortality and had an LT50 of 3.0 days while Steinernema sp. (T1) caused 50.5 ± 3.2% mortality and had an LT50 of 4.1 days (Fig. 4B). There was no cockroach mortality in the control treatment.
Susceptible stages of American cockroach and German cockroach. For the American cockroach, mortality caused by either EPN was lower with the fourth instar nymph than with the other stages (F = 19.3, P < 0.05); mortality was similar with both EPNs (Fig. 5A). For the German cockroach, mortality was unaffected by cockroach instar (F = 1.2, P > 0.05) and was similar for both EPNs (Fig. 5B).
Conclusions
Bowen and Ensign (1998) reported that Photorhabdus luminescens (Thomas & Poinar, 1979), a symbiotic bacteriumdae, produces a protein complex that is lethal when fed to or injected into the haemolymph of several insect species. When fed to cockroaches, however, the toxin caused only 30% mortality of the German cockroach and 0% mortality of the American cockroach. This is consistent with our results, which indicated poor control of these cockroaches by two EPN species in the Heterorhabditidae. The intestines of EPN species in Steinernematidae contain the symbiotic bacterium Xenorhabdus spp., which also produces a toxin. Based on our results, which documented high mortality by two species of Steinernema, we suspect that the toxin produced by Xenorhabdus spp. might be more effective against cockroaches than the toxin produced by P. luminescens. The cause for the poor performance of S. glaseri is unclear.
Attapulgite clay was used because, unlike diatomaceous earth or dry silica gel, does not have acute or dermal effects on insects (at least these effects have not been reported); we did not want the mortality caused to the cockroaches to be confounded by mortality caused by another bait component different to the EPNs. However, the clay particle size used in the current study might have been so fine that there was low gas exchange in the baits and therefore inadequate oxygen available for the EPNs. This could explain why EPN survival dropped so rapidly in the absence of cockroaches (Fig. 3). In addition, the experimental open containers were maintained uncovered, which allowed moisture in the bait to be released into the atmosphere, and the drop in moisture could have reduced EPN survival.
Morphological studies revealed that Steinernema sp. (T1) is not one of the species of Steinernema that was imported to Thailand nor is it S. siamkayai Stock, Somsook and Reid, 1998, a new Thai species reported a decade ago (Stock et al. 1998). Additional research is required regarding the identi-fication of this species and its efficacy in controlling cockroaches and others insect pests.
Acknowledgements
The authors would like to thank the following people and institutions for their assistance: Mrs. Vatcharee Somsook, Thai Department of Agriculture, for providing three EPNs; Mr. Kasin Supaprathom, Thai Department of Health Science, for cockroaches’ specimens; and Dr. S.P. Stock, The University of Arizona, for identifying the EPN species.
Literature cited
APPEL, A. G. 2003. Laboratory and field performance of an indoxacarb bait against German cockroaches (Dictyoptera: Blatellidae). Journal of Economic Entomology 96 (3): 863-870. [ Links ]
APPEL, A. G., BENSON, E. P. 1995.- Performance of avermectin bait formulations against German cockroaches (Dictyoptera: Blattellidae). Journal of Economic Entomology 88 (4): 924- 931. [ Links ]
APPEL, A. G., STANLEY, M. J. 2000.- Laboratory and field performance of an imidacloprid gel bait against german cockroaches (Dictyoptera: Blatellidae). Journal of Economic Entomology 93 (1): 112-118. [ Links ]
APPEL, A. G.; BENSON, E. P.; ELLEENBERGER, J. M.; MANWELLER, S. A. 1993. Laboratory and field evaluation of entomogenous nematode (Nematoda: Steinernematidae) for German cockroach (Dictyoptera: Blattidae) control. Journal of Economic Entomology 86 (3): 777-784. [ Links ]
