Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488On-line version ISSN 2665-4385
Rev. Colomb. Entomol. vol.36 no.2 Bogotá July/Dec. 2010
Top-down, bottom-up, and horizontal mortality variation in a generalist seed beetle
Variación en mortalidad “top-down”, “bottom-up” y horizontal en un escarabajo generalista comedor de semillas
Ángela R. Amarillo-Suárez1
1 Ph. D. Departamento de Ecología y Territorio, Pontificia Universidad Javeriana. Transv. 4 No. 42-00, piso 8. aamarillo@javeriana.edu.co
Recibido: 10-oct-2009 • Aceptado: 16-nov-2010
Abstract: The study of the interacting factors that constrain resource use in organisms and promote diversity is an important task, especially in a mega diverse area, in which the increasing transformation of ecosystems modifies the interactions among organisms. Among the hypotheses that explain resource use and diversity of insects are the Topdown and the Bottom-up hypotheses, in which experimental studies have shown trade-offs between these factors. The influence of parasitism, host plant and competition was evaluated to determine their effect on mortality of the seed beetle Stator limbatus from populations adapted to different host plants. Mortality of eggs caused by parasitism, and mortality of larvae caused by competition was recorded for seven populations that use a single host seed, and for one population that uses two hosts. Populations that use Acacia greggii experienced the lowest mortality, and populations that use Parkinsonia florida suffered the highest mortality, demonstrating no evidence of trade-offs between bottom-up and top-down factors. Interactions between host and larval density, and between host and number of eggs on seeds, showed variation between hosts in the mortality of beetles caused by competition and by parasitism, respectively. In addition, there was no evidence of egg size affecting parasitism of eggs. These results show the need of including in the traditional bottom-up and top-down explanations, the study of factors that could be mediating their outcome such as the one examined here (competition). This need is more urgent now that we are exposing ecosystems to accelerated changes in structure, functioning, and composition.
Key words: Parasitism. Competition. Host plant. Natural enemies. Trade-offs.
Resumen: El estudio de los factores interrelacionados que restringen el uso de recursos en los organismos y que promueven la diversidad, es una tarea importante, especialmente en una región megadiversa, en donde el incremento en la transformación de los ecosistemas modifica las interacciones entre los organismos. “top-down” y “bottom-up,” están entre las hipótesis que explican el uso de los recursos y la diversidad de insectos y sus estudios experimentales han mostrado compromisos (“trade-offs”) entre estos factores. Se evaluó la influencia del parasitismo, la planta hospedera y la competencia sobre la mortalidad del escarabajo comedor de semillas Stator limbatus en poblaciones adaptadas a diferentes hospederos. Se registró la mortalidad de huevos debida a parasitismo y la mortalidad de larvas debida a competencia en siete poblaciones que emplean solo un hospedero semilla y en una que emplea dos hospederos. Las poblaciones que emplean Acacia greggii presentaron la mortalidad más baja y las poblaciones que emplean Parkinsonia florida experimentaron la mortalidad más alta, sin evidencia de “trade-offs” entre factores “bottom-up” y “top-down”. Interacciones entre hospedero y densidad de larvas y entre hospedero y número de huevos en las semillas mostraron variación entre los hospederos en la mortalidad de los escarabajos debido a la competencia y al parasitismo, respectivamente. No hubo evidencia de que el tamaño de los huevos afectara su parasitismo. Estos resultados muestran la necesidad de incluir en las tradicionales explicaciones “bottom-up” y “top-down” el estudio de factores que podrían influir en su resultado como lo examinado acá (competencia). Esta necesidad es más urgente ahora que estamos exponiendo los ecosistemas a cambios acelerados en estructura, funcionamiento y composición.
Palabras clave: Parasitismo. Competencia. Planta hospedera. Enemigos naturales. Trade-offs.
Introduction
The understanding of the interacting factors that constrain resource use in organisms is an important task, especially in a mega diverse country (Mainka 2002), but with a rapid transformation of ecosystems (Etter et al. 2008) that also modifies the interactions between and among organisms and their environment. Thus, when studying the ways in which organisms adapt to their host plants, it is necessary to examine the factors that restrict or favor host use. In herbivorous insects, such factors incorporate the biology and behavior of insects, and environmental factors such as host availability, spatial distribution and nutritional value of the host, and the diversity and abundance of natural enemies and competitors, among others (Bernays and Chapman 1994; Fox et al. 1996; Camara 1997).
