Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488On-line version ISSN 2665-4385
Rev. Colomb. Entomol. vol.37 no.2 Bogotá July/Dec. 2011
Scientific note
Susceptibility of the predator Euborellia annulipes (Dermaptera: Anisolabididae) to mycoinsecticides
Susceptibilidad del depredador Euborellia annulipes (Dermaptera: Anisolabididae) a micoinsecticidas
FLÁVIA QUEIROZ DE OLIVEIRA1, JACINTO DE LUNA BATISTA1, JOSÉ BRUNO MALAQUIAS3, CARLOS HENRIQUE DE BRITO4, and EMMERSON PEREIRA DOS SANTOS5 1 M.Sc. Student in Environmental Science and Technology, Universidade Estadual da Paraíba (UEPB), Brazil. e-mail: flavinha2010@ibest.com.br corresponding author; 2 Ph.D., Universidade Federal da Paraíba (UFPB). Campus II - Areia- PB. e-mail: jacinto@cca.ufpb.br; 3 M.Sc. Student in Entomology, Universidade de São Paulo (USP), Brazil. e-mail: jbmalaquias@ig.com.br; 4 Researcher/Professor, Universidade Federal da Paraíba (UFPB), Campus II - Areia- PB. e-mail: chbrito1@hotmail.com; 5 B.Sc. Universidade Federal da Paraíba (UFPB), Campus II - Areia - PB. e-mail: emmersonbiozoo@hotmail.com. Received: 23-dec-2010 - Accepted: 2-jun-2011
Abstract: To evaluate the infuence of Beauveria bassiana (Balsamo) Vuill and Metarhizium anisopliae (Metsch) Sorok on the survival of the predator Euborellia annulipes Lucas (Dermaptera: Anisolabididae) were used product concentrations 5.00x109; 7.50x109; 10.00x109; 12.50x109 conidia/L from isolates of B. bassiana and M. anisopliae which were obtained, respectively, through products Boveril® and Metarril® and control (0.0 conidia/L). High rates of hatching nymphs of E. annulipes have been recorded in clutches that received applications of M. anisopliae. Moreover, B. bassiana has affected negatively the hatching rate of nymphs of E. annulipes. A slightly harmful effect has been observed in all concentrations of conidia B. bassiana in clutches of E. annulipes. The survival was 100% in all tested concentrations of M. anisopliae in females of E. annulipes. The females which received topical application of B. bassiana have shown a survival rate ranging from 80.30 (12.50x109 conidia) to 100% (5.00x109 conidia). In males of E. annulipes, the survival rate in insects treated with B. bassiana ranged from 95.00 (12.50x109 conidia) to 100% (5.00x109 conidia), while with M. anisopliae applications this rate was 96.02 (12.50x109 conidia) to 100% (5.00x109 conidia). The entomopathogenic fungi B. bassiana and M. anisopliae did not affect the mortality of nymphs and adults of E. annulipes. However, careful measures should be adopted in applications of B. bassiana directly into clutches of E. annulipes.
Key words: Pathogenicity. Entomopathogenic fungi. Selectivity. Natural enemy.
Resumen: Se evaluó la infuencia de Beauveria bassiana (Balsamo) Vuill y Metarhizium anisopliae (Metsch) Sorok en la supervivencia del depredador Euborellia annulipes Lucas (Dermaptera: Anisolabididae). Las concentraciones de los productos evaluados fueron 5,00x109; 7,50x109; 10,00x109; 12,50x109 conídios/L de aislamientos de B. bassiana y M. anisopliae, respectivamente, con los productos Metarril® y Boveril® y un control (0,0 conidios / L). Se registraron altas tasas de eclosión de ninfas de E. annulipes en especímenes que recibieron tratamientos con M. anisopliae. Se observó un efecto levemente perjudicial en todas las concentraciones de conidios de B. bassiana en posturas de E. annulipes, pues B. bassiana afectó negativamente la tasa de eclosión de ninfas de E. annulipes. La supervivencia fue del 100% en hembras de E. annulipes que recibieron todas las concentraciones probadas de M. anisopliae. Las hembras que recibieron aplicaciones tópicas de B. bassiana presentaron sobrevivencia entre 80,3 (12,50x109 conidios) y 100% (5,00x109 conidios). Los machos de E. annulipes presentaron una tasa de supervivencia con B. bassiana entre 95 (12,50x109 conidios) y 100% (5,00x109 conidios) y de 96,0 (12,50x109 conidios) a 100% (5,00x109 conidios) al recibir aplicaciones de M. anisopliae. Los hongos entomopatógenos B. bassiana y M. anisopliae no afectaron la mortalidad de ninfas y adultos de E. annulipes. Sin embargo, se deben adoptar medidas precisas en aplicaciones de B. bassiana directamente en posturas de E. annulipes.
Palabras clave: Patogenicidad de hongos. Hongos entomopatógenos. Seletividad. Enemigo natural.
