Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488On-line version ISSN 2665-4385
Rev. Colomb. Entomol. vol.37 no.2 Bogotá July/Dec. 2011
Screening of entomopathogenic nematodes to control Mahanarva fimbriolata (Hemiptera: Cercopidae)
Selección de nematodos entomopatogenicos para el control de Mahanarva fimbriolata (Hemiptera: Cercopidae)
ELDER SIMÕES DE PAULA BATISTA1, ALEXANDER M. AUAD2*, TIAGO TEIXEIRA DE RESENDE3, and CAIO MÁRCIO DE OLIVEIRA MONTEIRO1
1 M. Sc. Pos-graduation Animal Biology and Behavior, Federal University of Juiz de Fora - Campus Universitário - Martelos, 36036-900 - Juiz de Fora, Minas Gerais, Brazil; elderspb@gmail.com, caiosat@gmail.com.
2Ph. D. Entomology Laboratory, Embrapa Dairy Cattle - Eugênio do Nascimento Street, 610, Dom Bosco, 36038-330, Juiz de Fora, Minas Gerais, Brazil, amauad@cnpgl.embrapa.br * Corresponding author.
3Agronomist Entomology Laboratory, Embrapa Dairy Cattle, tiago@cnpgl.embrapa.br
Received: 23-dec-2010 - Accepted: 12-sep-2011
Resumen: Se seleccionaron aislamientos de nematodos entomopatogénicos (NEPs) contra el salivazo de los pastos, Mahanarva fimbriolata. Se desarrolló un experimento en laboratorio donde ninfas de salivazo se expusieron a siete aislamientos (Steinernema anomali, S. carpocapsae, S. feltiae, S. riobravis, Heterorhabditis amazonensis RSC5, H. bacteriophora HP88 y H. baujardi LPP7) en tres concentraciones (200, 400 y 800 NEP/ninfa). Luego de la selección de los aislamientos más virulentos en laboratorio, se efectuó un experimento en invernadero. El aumento de la concentración no proporcionó incremento en la mortalidad ninfal de M. fimbriolata en laboratorio. Las ninfas de salivazo fueron susceptibles a todos los aislamientos, destacándose S. riobravis con un 90% de mortalidad, y S. feltiae con un 80%. El desempeño en invernadero no fue diferente entre los aislados, alcanzando una mortalidad ninfal de 48% (S. carpocapsae) y 72% (S. feltiae). Hay disminución de la eficiencia de los nematodos en invernadero comparada con la eficiencia en laboratorio.
Palabras clave: Caña de azúcar. Control microbiano. Salivazo de los pastos. Heterorhabditis. Steinernema.
Abstract: We aimed to screen entomopathogenic nematodes (EPNs) strains against the root spittlebug, Mahanarva fimbriolata. An assay was performed at laboratory in which nymphs of the insect were exposed to seven EPNs strains (Steinernema anomali, S. carpocapsae, S. feltiae, S. riobravis, Heterorhabditis amazonensis RSC5, H. bacteriophora HP88 and H. baujardi LPP7) under three concentrations (200, 400 and 800 EPNs/nymph). After the laboratory screening a greenhouse assay was carried out using the most pathogenic strains. Increasing concentration didn't increase nymph mortality at laboratory. The root spittlebug nymphs were susceptible to all strains, with 48% (S. carpocapsae) and 72% (S. feltiae) pathogenicity extremes. A decrease in efficiency was observed in greenhouse tests compared with laboratory tests.
Key words: Sugarcane. Microbial control. Spittlebug. Heterorhabditis. Steinernema.
Introduction
Brazil is the leading producer of sugarcane and products made from it worldwide, and cane is the third leading crop by area cultivated in the country. Besides sugar, the main products derived from cane are biofuels such as ethanol and biodiesel. The outlook is for greatly expanded production of these fuels because they are more environmentally friendly and sustainable than fossil fuels (Macedo 2005).
The root spittlebug, Mahanarva fimbriolata (Stal, 1854) (Hemiptera: Cercopidae), stands out among the pests that attack sugarcane. The gradual replacement of manual harvesting, after burning off the litter, by mechanical harvesting without burning favors increasing populations of these pests, because the leaf trash cover left afterward provides an ideal environment for the development of nymphs, which feed off the sap in the roots of the new cane plants growing from the ratoons (Ravaneli et al . 2006). According to Madaleno et al . (2008), the feeding of nymphs and adults can reduce sugarcane productivity by 29.8% when the number of nymphs reaches 7.3 per linear meter.