In general, there are two hypotheses that explain the diversity, abundance and diet breadth of herbivores. The Top-down hypothesis proposes that natural enemies such as predators and parasitoids are the main forces controlling diversity and abundance of insect populations (Hairston et al. 1960). Meanwhile, the Bottom-up hypothesis proposes that the main factor that control insects on plants is the characteristics of hosts such as nutritional value, temporal and spatial availability, and type of secondary compounds, among others (Root 1973; Schowalter 2006). With the exception of studies performed in some pest insects, few studies have been conducted to determine the simultaneous effects of natural enemies and host quality in the structure of communities around a biological resource (Aquilino et al. 2005 and references there in), in natural conditions, and comparing habitats or sites (Gripenberg and Roslin 2007).
A daptive responses of organisms are a consequence of the synergic, and sometimes, the antagonistic interaction between the factors that influence resource use. Sometimes, these interactions result in trade-offs between top-down andbottom-up factors, and in the evolution of life histories. For example, some populations of insects use hosts that while suboptimal for progeny development, offer enemy free space, reducing the risk of predation and parasitism (Mira and Bernays 2002).
On the other hand, a number of studies have demonstrated the negative effects of competition in fitness and fitness related traits of organisms. For example, when competition increases, body size and in consequence, fecundity decreases (Bai and Mackauer 1992; Hardy et al. 1992; Fox et al. 1996; Ode et al. 1996; Mackauer and Chau 2001). Thus, it is expected that females exposed to competition during oviposition minimize negative effects by, for example, distributing eggs uniformly among the available hosts (Messina and Mitchel 1989), reducing oviposition rate, and choosing to oviposit on hosts with a lower number of competitors (Messina and Renwick 1985). Stilwell et al. (2007) found that host plants are the main factor that explains adult body size variation of Stator limbatus (Horn, 1873) along its distribution range. Studies done with this beetle also found that females modify egg size in response to seed quality, increasing survivorship of progeny (Fox et al. 2001).
Little is known about the influence of other selection factors that like host availability, parasitism, and competition influence host use in herbivorous insects in natural conditions. Given that competition, natural enemies, and host plant simultaneously affect resource use, in this study I evaluated and compared the effects of host plant, density of eggs and density of larvae on the mortality caused by parasitism and by competition on S. limbatus. I compared populations of S. limbatus that use one out of four host plants, and that belong to the extremes of the distribution range of the species. This beetle constitutes a very good model to understand the ecological mechanisms that constrain host plant use, the colonization of new hosts and in a broader sense, the factors that facilitate diet expansion in organisms that alike S. limbatus, have broad distribution and are generalists, but restricted locally to a few host plants.
Materials and Methods
Study organism. S. limbatus (Coleoptera: Chrysomelidae) is a seed feeding beetle with a broad distribution in the Americas (Johnson and Kingsolver 1976). Populations are distributed from the Southwestern in the United States to the Northwestern Argentina (Johnson et al. 1989). Besides being considered a generalist species because it feeds on more than 70 legume species (Morse and Farrel 2005), populations use just a few available hosts in each area with indication of minor local adaptation in some populations (Amarillo-Suárez and Fox 2006). Females oviposit directly on the seed coat, and development of larvae and pupae occurs completely inside the seed host. Thus, factors such as the host selected by mothers to oviposit, natural enemies, competition, and host quality and size are easier to identify than in organisms that develop and move among hosts.
Host plants. Acacia greggii (Gray, 1852) (Fabaceae), commonly known as cat claw, is a shrub distributed along the most part of the southwest of the United States and northern Mexico (Sargent 1965). It grows in gravelly and sandy areas at the side of roads, canyons and streams. Seed pods contain between one and five brown, round, and laterally compressed seeds. Seed mass varies between 600 and 300 mg. S. limbatus colonizes seeds through holes in the pods made by other insects, by partial dehiscence of the legume, or by cracks in the seed pod.
