Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488On-line version ISSN 2665-4385
Rev. Colomb. Entomol. vol.36 no.2 Bogotá July/Dec. 2010
Laboratory rearing technique and biology of Mycotretus apicalis (Coleoptera: Erotylidae) on dried Pleurotus sajor-caju mushrooms
Técnica de cría y biología de Mycotretus apicalis (Coleoptera: Erotylidae) en el hongo Pleurotus sajor-caju seco
GRAZIELLE F. MOREIRA1, CAMILA C. MOREIRA2, VANESSA ANDALÓ2, ALCIDES MOINO JUNIOR2, MARCELA M. CARDOSO-FREIRE2 and EUSTÁQUIO SOUZA DIAS2
1 Universidade Federal de Lavras (UFLA), Department of Entomology; P.O. Box 3037, Zip Code 37200-000, Lavras - MG - Brasil. grabiologia@yahoo.com. br Corresponding author,
2 Universidade Federal de Lavras (UFLA), Department of Biology; P.O. Box 3037, Zip Code 37200-000, Lavras - MG - Brasil
Recibido: 11-feb-2010 • Aceptado: 16-nov-2010
Abstract: Mycotretus apicalis is an erotylid beetle that is commonly found in cultivations Pleurotus sajor-caju in Minas Gerais, Brazil and could potentially be a pest. The life cycle is studied for the first time by laboratory rearing using dried P. sajor-caju mushrooms as both a feeding resource and an oviposition substrate. M. apicalis species has a generation time of about three weeks, with the egg-adult period was about 22 days, four larval instars, and the average longevity for adults of 89.7 ± 32.89 days. These results suggest that the M. apicalis has a potentially high rate of population increase, indicating its great potential to be a pest insect.
Key words: Life cycle. Mushroom pests. Edible mushrooms. Life table. Pleasing fungus beetles.
Resumen: Mycotretus apicalis es un erotilído comúnmente encontrado en cultivos de Pleurotus sajor-caju, al sur del Estado de Minas Gerais, Brasil, y podría ser una plaga. El ciclo de vida es estudiado por primera vez, teniendo como recurso alimenticio y substrato de oviposición al hongo P. sajor-caju seco. M. apicalis tiene un tiempo promedio de una generación de tres semanas y el periodo huevo-adulto fue, aproximadamente, 22 días, con cuatro instares larvales y la longevidad promedio del adulto de 89,7±32,89 días. Esos resultados demuestran que la especie M. apicalis posee alta tasa de aumento poblacional, indicando gran potencial como insecto plaga.
Palabras clave: Ciclo de vida. Plagas de hongos. Tabla de vida. Hongos comestibles. Erotilídos.
Introduction
The family Erotylidae, with about 3,200 reported species, comprises exclusively mycetophagus insects that feed mainly on the fruiting bodies of basidiomycetes (Wegrzynowicz 2002). According to Skelley (1988), a wide variety of basidiomycete fungi serve as a host to the family; however, each species seems to be specific to a particular group of fungi. Some species have been reported to cause economic losses in commercial edible mushroom cultivation, since the larvae and adults consume the fruiting body (Valencia and Lopes 2005; Gaitán-Hernandez et al. 2006; Sato 2003).
The genus Pleurotus is one of the most edible mushroom cultivated in Brazil due to low cost, easy availability of substrates for its cultivation and high biological efficiency, adapting well to the condition of tropical climate (Azevedo et al. 2009; Souza Dias et al. 2003). Sciarid fly and coleopteran species are the major pests in Pleurotus cultivation.
Mycotretus apicalis Lacordaire, 1842 has been found to cause injuries and economic losses in commercial cultivations of Pleurotus sajor-caju (Fr.) Singer in southern Minas Gerais, Brazil (Moreira et al. 2009). Thus, data on the rearing and life cycle of this species may contribute significantly to the implementation of an effective program to control this pest insect. Moreover, the life cycle of M. apicalis is unknown so the results can contribute in further studies like systematic and ecology of erotylids.
Materials and Methods
The experiments were carried out under controlled temperature (25 ± 2°C), humidity (70%) and photoperiod (14 h) conditions at the Laboratory of Insect Pathology, Department of Entomology, Federal University of Lavras (UFLA), Lavras, MG, Brazil.
Rearing technique: Specimens of erotylid beetles were collected from the cultivation area of P. sajor-caju at the Laboratory of Edible Mushrooms, Department of Biology, Federal University of Lavras (UFLA), Lavras, MG, Brazil and some individuals were sent to the Museum of Zoology, Universidade de São Paulo (MZUSP), São Paulo, SP, Brazil for species identification. These specimens were incorporated into the entomological collection of this museum an identified as belonging to the species M. apicalis.