Efforts to control this pest vary widely, often in various combinations, include strategic harvest timing, physical removal of insects and litter, use of resistant genotypes, application of pesticides and biological control by entomopathogenic fungi (Dinardo-Miranda et al . 2001; Dinardo-Miranda et al . 2002; Almeida et al . 2003; Batista-Filho et al . 2003; Souza et al . 2008). Integrated pest management calls have been done for reduced use of chemicals due to the environmental and economic problems caused by their indiscriminate use. The growing concern on the problems of overuse of pesticides has attracted greater attention to the use of plant varieties resistant to root spittlebugs (Auad et al . 2007) and the use of biological control agents, of which entomopathogenic fungi have attracted most of the attention (Batista-Filho et al . 2003; Loureiro et al . 2005). However, these measures have certain limitations, hence the need to develop new strategies to manage this pest.
Some characteristics make entomopathogenic nematodes (EPNs) promising for control of crop pests, such as the speed with which they attack the host, the ease of mass producing them at low cost and the wide spectrum of susceptible host pests (Georgis et al . 2006; Grewal et al . 2001). They also are compatible with many pesticides, allowing them even to be applied in combination with chemicals in some situations (Koppenhöfer et al . 2002; Negrisoli Jr. et al ., 2008; Reis-Menini et al . 2008). Finally, they are able to seek out hosts that have cryptic habits (Kaya and Gaugler 1993) such as the root spittlebug. In the case of M. fimbriolata, the only reports in the literature are the studies by Leite et al . (2002) and Leite et al . (2005), in which nematodes were shown to be potential control agents.
The life cycle of these nematodes includes three development phases: egg, juvenile and adult (females and males of Steinernematidae and a generation of hermaphrodites in the case of Heterorhabditidae). The juvenile phase is composed of four stages (J1, J2, J3 or Infective Juvenile and J4). The infective juvenile (IJ) stage contains symbiont bacteria and is when the nematode is found on soil. These juveniles locate their hosts by detecting excretion products, CO2 levels and temperature gradients. The nematodes then penetrate the host through natural openings (mouth, anus or spiracles). Once inside, they migrate to the hemolymph and release bacteria. These produce toxins that kill the host by septicemia in 24 to 48 hours. Then the IJs start to multiply and later feed on the bacteria and tissues decomposed by the bacteria and advance to stage J4. From this stage they become first-generation adults and the females lay eggs, giving rise to the second generation (Kaya and Gaugler 1993). Some EPN species, called “ambushers”, wait on the soil surface for a host to approach, while others, called “cruisers”, are adapted to search for hosts in the soil. EPNs in the second group are very active, relying on chemical trails to find distant hosts, and are thus more suitable to combat sedentary hosts (Campbell and Gaugler 1993; Grewal et al . 1994).
There are substantial behavioral differences among ENP strains in relation to penetration in the host and later emergence and the strategy of finding hosts. Besides these, there are also important morphological and physiological differences. Finally, the concentration of these pathogens in the environment and the target host are also factors that influence their action (Lewis et al . 2006). This explains the specificity of each strain and requires conducting experiments to choose adequate strains to fight the pest species of interest. Therefore, EPNs can be included in integrated pest management programs, to optimize sugarcane production, since they exploit the same environment as spittlebug nymphs. The study reported here screened different EPN strains for their effectiveness against nymphs of the root spittlebug, M. fimbriolata, in laboratory and greenhouse conditions.
Material and Methods
We used M. fimbriolata nymphs that had been maintained in a greenhouse on sugarcane plants (Saccharum spp. cv. RB739735). The nematodes were multiplied according to the method proposed by Kaya and Stock (1997) on larvae of Tenebrio molitor L., 1758 (Coleoptera: Tenebrionidae) as the host. Seven strains were tested: Steinernema anomali Kozodoi, 1984; S. carpocapsae (Weiser, 1955); S. feltiae (Filipjev, 1934); S. riobravis Cabanillas, Poinar & Raulston, 1994; Heterorhabditis amazonensis RSC5 Andaló, Nguyen & Moino Jr. 2006; Heterorhabditis bacteriophora HP88 Poinar 1976; and Heterorhabditis baujardi LPP7 Phan, Subbotin, Nguyen & Moens 2003.