Acacia berlandieri Benth, 1842 (Fabaceae), commonly known as guajillo, is a small to medium size shrub distributed from Mexico to the southwest of the United States in Texas (Hatch and Pluhar 1993). It grows in roadsides and sandy areas. Seed pods contain around five large, brown, and round to square seeds that are about 40% larger than A. greggii seeds. S. limbatus colonizes seeds through holes in the pods made by other insects or created by cracks in the seed pod.
Parkinsonia florida (Benth. ex A. Gray, 1876) (Fabaceae), commonly known as blue paloverde, is a native tree distributed in California, Arizona and Nevada of the United States and in the Sonora desert region from Mexico and USA. It contains between one and five large, laterally compressed and oval seeds of similar size than A. greggii seeds. These seeds have a toxic seed coat that causes large mortality of S. limbatus larvae when borrowing (Siemens et al. 1992; Siemens et al. 1994). Beetles colonize seeds through holes in the pods made by other insects or created by cracks in the seed pod.
Pseudosamanea guachapele (Kunth, 1930) (Fabaceae) is a medium to large tree that usually grows in pastures and dry areas from Central America to Northern South America (Bartholomaüs et al. 1990). The dehiscent seed pods contain between 10 and 25 white, oval and laterally compressed seeds. Seed mass vary between 18 and 46 mg. Because seed pods are dehiscent, S. limbatus colonizes seeds directly when they are exposed, and still on the tree.
Collection of seeds in the field. Seeds during two field trips were collected. The first field trip occurred between December of 2002 and January of 2003 to the Municipios of Anapoima (Cundinamarca), and Melgar (Tolima) in Colombia, and the second one was between July and august 2003 to the Counties of Verde, Wenden, Roosevelt, Phoenix (Arizona), and Del Rio (Texas) in The United States of America. 10- 20 plants from each locality were inspected, their seeds were collected and deposited in 1000 cc hermetic plastic bags, and labeled to be transported to laboratory. Seeds were collected from a total of four host plants: P. florida, A. greggii , A. berlandieri and P. guachapele. Table 1 shows the locations and host plant of each population.
Data recording and analysis. Once in the lab, seed pods were split open and seeds containing eggs were placed in a chamber at 28oC, 80% humidity. Seeds were inspected daily and emerging organisms were collected and stored in vials with alcohol. Inspection of seeds was done until no individuals were obtained for seven continuous days in order to make sure no organisms emerged later.
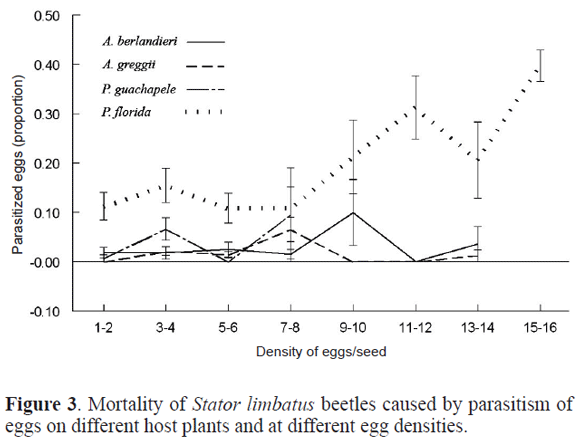
Once all individuals emerged from the seeds, a random sample of 200 seeds from each host/locality was taken, and the following data were recorded for each individual seed: number of eggs laid on the seed, number of eggs hatched, number of eggs parasitized and number of exit holes. The difference between hatched and non hatched eggs is easily determined because hatched eggs are evenly cream colored; meanwhile non hatched eggs are transparent. Parasitized eggs are silver color and have a conspicuous exit hole on the top (Figs. 1A-F).
Mortality caused by competition of larvae was scored for each seed as the number of exit holes out of the total number of hatched eggs on the seed. Mortality caused by parasitism was scored for each seed as the number of parasitized eggs out of the number of laid eggs on the seed. Data matrix for the analyses was expanded to accounts for mortality on each individual egg. Logistic regression analyses were performed to determine the effects of host plant, population origin, density of eggs, and egg size in mortality of eggs by parasitism. In the case of mortality of larvae by competition I used for the analysis the number of hatched eggs per seed instead of density of eggs, because the former takes in account the effective number of larvae under competition inside each seed.