Thirty adults of M. apicalis were collected in the same cultivation area of P. sajor-caju and kept in a beaker (500 mL) closed with a filter paper disk, which contained pieces of fresh mushrooms and two filter paper disks at the bottom of the vial. After 48 h, the adults were removed with a glass tube, and the presence of eggs and larvae were observed.
All of the rearing were done in polystyrene Petri dishes (15 cm diameter) with holes at the top that were covered with moistened hydrophilic cotton pieces, and two filter paper disks, that were moistened with 3 mL of distilled water, to maintain humidity. About 4 g of dried basidiocarps of P. sajor-caju were used as a feeding substrate, as suggested by Sato et al. (1999). First instar larvae hatched were transferred to the dishes using a brush. The maintenance of the reared population was done every 48 h, with cleaning and provision new food. After their feeding, the larvae, and afterwards the pupae, were transferred to dishes containing only 2 mL of distilled water. Once emerged, adults were transferred to dishes containing dried mushrooms. Dishes that had eggs in the gills of the mushrooms were kept for up to four days in a B.O.D chamber (T: 25 ± 2°C, RH: 70% and 14L10D), then the larvae hatched were transferred to Petri dishes repeating the rearing process.
Biology of Mycotretus apicalis on mushrooms: Adults of M. apicalis reared by the Insect Pathology Laboratory / UFLA, were placed in a beaker (500 mL) containing filter paper at the bottom and fresh P. sajor-caju mushrooms. After twelve hours, the adults were removed with a glass tube. We noticed that there were eggs in the gills of the fresh mushrooms. The eggs were incubated for 24 h, and then the first instar larvae were transferred to ELISA® plates with 24 cavities (1.5 x 1.5 cm), using 12 cavities per plate, with one larvae per cavity. Each cavity contained two filter paper disks that were moistened with 50 μl of distilled water with pieces of dried mushrooms at the bottom, giving a total of 108 replicates. The assessment was done every 12 h until the larvae reached the fourth instar. The presence of exuvia, which was removed using a brush at each molt, was the criterion used to determine the instar change.
Upon reaching the fourth instar, each larva was transferred to 5-cm-diameter Petri dishes. The dishes had holes in their lids, which were covered with moistened cotton. Two filter-paper disks at the bottom, moistened with 200 μl of distilled water, also contained pieces of dried mushrooms. After pupation, they were transferred - as described above - to Petri dishes that had only two filter paper disks moistened with 150 μl of distilled water.
About seven days after the emergence of adults (as the species lacks dimorphism), they were marked and randomly separated into pairs so that the mating behavior could be observed. Copulating couples were then set aside in 5 cmdiameter Petri dishes with pieces of dried mushrooms, as described previously. The presence or absence of eggs was observed after 24 h. The adults on whose dish we found eggs were confirmed to be a couple. We obtained a total of 19 couples. The number of eggs laid by the female was observed daily. The adults were sexed by dissection after their death, and their longevity was then evaluated.
The life table of fertility was drawn using the obtained data, as suggested by Silveira Neto et al. (1976), where: x = the midpoint of age range; lx = the age specific survival rate during stage x; and mx = the age specific fertility rate, that is, the number of offspring produced per female at age x that originates females. The parameters of population growth, Ro (net reproductive rate), T (mean generation time), rm (intrinsic rate of increase) and λ (finite rate of increase), were calculated according to Maia et al. (2000).
Results and Discussion
Rearing technique: The methodology for the rearing of M. apicalis has proved effective for the maintenance of the insect in the laboratory. The use of dried mushrooms as a feeding substrate enabled the rearing of these insects. Larger intervals are possible when performing maintenance of a reared population, since dried mushrooms are not perishable and do not deteriorate quickly like fresh mushrooms. According to Cline and Leschen (2005), mushrooms of the genus Pleurotus are one of the few edible mushrooms that can be dried and rewetted without the loss of flavor and constitution. Moreover, P. sajor-caju mushrooms, when dried, can be stored for long periods in the refrigerator, which allows a constant supply of food for the reared population, regardless of the availability of fresh mushrooms.
These insects consumed large amounts of dried mushrooms during the larval stage. We also observed that at the end of the fourth instar, after the larvae had ceased their feeding, they gathered at the edges of the dishes. This behavior is commonly found in some members of the family Erotylidae, as cited by Robertson et al. (2004).
Despite consuming mushrooms in smaller quantities, adults use their lamellae for oviposition. According to Wegrzynowicz (2002), erotylid couples copulate near or on the fruiting body. The females prefer to deposit their eggs in dried mushrooms with intact gills, and this pattern is obtained when using mushrooms harvested before the beginning of the senescence process (margin of pileus plane). Sato et al. (1999) observed a similar behavior in the female of Dacne picta Crotch, 1873 (Erotylidae). According to these authors, females of D. picta can distinguish the quality of mushrooms, so they oviposit on the freshest mushrooms, which are more favorable for the development of their offspring.