Nymph mortality in laboratory. The experimental units consisted of Petri dishes (9cm in diameter) lined with two sheets of filter paper as substrate, moistened with 2mL of an aqueous solution of EPNs (treatments) or distilled water without pathogens (control). Then, five nymphs of the fourth or fifth instar were placed in each dish, which was sealed with plastic film and placed an incubation chamber at a temperature of 25±1°C and 80±10% relative humidity. The nematodes were applied at concentrations of 200, 400 and 800 EPNs/nymph. Hence, there were 21 treatments in a factorial setup (seven strains x three concentrations) with five repetitions, for a total of 25 nymphs per treatment. A control group was exposed to distilled water without nematodes to confirm that the nymphs had no previous contact with the pathogens and that the mortality was caused exclusively by the treatments. After seven days the dead nymphs were dissected and observed under stereomicroscope. Those that contained EPNs were considered to have been killed by these pathogens. The mean mortality figures were submitted to variance analysis and compared by the Tukey test at 5% significance, using the SAS 9.2 (SAS institute) statistical software.
Nymph mortality in greenhouse. Five nymphs of the fourth or fifth instar were placed at the base of a sugarcane seedling (cv. RB739735) planted in a 500mL plastic cup containing soil and cattle manure (1:1) as substrate, with the roots previously exposed, and kept in a greenhouse at an average temperature of 25,5±1°C and relative humidity of 94±5%. After 24 hours, when the nymphs were fully protected by their froth, the nematodes that had presented pathogenicity greater than or equal to 80% in the laboratory assay were applied at a concentration of 200 IJs/nymph in a total volume of 2 mL per cup, using a sprayer adjusted to the squirt mode, with the stream directed at the froth.
There were five repetitions with the strains that had shown 80% or better pathogenicity, for a total of 25 nymphs per treatment. As in the laboratory, there was a control group with five repetitions also, to confirm that the substrate and nymphs used were free of nematodes, so that the nymph deaths could be attributed to the EPNs. The nymphs were monitored daily until emergence of the last adult and the dead ones were dissected and observed under stereomicroscope to determine the presence of nematodes inside, in which case this presence was attributed as the cause of death. As before, the mortality data were submitted to variance analysis and the means were compared by the Tukey test at 5% significance, using the SAS (version 9.2) statistical software. The nymph mortality rates caused by the EPNs were also compared between the laboratory and greenhouse through the Tukey test at the same significance level.
Results
Nymph mortality in laboratory. No nymphs were killed by the nematodes in the control group, confirming that there had been no previous contact between hosts and pathogens and that the results were due to the treatments. All the EPN strains caused mortality to the M. fimbriolata nymphs, showing its potential to control by this pest.
Irrespective of concentration, S. riobravis produced 90% nymph mortality, followed by S. carpocapsae, S. feltiae and H. baujardi LPP7 (80%), S. anomali (70%), H. amazonensis RSC5 (68%) and H. bacteriophora HP88 (38%). This last strain was statistically inferior to the others (Table 1). The concentrations did not significantly affect the efficiency of the treatments. The lowest of them (200 EPNs/nymph) provided a maximum control level, so there appears to be no need to increase the concentration to achieve the desired effects, whereas the lowest concentration reached statistically the same nymph mortality than the greatest (800 EPNs/nymph).
Steinernema anomali, H. bacteriophora HP88 and H. amazonensis RSC5, were less efficient at the intermediate concentration (400 EPNs/nymph), than the other strains at this same concentration. This also occurred for H. bacteriophora HP88 at the other concentrations, reflecting its substantially lower efficiency in relation to the strains, considering the average of all concentrations tested.