Survivorship to parasitism was scored for each single egg as “1” if given the laid egg, it hatched. It was scored as “0” if the egg presented a silver color and an exit hole in the chorion. Survivorship to competition was scored as “1” if given the hatched egg, there was a corresponding emergence hole in the seed. It was scored as “0” if given the hatched egg, there was not an emergence hole. For example, a seed with five eggs, could have three emergence holes, one parasitized egg and four hatched eggs. In this case, survivorship due to parasitism was scored 0 for egg number 1, and 1 for eggs 2-5, under the treatment density = 5 (number of eggs laid on the seed). Survivorship to competition was scored 0 for egg number 2, and 1 for eggs 3-5 under the treatment density = 4 (number of hatched eggs).
L ogistic regression analyses were performed to test for the effects of density and host plant in mortality caused by parasitism and by competition. Analyses were performed with SAS (SAS institute, ver. 8.2). Graphs were done using mean proportional mortality.
Results
In general, there was higher mortality caused by competition (Total mean mortality of larvae = 0.27±0,99) than to by parasitism (Total mean mortality of eggs = 0.018±0.003 P < 0.05).
Table 2 shows the parasitoids that emerged from each host. Since parasitoids from Verde and Wenden populations were lost during their storing, this table shows parasitoids from another A. greggii population (Oracle, Arizona) that was not considered in this study. In the case of USA populations, all parasitoids may not be parasitoids of S. limbatus. Seeds of A. greggii are hosts of other species of seed feeders such as Stator pruininus (Horn, 1873), and Merobruchus julianus (Horn, 1894), though less abundant than S. limbatus (Siemens et al. 1991); and seeds of P. florida are hosts of Mimosestes spp. (Hetz and Johnson 1988). In the case of the Colombia populations, only S. limbatus were found emerging from seeds of P. guachapele.
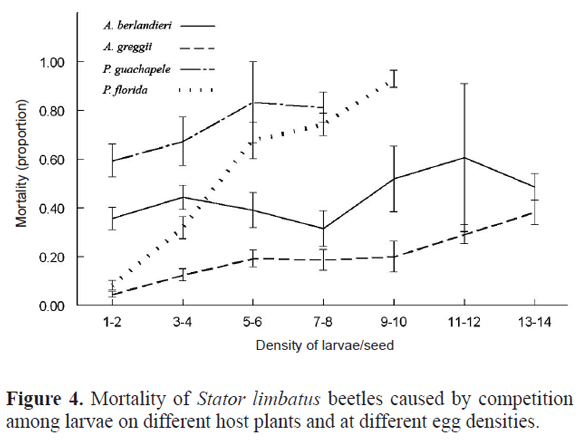
Host plant effects in mortality due to parasitism and to competition. Irrespective of the density of eggs on the seeds, parasitism was significantly higher on P. florida followed by A. greggii , A. berlandieri, and P. guachapele (Fig. 2A, Table 3). Mortality caused by competition was higher in seeds of A. greggii , followed by P. florida, A. berlandieri, and P. guachapele (Fig. 2B, Table 3) irrespective of the rearing density of larvae. In addition, there was a certain amount of eggs that did not hatch due to unknown reasons, but associated to the embryo death, or to the first instar larvae death before burrowing into the seeds, the last being the case of larvae in P. florida.
Density effects in mortality due to parasitism and to larval competition. There was a significant effect of density of eggs in parasitism of eggs. However, the pattern of response was different among hosts (Table 3). While in P. florida parasitism increased about 40% on seeds with higher density of eggs, in the remaining hosts mortality did not increase higher than 85%, and there was not a gradual increase in parasitism (Fig. 3).
Mortality of larvae also increased with larval competition (Table 3). However, the pattern of mortality with density was different among hosts (Table 3). Mortality in P. florida increased sharply up to 90% in seeds with higher density of eggs. In the other three hosts there was a smoother increase in mortality, but it did not past over an 80% in P. guachapele, over a 40% in A. berlandieri, and over a 30% in A. greggii (Fig. 4).