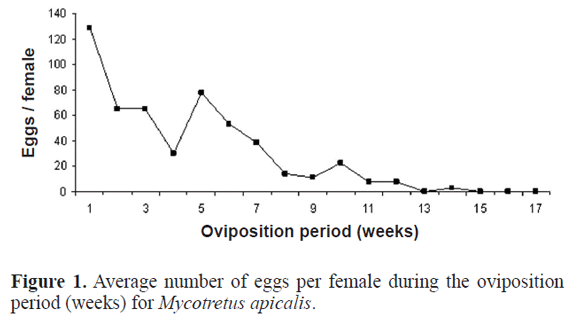
Biology of Mycotretus apicalis: Egg laying of M. apicalis was not continuous; oviposition activity alternated with intervals when no oviposition occurs. The highest average of eggs per female (129) was observed in the first week of oviposition, and the average decreased with age (Fig. 1). The average of eggs per female was 522. M. apicalis has four larval instars. According to Skelley and McHugh (2002), Erotylidae larvae that feed on mushrooms that quickly start the senescence process exhibit rapid development. In our study, the initial stages of development were fairly rapid, occurring at 13.0 ± 3.33, 17.29 ± 7.08, 21.33 ± 6.98 h for the first, second and third instars, respectively. The fourth larval stage was the longest (12.99 ± 1.63 days) and the one that consumed the greatest amount of dried mushrooms. The duration of the pupal period was, on average, 6.46 ± 0.74 days (Table 1). Thus, complete development from egg to adult was about 22 days. The highest mortality rate, 27.03% was observed in the first larval instar (Table 1). We may infer that the feeding activity was among the possible causes of death. As noted by Sato et al. (1999), the use of dried mushrooms as a feeding substrate, because of its texture, can harm the feeding process of first instar larvae.
The longevity of adults was, on average, 89.7 ± 32.89 days. In 82% of the studied couples, the males showed greater longevity (102.95 ± 26.68) than the females (76.47 ± 33.8). As indicated by Townsend et al. (2006), the process of reproduction may have a high energy cost. Thus, one of the possible causes of lower longevity in the female is the high energy consumption involved in their oviposition process. The biological parameters determined in this study indicate that the population of M. apicalis may increase 230.78 times (Ro) in each generation, and the mean generation time (T) is about three weeks. The population had a weekly increase of 46% [finite rate of increase (λ) = 5.46], and the intrinsic rate of increase (rm) was 1.69.
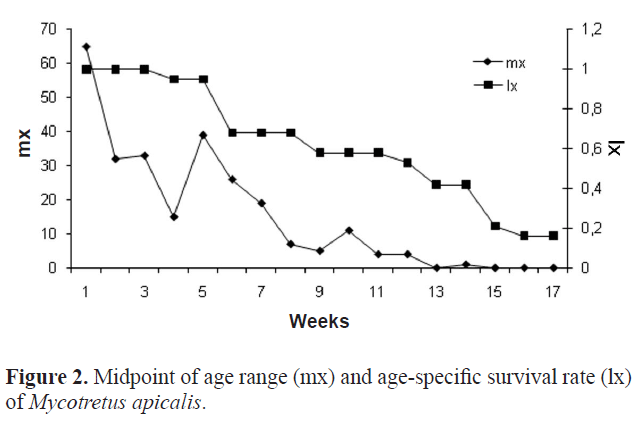
The highest rate of population increase, which corresponds to the meeting point of the age-specific fertility rate (mx) and age-specific survival rate (lx), occurred in the first week (Fig. 2).
According to Price (1997), the intrinsic rate of increase (rm) can be used to compare growth rates among different species or growth rates of the same species under different conditions. Therefore, this datum will be of great importance, as it facilitates the selection of natural enemies to control this pest in Integrated Pest Management (IPM) programs. It is important to note that these parameters of population growth were obtained under optimum conditions of humidity, temperature and the amount of resources for the development of the insect (similar to mushrooms house conditions). According to Odum (1988), under natural conditions, interactions within the population, as well as external environmental resistance, slow the growth rate, interfering with the determination of the form of population growth.
The results of this study show that M. apicalis has potential for a high rate of population growth, indicating its great potential as an insect pest of edible mushrooms. These data can be used to implement programs of integrated pest management and to contribute in further studies like systematic and ecology of erotylids.
Acknowledgments
The authors would like to thank to "Conselho Nacional de Desenvolvimento Científico e Tecnológico" (CNPq) for granting a scholarship to the first author, the "Fundação de Amparo à Pesquisa do Estado de Minas Gerais" (FAPEMIG) for their financial support and Sandro Carmelino for his help in the abstract translation.