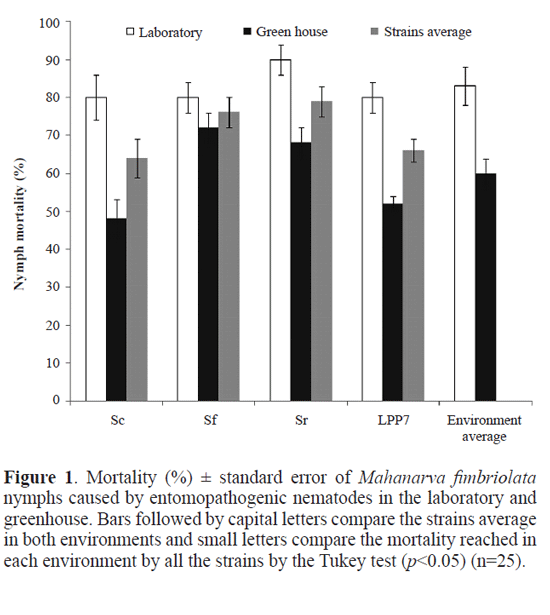
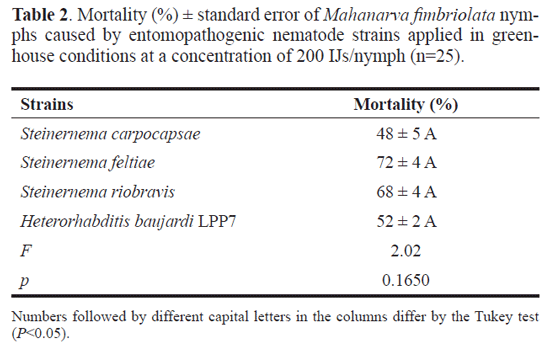
Nymph mortality in greenhouse. All strains were pathogenic to M. fimbriolata nymphs in the greenhouse experiment with the nymph mortality caused by S. feltiae (72%), S. riobravis (68%), H. baujardi LPP7 (52%) and S. carpocapsae (48%) with no statistical difference between them (Table 2). While there were no significant differences between strains performance within the environments, there were significant differences comparing the influence of the environments on nymph mortality, with lower rates of nymph mortality caused by S. carpocapsae (F = 2.899, p = 0.008) and H. baujardi LPP7 (F = 9.391, p = 0.004) in the greenhouse than in the laboratory (Fig. 1).
Discussion
Nymph mortality in laboratory. The concentrations did not significantly affect the efficiency of the treatments, with the lowest of them providing a maximum control level. A similar finding was reported by Leite et al . (2007), testing the virulence of EPNs on Bradysia mabiusi (Lane, 1959) (Diptera: Sciaridae), where there was no difference in mortality of the larvae between concentrations of 10 and 50 IJs/cm2. However, Vasconcelos et al . (2004) found that only the highest concentration tested (1200 IJs/female) was able to significantly reduce the egg mass produced by Rhipicephalus (Boophilus) microplus (Canestrini, 1888) (Acari: Ixodidae). Furthermore, Monteiro et al . (2010) reported that the six concentrations tested produced different effects on some reproductive parameters of R. (B.) microplus but similar effects on other parameters.
Some strains at the intermediate concentration were less efficient than others at this concentration. This difference can reflect a greater sensitivity of these strains regarding the concentration at which they are applied. This also occurred for H. bacteriophora HP88 at the other concentrations, reflecting their substantially lower efficiency in relation to the strains, considering the average of all concentrations tested. Our data agree with Leite et al . (2005), where strains of Heterorhabditis sp. (CB-n5), Steinernema sp. (CB-n6) and Heterorhabditis sp. (CCA) presented 100%, 98% and 96% efficiency, respectively, when applied at a concentration of 2000 IJs/mL under laboratory conditions, showing that M. fimbriolata is susceptible to both EPN genera.
The species S. riobravis, S. feltiae, S. carpocapsae and H. baujardi LPP7 were the most efficient against M. fimbriolata and have also been shown to be promising for use against the boll weevil, Anthonomus grandis Boheman, 1843 (Coleoptera: Curculionidae), the mound-building termite, Cornitermes cumulans (Kollar, 1832) (Isoptera: Termitidae), the lesser mealworm, Alphitobius diaperinus (Panzer, 1979) (Coleoptera: Tenebrionidae) and the guava weevil, Conotrachelus psidii Marshall, 1922 (Coleoptera: Curculionidae), respectively, demonstrating the broad spectrum of hosts of these pathogens (Cabanillas 2003; Alves et al . 2005; Del- Valle et al . 2008).
Moreover, Giometti et al . (2011) tested the virulence of 17 EPN strains against another sugar cane pest and founded a maximum of 74.3% of Sphenophorus levis Vaurie, 1978 (Coleoptera: Curculionidae) adults mortality with an Heterorhabditis strain in laboratory conditions. A similar assay was developed by Tavares et al . (2007) with different strains against the same insect and they founded a maximum of 95% of larvae mortality, also with an Heterorhabditis strain.
Nymph mortality in greenhouse. No differences were observed between the strains tested in greenhouse. This is possibly because they had been previously selected in the laboratory as being the most virulent. Although they have different foraging strategies (cruisers and ambushers), possibly there was no difference because they were all applied directly on the nymphs' froth, thus minimizing the need either to go find the host or wait for it to approach.