BOWEN, D. J.; ENSIGN, J. C. 1998. Purification and characterization of a high-molecular-weight insecticidal protein complex produced by the entomopathogenic bacterium Photorhabdus luminescens. Applied and Environmental Microbiology 64 (8): 3029-3035. [ Links ]
CHANBANG, Y. 1997. Monitoring of cockroaches (Orthoptera: Blattidae) population in Bangkok urban area and effective used of insecticides. Ph. D. Thesis, Kasetsart University, Bangkok, 57 p. [ Links ]
HWANG, S. Y.; CHEN, L. M. 2004. Effects of four physical treatments of oothecae of Periplaneta americana on parasitism and development of parasitic wasp Evania appendigaster. Environmental Entomology 33 (5): 1321-1326. [ Links ]
JIANG, H.; ZHOU, L.; ZHANG, J. M.; DONG, H. F.; HU, Y. Y.; JIANG, M. S. 2008. Potential of Periplaneta fuliginosa densovirus as a biocontrol agent for smoky-brown cockroach, P. fuliginosa. Biological Control 46 (2): 94-100. [ Links ]
KOCHLER, P. G.; PATTERSON, R. S.; MARTIN, W. R. 1992. Susceptibility of cockroaches (Dictyoptera: Blattellidae, Blattidae) to infection by Steinernema carpocapsae. Journal of Economic Entomology 85 (4): 1184-1187. [ Links ]
LEBECK, L. M. 1991. A review of the hymenopterous natural enemies of cockroaches with emphasis on biological control. Bio- Control 36 (3): 335-352. [ Links ]
NGUYEN, K. B.; SMART, G. C. Jr. 1996. Identification of entomopathogenic nematodes in the Steinernematidae and Heterorhabditidae (Nematoda: Steinernematidae) for German cockroach (Dictyoptera: Blattelidae) control. Journal of Nematology 28 (3): 286-300. [ Links ]
PATHAK, S. C.; KULSHRESTHA, V. 1998. Experimental aspergillosis in the German cockroach Blattella germanica: a histopathological study. Mycopathologia 143 (1):13-16. [ Links ]
PAYNE, J. M.; KENNEDY, K. M.; RANDALL, J. B.; BROWER, D. O. 1994. Bacillus thuringiensis isolates active against cockroaches and genes encoding cockroach-active toxins. U. S. Patent No. 5302387. [ Links ]
PRIDGEON, J. W.; APPEL, A. G.; MOAR, W. J.; LIU, N. 2002. Variability of resistance mechanisms in pyrethroid resistant German cockroaches (Dictyoptera: Blattellidae). Pesticide Biochemistry and Physiology 73 (3): 149-156. [ Links ]
RAMOS-RODRÍGUEZ, O. ; CAMPBELL, J. F.; RAMASWAMY, S. B. 2006. Pathogenicity of three species of entomopathogenic nematodes to some major stored-product insect pests. Journal of Store Product Research 42 (3): 241-252. [ Links ]
RUST, M. K.; REIERSON, D. A.; HANSGEN, K. H. 1991. Control of American cockroaches (Dictyoptera: Blattidae) in sewers. Journal of Medical Entomology 28 (2): 210-213. [ Links ]
SHAMIM, S. A.; ISLAM, W.; MONDAL, K. A. M. S. H. 2001. Biological control potential of the cockroach parasitoid Aprostocetus asthenogmus (Waterson) (Hymenoptera: Eulophidae). International Pest Control 43 (2): 68-71. [ Links ]
SOMSOOK, V. 1991. Entomopathogenic nematodes for agricultural pests control. Department of Agriculture. Bangkok. 146 p. [ Links ]
STOCK, S. P.; SOMSOOK, V.; REID, A. P. 1998. Steinernema siamkayai n. sp. (Rhabditida: Steinernematidae), an entomopathogenic nematode from Thailand. Systematic Parasitology 41: 105-113. [ Links ]
VALLES, S. M.; YU, S. J. 1996. Detection and biochemical characterization of insecticide resistance in the German cockroach (Dictyoptera: Blattellidae). Journal of Economic Entomology 89 (1): 21-26. [ Links ]
WANG, C.; BENNETT, G. W. 2006. Efficacy of noviflumuron gel bait for control of the German cockroach (Dictyoptera: Blattellidae) in laboratory studies. Pest Management Science 62 (5): 434-439. [ Links ]
WEI, Y.; APPEL, A. G.; MOAR, W. J.; LIU, N. 2001. Pyrethroid resistance and cross-resistance in german cockroach, Blattella germanica (L.). Pest Management Science 57 (11): 1055-1059. [ Links ]
WHITE, G. F. 1927. A method for obtaining infective nematode larvae from cultures. Science 66: 302-303. [ Links ]