Discussion
Despite the numerous studies showing the effects of competition, parasitism and host plant in life history traits ofherbivores (Lewinsohn et al. 2005; Ode 2006), just a few of them have considered bottom-up, and top-down mortality factors simultaneously (Aquilino et al. 2005), and even fewer, have considered the analysis of these factors together with intraspecific competition and in field conditions (Mira and Bernays 2002). Overall it was found that competition and host plant had a greater impact than parasitism in survivorship of larvae.
Uscana semifumipennis Girault, 1911, the single egg parasitoid that has being reported to attack S. limbatus in P. florida (Siemens and Johnson 1992), was not found in this study. Hetz and Johnson (1988) report Stenocorse bruchivora (Crawford, 1909), and Urosigalphus neobruchi Gibson, 1972 as parasitoids of larvae of S. limbatus feeding on P. florida. In this study we recorded two species of these genera emerging from P. florida, but the specimens were not determined to species level. Heterospilus bruchi Viereck, 1910, Urosigalphus bruchi Crawford, 1907, U. neobruchi, Lariophagus texanus Howard, 1898, Zatropis incertus (Ashmead, 1864) are recorded in the same paper as parasitoids of larvae of bruchids on A. greggii , but there is not specific reference to these Hymenoptera attacking S. limbatus. However, in this study we recorded Urosigalphus sp. 1, emerging from A. greggii . Other parasitoids of larvae are reported, but they belong to parasitoids on hosts and localities other than the localities studied here.
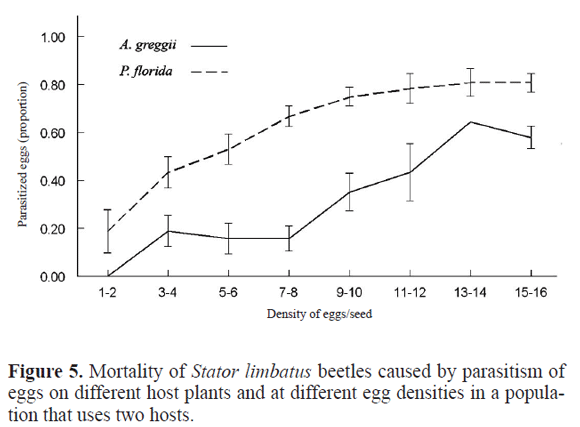
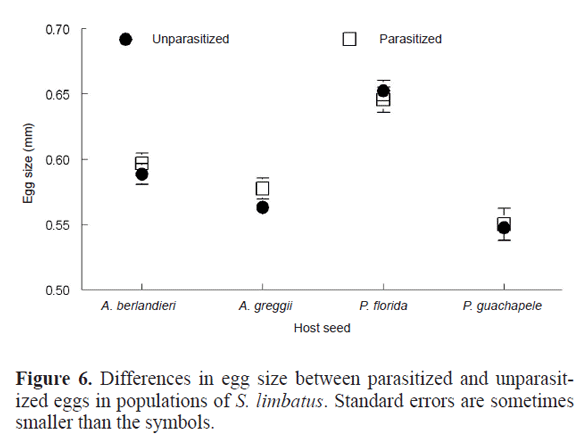
Mortality caused by egg parasitism was higher in P. florida, and its effect increased with density of eggs. An explanation for this pattern remains unclear. Because S. limbatus females lay larger eggs in this host, it could be considered that egg size could play an important role in parasitism of eggs. In fact, a preliminary examination of the relationship between egg size and parasitism in a population in which P. florida and A. greggii are sympatric, with many cases of trees overlapping branches, shows that there is higher parasitism of eggs laid on P. florida than on eggs of A. greggii (Fig. 5). This is a host in which females lay larger eggs due to the toxicity of the seed coat that causes mortality up to a 40% in some populations (Siemens and Johnson 1990; Fox 2000). As a consequence, there is high mortality of larvae when entering the seed, with larger eggs having a higher probability of survivorship during the hatching process. However, and contrary to what some experiments done in laboratory conditions show (Deas and Hunter 2008), a preliminary examination of the effect of egg size in parasitism within hosts shows that there are not significant differences in egg size between parasitized and unparasitized eggs collected in the field (Fig. 6. Table 4). This surprising result could imply that there are factors other than egg size involved in the higher risk of mortality by parasitism in P. florida. One hypothesis to test would be that this species of plant attracts more natural enemies than the other hosts.