After selecting the most virulent strain against M. fimbriolata in the laboratory, Leite et al . (2005) tested its efficiency in the field at different concentrations and found that Heterorhabditis sp. provided the maximum control of the spittlebugs (70%) irrespective of the concentrations. Both that study and ours thus show the capacity of EPNs for control of root spittlebugs. Furthermore, Tavares et al . Machado et al . (2005) evaluated EPNs against another sugarcane pest and showed their potential to control Migdolus fryanus (Westwood, 1863) (Coleoptera: Vesperidae) larvae, causing a maximum mortality of 76.43% with a S. glaseri strain and even that the nematodes' IJs can penetrate the egg of this coleopteran trough its aeropile.
The negative influence of the greenhouse environment can possibly be explained by the fact that the laboratory environment was ideal for them (good substrate for locomotion, constant and optimized temperature/relative humidity, absence of antagonistic organisms, no refuge for the hosts). This was not the case in the greenhouse, where the conditions were chosen to simulate those in the field. Nevertheless, two of the strains tested (S. feltiae and S. riobravis) maintained high pathogenicity in the greenhouse, indicating they are good candidates for tests in the field. Aiming to know the EPN action against the sugarcane weevil, S. levis, Tavares et al. (2007), applied two strains in greenhouse after a initial assay in laboratory, and founded a larvae mortality ranging from 42% to 85%, with these percentages directly related with the EPNs dose. These authors also noted that the laboratory environment provided a higher better condition to the EPNs to show their potential, with higher mortality values in this case. These and our results reinforce the possibility of use of these pathogens to control pests of this crop.
Acknowledgements
The first author wish to thank the Insect Pathology Laboratory of Federal University of Lavras for the entomopathogenic nematodes isolates used in this study, Embrapa Dairy Cattle for the structure to develop this study and Federal University of Juiz de Fora for providing financial support and to Julian Penzi for the spanish version of the abstract.
Cited literature
ALMEIDA, J. E. M.; BATISTA FILHO, A.; SANTOS, A. S. 2003. Avaliação do controle biológico de Mahanarva fimbriolata (Hom., Cercopidae) com o fungo Metarhizium anisopliae em variedades de cana-de-açúcar e diferentes épocas de corte. Arquivos do Instituto Biológico 70: 101-103. [ Links ]
ALVES, L. F. A.; ROHDE, C.; ALVES, V. S. 2005. Patogenicidade de Steinernema glaseri e S. carpocapsae (Nematoda: Rhabdita) contra o cascudinho, Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae). Neotropical Entomology 34: 139-141. [ Links ]
AUAD, A. M.; SIMÕES, A. D.; PEREIRA, A. V.; BRAGA, A. L. F.; SOBRINHO, F. S.; LÉDO, F. J. S.; PAULA-MORAES, S. V.; OLIVEIRA, S. A.; FERREIRA, R. B. 2007. Seleção de genótipos de capim-elefante quanto à resistência à cigarrinha-daspastagens. Pesquisa Agropecuária Brasileira 42: 1077-1081. [ Links ]
BATISTA-FILHO, A.; ALMEIDA, J. E. M.; SANTOS. A. S.; MACHADO, L. A.; ALVES, S. B. 2003. Eficiência de isolados de Metarhizium anisopliae no controle de cigarrinha-da-raiz da cana-de açúcar Mahanarva fimbriolata (Hom: Cercopidae). Arquivos do Instituto Biológico 70: 309-314. [ Links ]
CABANILLAS, H. E. 2003. Susceptibility of the boll weevil to Steinernema riobrave and other entomopathogenic nematodes. Journal of Invertebrate Pathology 82: 188-197. [ Links ]