Cited literature
AZEVEDO, R. S.; ÁVILA, C. L. S.; SOUZA DIAS, E.; BERTECHINI, A. G.; SCHWAN, R. F. 2009. Utilização do composto exaurido de Pleurotus sajo-caju em rações de frangos de corte e seus efeitos no desempenho dessas aves. Acta Scientiarum 31(2): 139-144. [ Links ]
CLINE, A. R.; LESCHEN, R. A. B. 2005. Coleoptera associated with the oyster mushroom, Pleurotus ostreatus Fries, in North America. Southeastern Naturalist 4 (3): 409-420. [ Links ]
GAITÁN-HERNÁNDEZ, R.; SALMONES, D.; MERLO, R. P.; MATA, G. 2006. Manual práctico del cultivo de setas - Aislamiento, siembra y producción. Instituto de Ecología, A.C. Xalapa, Ver., México. 56 p. [ Links ]
MAIA, H. N. A.; LUIZ, A. J. B.; CAMPANHOLA, C. 2000. Statistical inference on associated fertility life table parameters using jackknife technique: computational aspects. Journal of Economic Entomology 93(2): 511-518. [ Links ]
MOREIRA, G. F.; MOREIRA, C. C.; ANDALÓ, V.; MOINO JUNIOR, A.; MARTOS, E. T.; SOUZA DIAS, E.; LOPES, P. L. 2009. Occurrence and characterization of injuries caused by Mycotretus apicalis Lacordaire, 1842 (Coleoptera: Erotylidae) on cultivation of Pleurotus sajor-caju. World Journal of Microbiology and Biotechnology 26 (3): 573-575. [ Links ]
ODUM, E. P. 1988. Ecologia. Guanabara Koogan, Rio de Janeiro. 434 p. [ Links ]
PRICE, P. W. 1997. Insect ecology. 3rd ed. J. Wiley, New York. 874 p. [ Links ]
ROBERTSON, J. A.; MCHUGH, J. P.; WHITING, M. F. 2004. A molecular phylogenetic analysis of the pleasing fungus beetles (Coleoptera: Erotylidae): evolution of colours patterns, gregariousness and mycophagy. Systematic Entomology 29 (2): 173- 187. [ Links ]
SATO, T. 2003. Effects of photoperiod and temperature on development and larval diapause of Dacne picta (Coleoptera: Erotylidae). Applied Entomology and Zoology 38 (1): 117-126. [ Links ]
SATO, T.; SHINKAJI, N.; AMANO, H. 1999. Selective oviposition by adult females and larval growth of Dacne picta Crotch (Coleoptera: Erotylidae) on diferrent growing stages of the shiitake mushroom, Lentinula edodes. Applied Entomology and Zoology 34 (1): 1-7. [ Links ]
SILVEIRA NETO, S.; NAKANO, O.; BARBIN, D.; VILLA NOVA, N. D. 1976. Manual de ecologia dos insetos. Ceres, São Paulo. 419 p. [ Links ]
SKELLEY, P. E. 1988. Pleasing fungus beetles (Coleoptera: Erotylidae). Florida Department of Agriculture & Consumer Services, Division of Plant Industry, Entomology Circular 313. 2 p. [ Links ]
SKELLEY, P. E.; MCHUGH, J. V. 2002. Erotylidae Leach 1815, p. 348-353. In: Arnett Jr., R. H.; Thomas, M. C.; Skelley, P. E. & J. H. Frank, eds. American Beetles 2. Polyphaga: Scarabaeoidea through Curculionoidea. CRC Press, Boca Raton, Florida. 861 p. [ Links ]
SOUZA DIAS, E.; KOSHIKUMO, E. M. S.; SCHWAN, R. F.; SILVA, R. 2003. Cultivo do cogumelo Pleurotus sajor-caju em diferentes resíduos agrícolas. Ciência e Agrotecnologia 27(6): 1363-1369. [ Links ]
TOWNSEND, C. R.; BEGON, M.HARPER, J. L. 2006. Fundamentos em ecologia. 2nd ed. Artmed, Porto Alegre. 592 p. [ Links ]
VALENCIA, N. R.; LÓPEZ, C. J. 2005. Cultivo de hongos comestibles del gênero Pleurotus sobre residuos agrícolas de la zona cafetera. FNC-Cenicafé, Colômbia. 56 p. [ Links ]
WEGRZYNOWICZ, P. 2002. Morphology, phylogeny and classification of the family Erotylidae based on adult characters (Coleoptera: Cucujoidea). Genus 13 (4): 435-504. [ Links ]