In addition to being the host in which parasitism increased broadly with density of eggs, P. florida was also the host where beetles had higher mortality caused by competition. These results contrast with literature reports regarding tradeoffs between bottom-up and top-down sources of mortality in herbivorous insects, in which ovipositing in a low quality host or in a less preferred host would provide, for example, enemy free space (Feder 1995; Mira and Bernays 2002), being this, one of the underlying reasons for specialization in resource use, and colonization of new hosts (Futuyma and Moreno 1988; Jaenike 1990).
In addition to the differences in mortality mentioned before, there was variation in the response to each source of mortality between hosts. Thus, parasitism and competition differed in the magnitude of their impact in each population adapted to different hosts, perhaps as a result of local adaptation to their hosts, which has been proved as one of the main factors determining life history differences among populations (Van Zandt and Mopper 1998; Amarillo-Suárez and Fox 2006; Stillwell et al. 2007). Thus, P. florida is the host in which there is larger mortality risk by bottom-up and top-down factors, and A. greggii is the host in which beetles experienced the lowest mortality. Seed quality may also play an important role in the variation observed. Previous studies show that beetles from the same populations examined here experienced longer development time and higher mortality due to competition in P. guachapele, though the response from each population was not symmetrical (Amarillo-Suárez et al., submitted).
Trade-offs are one of the main factors proposed as the cause of specialization and local adaptation in host use (Futuyma and Moreno 1988; Jaenike 1990; Mira and Bernays 2002). Thus it would be expected that adaptation to hosts plants would result in an antagonistic balance between mortality by top-down and bottom-up factors. In the present study, there was no evidence of trade-offs between top-down and bottom-up mortality factors in the field in populations of S. limbatus using different host plants. The results show a host in which total mortality by these factors is reduced, and another in which mortality is the highest. One of the explanations to this unusual result may be in the examination of additional factors, and in natural conditions. One of them analyzed here is competition. Recent papers show that competition, predation risk, intraspecific variation, and spatial variation, among others could be affecting the outcome of mortality by natural enemies and by host plant characteristics (Heisswolf et al. 2006). These results post the need of including in the traditional bottom-up top-down paradigm, the study of factors that could be mediating their outcome. Some of them to explore are intraspecific and interspecific competition, the spatial distribution of resources and its temporal availability, a necessity more urgent now when we humans are exposing ecosystems to accelerated changes in structure, functioning, and composition.
Acknowledgements
I thank Charles Fox for comments on the early version of the manuscript. To Craig Stillwell, Bill Wallin, Jordi Moya- Laraño, and Kristy Schelby, for their help with the experiments and field collecting in Arizona and Texas. I also thank Celestino Amarillo, Fidelia Suárez, Carlos Sarmiento-M, María A. Sarmiento-Amarillo, Adela Suárez, and Wilson Yaya for help with field collecting in Colombia. Entomologist Helmuth Aguirre made the identification of parasitoids. The anonymous reviewers provided insightful comments and supporting literature that improved the quality of the manuscript. Support for this study was provided by Grants DEB 01-10754 from Nacional Science Foundation, USA (to C.W. Fox), grants 1568 and 620 from Fundación para la Promoción de la Investigación y la Tecnología, Banco de la República, Colombia (to A. Amarillo-Suárez), and by grant ID PRY 001932 from Departamento de Ecología y Territorio, Pontificia Universidad Javeriana, Bogotá, Colombia (to A. Amarillo-Suárez).