CAMPBELL, J. F.; GAUGLER, R. 1993. Nictation behaviour and its ecological implications in the host search strategies of entomopathogenic nematodes (Heterorhabditidae and Steinernematidae). Behaviour 126: 3-14. [ Links ]
DEL VALLE, E. E.; DOLINSKI, C.; BARRETO, E. L. S.; SOUZA, R.M.; SAMUELS, R. I. 2008. Efficacy of Heterorhabditis baujardi LPP7 (Nematoda: Rhabditida) applied in Galleria mellonella (Lepidoptera: Pyralidae) insect cadavers to Conotrachelus psidii, (Coleoptera: Curculionidae) larvae. Biocontrol Science and Technology 18: 33-41. [ Links ]
DINARDO-MIRANDA, L. L.; FERREIRA, J. M. G.; CARVALHO, P. A. M. 2001. Influência da época de colheita e do genótipo de cana-de-açúcar sobre a infestação de Mahanarva fimbriolata (Stal) (Hemiptera: Cercopidae). Neotropical Entomology 30: 145-149. [ Links ]
DINARDO-MIRANDA, L. L.; GARCIA, V.; PARAZZI, V. J. 2002. Efeito de inseticidas no controle de Mahanarva fimbriolata (Stal) (Hemíptera: Cercopidae) e de nematóides fitoparasitos na qualidade tecnológica e na produtividade da cana-de-açúcar. Neotropical Entomology 31: 609-614. [ Links ]
GEORGIS, R.; KOPPENHOFER, A. M.; LACEY, L. A.; BÉLAIR, G.; DUNCAN, L. W.; GREWAL, P. S.; SAMISH, M.; TAN, L.; TORR, P.; VAN TOL, R. W. H. M. 2006. Successes and failures in the use of parasitic nematodes for pest control. Biological Control 38: 103-123. [ Links ]
GIOMETTI, F. H. C.; LEITE, L. G.; TAVARES, F. M.; SCHMIT, F. S.; BATISTA-FILHO, A.; DELL'ACQUA, R.2011 Virulência de nematóides entomopatogênicos (Nematoda: Rhabditida) a Sphenophorus levis (Coleoptera: Curculionidae). Bragantia 70 (1): 81-86. [ Links ]
GREWAL, P. S.; LEWIS, E. E.; GAUGLER, R.; CAMPBELL, J. F. 1994. Host find behavior as a predictor of foraging strategy in entomopathogenic nematodes. Parasitology 108: 207-215. [ Links ]
GREWAL, P. S.; NARDO, E. A. B.; AGUILLERA, M. M. 2001. Entomopathogenic nematodes: Potential for exploration and use in South America. Neotropical Entomology 30: 191-205. [ Links ]
KAYA, H. K.; GAUGLER, R. 1993. Entomopathogenic nematodes. Annual Review of Entomology 38: 181-206 [ Links ]
KAYA, H. K.; STOCK, S. P. 1997. Techniques in insect nematology. In: LACEY LA (ed) Manual of Techniques in Insect Pathology, Academic Press, San Diego. p. 281-234. [ Links ]
KOPPENHÖFER, A. M.; COWLES, R. S.; COWLES, E. A.; FUZY, E. M.; BAUMGARTNER, L. 2002. Comparison of neonictinoid inseticides as synergists for entomopathogenic nematodes. Biological Control 24: 90-97. [ Links ]
LEITE, L. G.; MACHADO, L. A.; AGUILLERA, M. M.; RODRIGUES, R. C. D.; NEGRISOLI Jr, A. S. 2002. Patogenicidade de Steinernema e Heterorhabditis (Nematoda: Rhabditidae) contra ninfas da cigarrinha-das-raízes da cana-de-açúcar, Mahanarva fimbriolata (Hemiptera: Cercopidae). Revista Agricultura 78: 139-148. [ Links ]
LEITE, L. G.; MACHADO, L. A.; GOULART, R. M.; TAVARES, F. M.; BATISTA-FILHO, A. 2005 Screening of entomopathogenic nematodes (Nemata: Rhabditida) and the efficiency of Heterorhabditis sp. against the sugar cane root spittlebug Mahanarva fimbriolata (Fabr.) (Hemiptera: Cercopidae). Neotropical Entomology 34: 785-790. [ Links ]
LEITE, L. G.; TAVARES, F. M.; BUSSÓLA, R. A.; AMORIM, D. S.; AMBRÓS, C. M.; HARAKAVA, R. 2007. Virulência de nematóides entomopatogênicos (Nemata: Rhabditida) contra larvas da mosca-dos-fungos Bradysia mabiusi (Lane, 1959) e persistência de Heterorhabditis indica Poinar et al . 1992 em substratos orgânicos. Arquivos do Instituto Biológico 74: 337- 342. [ Links ]