Literature cited
AMARILLO-SUÁREZ, A.; FOX, C. W. 2006. Population differences in host use by a seed beetle: Local adaptation, phenotypic plasticity y maternal effects. Oecologia 150 (2): 247-258. [ Links ]
AMARILLO-SUÁREZ, A.; STILLWELL, R. C.; FOX, C. W. Host size and larval competition mediate natural selection on body size in a seed-feeding beetle. Submitted to Oikos in August 2010. [ Links ]
AQUILINO, K. M.; CARDINALE, B. J.; IVES, A. R. 2005. Reciprocal effects of host plant and natural enemy diversity on herbivore suppression: an empirical study of a model tritrophic system. Oikos 108 (2): 275-282. [ Links ]
BAI, B.; MACKAUER, M. 1992. Influence of super parasitism on development rate and adult size in a solitary parasitoid Aphidius ervi. Functional Ecology 6 (3): 302-307. [ Links ]
BARTHOLOMÄUS, A.; De la ROSA CORTÉS, A.; SANTOS GUTIÉRREZ, J. O.; ACERO DUARTE, L.E. MOOSBRUGGER, W. 1990. El manto de la tierra: flora de los Andes. Ediciones Lerner. 332 p. [ Links ]
BERNAYS, E. A.; CHAPMAN, F. F. 1994. Host-plant selection by phytophagous insects. Chapman & Hall. New York. 312 p. [ Links ]
CAMARA, M. D. 1997. A recent host range expansion in Junonia coenia Hubner (Nymphalidae): Oviposition preference, survival, growth, and chemical defense. Evolution 51 (3): 873-884. [ Links ]
DEAS, J.; HUNTER, M. S. 2008. Host egg-size plasticity affects preference and performance in the egg parasitoid Uscana semifumipennis (Hymenoptera: Trichogrammatidae) as a result of host size selection. Entomological Society of America. Annual Meeting. Available in: http://esa.confex.com/esa/2008/techprogram/ paper_39078.htm. [Consulted on July 10th. 2010]. [ Links ]
ETTER, A.; McALPINE, C.; POSSINNGHAM, H. 2008. Historical Patterns and drivers of dandscape change in Colombia since 1500: A regionalized spatial approach. Annals of the Association of American Geographers 98 (1): 2-23. [ Links ]
FEDER, J. L. 1995. The effects of parasitoids on sympatric host races of Rhagoletis pomonella (Diptera: Tephritidae). Ecology 76 (3): 801-813. [ Links ]
FOX, C. W.; MARTIN, J. D; THAKAR, M. S.; MOUSSEAU, T. 1996. Clutch size manipulations in two seed beetles: consequences for progeny fitness. Oecologia 108 (1): 88-94. [ Links ]
FOX, C. W. 2000. Natural selection on seed-beetle egg size in nature and the laboratory: Variation among environments. Ecology 81 (11): 3029-3035. [ Links ]
FOX, C. W.; CZESAK, M. E.; FOX, R. W. 2001. Consequences of plant resistance for herbivore survivorship, growth, and selection on egg size. Ecology 82 (10): 2790-2804. [ Links ]
FUTUYMA, D. J.; MORENO, G. 1988. The evolution of ecological specialization. Annual Review of Ecology and Systematics 19 : 207-233. [ Links ]
GRIPENBERG, S.; ROSLIN, T. 2007. Up or down in space? Uniting the bottom-up versus top-down paradigm and spatial ecology. Oikos 116 (2): 181-188. [ Links ]
HAIRSTON, N. G.; SMITH, F. E.; SLOBODKIN, L. B. 1960. Community structure, population control and competition. The American Naturalist 94 (879): 421-425. [ Links ]
HARDY, I. C.; GRIFFITHS, T; GODFRAY, H. C. J. 1992. Clutch size in a parasitoid wasp: A manipulation experiment. Journal of Animal Ecology 61 (1): 121-129. [ Links ]
HATCH, S. L.; PLUHAR, J. 1993. Texas range plants. Texas A&M Univ. Press. College Station. 326 p. [ Links ]
HEISSWOLF, A; JOACJIM, J.; OBERMAIER, E. 2006. Multitrophic influences on egg distribution in a specialized leaf beetle at multiple spatial scales. Basic and Applied Ecology 7 (6): 565- 576. [ Links ]
HETZ, M.; JOHNSON, C. D. 1988. Hymenopterous parasites of some bruchid beetles of North and Central America. Journal of Stored Products Research 24 (3): 131-143. [ Links ]
JAENIKE, J. 1990. Host specialization in phytophagous insects. Annual Review of Ecology and Systematics 21: 243-273. [ Links ]
JOHNSON, C. D.; KINGSOLVER, J. M. 1976. Systematics of Stator of North and Central America (Coleoptera: Bruchidae). USDA. Technical Bulletin 1537 101p. [ Links ]
JOHNSON, C. D.; KINGSOLVER, J. M; TERAN, A. L. 1989. Sistemática del género Stator (Insecta: Coleoptera: Bruchidae) en Sudamérica. Opera Lilloana 37 J. M.105 p. [ Links ]
LEWINSOHN, T.; NOVOTNY, V.; BASSET Y. 2005. Insects on plants: Diversity of herbivore assemblages revisited. Annual Review Ecology Evolution and Systematics 36: 597-620. [ Links ]
MACKAUER, M.; CHAU, A. 2001. Adaptive self superparasitism in a solitary parasitoid wasp: the influence of clutch size on offspring size. Functional Ecology 15 (3): 335-343. [ Links ]
MAINKA, S. 2002. Biodiversity, poverty and hunger - where do they meet? En: MAINKA, S.; TRIVEDI, M. Links between Biodiversity, Conservation, Livelihoods, and Food Security. The sustainable use of wild species for meat. IUCN, Gland, Switzerland and Cambridge, UK. 135 p. [ Links ]
MESSINA, F. J.; MITCHEL, R. 1989. Intraspecific variation in the egg-spacing behavior of the seed beetle Callosobruchus maculatus. Journal of Insect Behavior 2 (6):727-742. [ Links ]
MESSINA, F. J.; RENWICK, A. 1985. Ability of ovipositing seed beetles to discriminate between seeds with differing egg loads. Ecological Entomology 10 (2): 225-230. [ Links ]
MIRA, A.; BERNAYS, E. A. 2002. Trade-offs in host use by Manduca sexta: plant characters vs natural enemies. Oikos 97 (3): 387-397. [ Links ]
MORSE, G.: FARREL, B. 2005. Interspecic phylogeography of the Stator limbatus species complex: The geographic context of speciation and specialization. Evolution 59 (6): 1165-1388. [ Links ]
ODE, P. 2006. Plant chemistry and natural enemy fitness: Effects on herbivore and natural enemy interactions. Annual Review of Entomology 51: 163-85. [ Links ]
ODE, P. J.; ANTOLIN, M. F.; STRAND, M. R. 1996. Sex allocation and sexual asymmetries in intra-brood competition in the parasitic wasp Bracon hebetor. Journal of Animal Ecology 65 (6): 690-700. [ Links ]
ROOT, R. B. 1973. Organization of a plant-arthropod association in simple and diverse habitats: the fauna of collards (Brassica oleracea). Ecological Monographs 43 (1): 95-124. [ Links ]
SARGENT, C. S. 1965. Manual of trees of North America, vol. 2. Dover Publications Inc., New York. 934 p. [ Links ]
SCHOWALTER T. D. 2006. Insect ecology. Academic Press, Burlington. 536 p. [ Links ]
SIEMENS, D. H.; JONHNSON, C. D. 1990. Host-associated differences in fitness of a seed beetle (Bruchidae): effects of plant variability. Oecologia 82 (3): 408-413. [ Links ]
SIEMENS D. H.; JOHNSON, C. D. WOODMAN, R. L. 1991. Determinants of Host Range In Bruchid Beetles Ecology 72 (5): 1560-1566. [ Links ]
SIEMENS, D. H.; JOHNSON, C. D.; RIBARDO, K. J. 1992. Alternative seed defense mechanisms in congeneric plants. Ecology 73 (6): 2152-2166. [ Links ]
SIEMENS, D. H.; JONHNSON, C. D. 1992. Density-dependent egg parasitism as a determinant of clutch size in bruchid beetles (Coleoptera: Bruchidae). Environmental Entomology 21(3): 610-619. [ Links ]
SIEMENS, D. H., B. E. RALSTON, and C. D. JOHNSON. 1994. Alternative seed defense mechanisms in a palo verde (Fabaceae) hybrid zone - effects on bruchid beetle abundance. Ecological Entomology 19 (4): 381-390. [ Links ]
STILLWELL, R. C.; MORSE, G. E.; FOX, C. W. 2007. Geographic variation in body size and sexual size dimorphism of a seed beetle. The American Naturalist 170 (3): 358-369. [ Links ]
VAN ZANDT, P.; MOPPER, S. 1998. A meta-analysis of adaptive deme formation in phytophagous insect populations. The American Naturalist 152 (4): 597-606. [ Links ]