LEWIS, E. E.; CAMPBEL, J.; GRIFFIN, C.; KAYA, H.; PETERS, A. 2006. Behavioural ecology of entomopathogenic nematodes. Biological Control 38: 66-79. [ Links ]
LOUREIRO, E. S.; BATISTA-FILHO, A.; ALMEIDA, J. E. M.; PESSOA, L. G. A. 2005. Seleção de isolados de Metarhizium anisopliae (Metsch.) Sorok. contra a cigarrinha da raiz da canade- açúcar Mahanarva fimbriolata (Stal) (Hemiptera: Cercopidae) em laboratório. Neotropical Entomology 34: 791-798. [ Links ]
MACEDO, I. C. 2005. A energia da Cana-de-açúcar: Doze estudos sobre a agroindústria da cana-de-açúcar no Brasil e a sua sustentabilidade. Única - União da Agroindústria Canavieira de São Paulo, São Paulo. [ Links ]
MACHADO, L. A.; HABIB, M.; LEITE, L. G.; CALEGARI, L. C.; GOULART, R. M.; TAVARES, F. M. 2005. Patogenicidade de nematóides entomopatogênicos a ovos e larvas de Migdolus fryanus (Westwood, 1863) (Coleoptera: Vesperidae). Arquivos do Instituto Biológico 72: 221-226. [ Links ]
MADALENO, L. L.; RAVANELI, G. C.; PRESOTTI, L. E.; MUTTON, M. A.; FERNANDES, O. A. MUTTON, M. J. R. 2008. Influence of Mahanarva fimbriolata (Stal) (Hemiptera: Cercopidae) injury on the quality of cane juice. Neotropical Entomology 37: 68-73. [ Links ]
MONTEIRO, C. M. O.; PRATA, M. C. A.; FURLONG, J.; FAZA, A. P.; MENDES, A. S.; ANDALÓ, V.; MOINO Jr, A. 2010. Heterorhabditis amazonensis (Rhabditidae: Heterorhabditidae), strain RSC-5, for biological control of the cattle tick Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Parasitology Research 106: 19-21. [ Links ]
NEGRISOLI Jr, A. S.; BARBOSA, C. R. C.; MOINO Jr, A. 2008. Avaliação da compatibilidade de produtos fitossanitários com nematóides entomopatogênicos (Rhabditida: Steinernematidae, Heterorhabditidae) utilizando o protocolo modificado da IOBC/ WPRS. Nematologia Brasileira 32: 111-116. [ Links ]
RAVANELI, G. C.; MADALENO, L. L.; PRESOTTI, L. E.; MUTTON, M. A.; MUTTON, M. J. R. 2006. Spittlebug infestation in sugarcane affects ethanolic fermentation. Scientia Agricola 63: 534-539. [ Links ]
REIS-MENINI, C. M. R.; PRATA, M. C. A.; FURLONG, J.; SILVA, E. R. 2008. Compatibility between the entomopathogenic nematode Steinernema glaseri (Rhabditida: Steinernematidae) and an acaricide in the control of Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Parasitology Research 103: 15-19. [ Links ]
SAS Institute Inc. SAS: Version 9.2 for Windows. Cary (NC): SAS Institute, Inc. 2006. [ Links ]
SOUZA, Z. M.; PAIXÃO, A. C. S.; PRADO, R. M.; CESARIN, L. G.; SOUZA, S. R.; MONTANARI, R. 2008. Produtividade agrícola de variedades de cana-de-açúcar e incidência de brocacomum e cigarrinha da raiz em canavial colhido sem queima. Bragantia 67: 413-419. [ Links ]
TAVARES, F. M.; BATISTA-FILHO, A.; LEITE, L. G.; ALMEIDA, L. C.; SILVA, A. C.; AMBRÓS, C. M. G. 2007. Efeito de Heterorhabditis indica e Steinernema sp. (Nemata: Rhabditida) sobre larvas do bicudo da cana-de-açúcar, Sphenophorus levis (Coleoptera: Curculionidae), em laboratório e casa-de-vegetação. Nematologia Brasileira 31 (1): 12-19. [ Links ]
VASCONCELOS, V. O.; FURLONG, J.; FREITAS, G. M.; DOLINSKI, C.; AGUILLERA, M. M.; RODRIGUES, R. C. D.; PRATA, M. C. A. 2004. Steinernema glaseri Santa Rosa strain (Rhabditida: Steinernematidae) and Heterorhabditis bacteriophora CCA strain (Rhabditida: Heterorhabditidae) as biological control agents of Boophilus microplus (Acari: Ixodidae). Parasitology Research 94: 201-206. [ Links ]