Introduction
Earwigs or Dermaptera constitute several species with high predation capacity due to their aggresive behavior. Among these species, Euborellia annulipes (Lucas, 1847) (Dermaptera: Anisolabididae) is an important biological control agent of pests in agriculture. This insect species occurs throughout the neotropical zoogeographical region and has been an potential predator of several insect pests, particularly of eggs and immature stages of insects of the Lepidoptera, Hemiptera, Coleoptera and Diptera (Lemos et al. 1998, 1999). In 1998). Innortheast Brazil it is common occurrence of this insect preying on immature stages of pests that occur in soil or in plant structures lying on the ground as case of the larvae and pupae of boll weevil at feld of cotton. Entomopathogens are prevalent in natural systems and should receive greater attention in life-history studies (Hesketh et al. 2010; Roy et al. 2009). Mycoinsecticides compose only a small component of the biopesticide market, but their use is gaining acceptance as an alternative to the use of traditional contact insecticides for pest control (Ludwig and Oetting 2001). Beauveria bassiana (Balsamo) Vuill and Metarhizium anisopliae (Metsch) Sorok are among the most studied species of entomopathogenic and are used as biocontrol agents of agricultural pests (Alves 1992; Alves et al.1998).biological control of pests an important pathogen is that one who affects only the target insect and not their natural enemies (Flexner et al. 1986; Generoso 2002). The severity of the effects of microbial insecticides depends on the concentration of spores applied and the stage of the insect (Ginsberg et al. 2002). The use of low concentrations of alternative insecticides may increase the selectivity and help reduce costs of production (Oliveira 2008). In recent studies conducted by Oliveira et al. (2010), it was observed that low concentrations of mycoinsecticides isolates of B. bassiana and M. anisopliae promoted high efficiency control of larvae and pupae of fruit fies. However the direct effects of entomopathogenic fungi in natural enemies have not been well studied and probably have been overestimated. It is also necessary to develop techniques to express accuracy in doses of microbial insecticides to achieve maximum physiological compatibility. Due to the increased use of biological control in integrated pest management and considering that information regarding the selectivity of entomopathogenic fungi in biological control agents is incipient, the aim of this research is to evaluate the infuence of M. anisopliae and B. bassiana in the survival of the predator E. annulipes.
Material and Methods Insects. Specimens of the E. annulipes used in this study were obtained from laboratory rearing. The stock colony was maintained in an acclimatized room at 26±1°C, of 70±10% r.h. and 12L:12D photoperiod. The bioassays followed the methodology described by Lemos et al. (1999). This study used eggs, nymphs of 1st instar, (≤ 24h old), 2nd instar, and adult insects of both sexes of E. annulipes. Individuals have been placed in Petri dishes and kept at a constant temperature of 25±1°C, with 70±10% R.H., and a photoperiod of 12:12h (L:D). The insects or clutches has been kept in groups of 5 units per dish. Recently emerged adults (≤ 24h old) have been sexed by the characteristics of external morphology, the number of abdominal segments and the curvature of the forceps (Tomkins and Simmons 1998). Bioassay. The following concentrations of conidia tested were 5.00x109, 7.50x109; 10.00x109; 12.50x109 conidia/L, and control (0.0 conidia/L). These concentrations are recommended to the pests control, i.e. in applications at soil to control of larvae and pupae of fruit fies (Oliveira 2008). The isolates have been obtained from the products Boveril® and Metarril®, produced by the Company Itaforte Bio products. Patogenicity and virulence of isolates. The viability of isolated evidence of pathogenicity and virulence has been evaluated according to the method described by França et al. (2006). In the viability test has been used two Petri dishes containing PDA (potato dextrose agar) culture media, incubated in climatized chamber (25±2°C, 12h of photophase and relative humidity of 70±10%) for 24 hours, later to perform readings in a light microscope by determining the percentage of germinated conidia and not germinated. For proving the pathogenicity and virulence of the isolates tested in this study, we spraying a suspension of 5.00x109 conidia/L on the 100 larvae 1st-instar of Ceratitis capitata (Wiedemann, 1824) (Diptera: Tephritidae) for each species of fungus, because it is a highly susceptible to entomopathogenic stage (Oliveira 2008). Effects on survival and development stages of E. annulipes. Fungal suspensions of each fungal product concentration have been applied topically with deposition of 5μl from each suspension. The applications have been performed using a manual spray (1L) just once time on the entire structure of the dorsal body of the specimens, and in the case of the clutches around the whole egg chorion. After 10 days of period application was observed the survival rate. This period was established because the action time of two microorganisms have been also observed and the assessments made after 5 days of application to the insects as young and adult (Bustillo et al. 1999) as well as after 10 days, which was the maximum period of hatching in this conditions (Lemos 1998). The mortality was confrmed by sporulation in humide chamber. Then, mortality has been calculated and corrigid to control (untreated) group. Data analysis. The experimental design was completely randomized with fve replications per concentration/treatment, with each replication consisting of fve insects or clutches (100 eggs per clutch). The survival of insects in treatments was calculated using the formula: S (%) = 100 - Ma (%). The mortality data (Ma %) of the treatments and control were submitted to Abbotts formula (Abbott 1925). Data were also subjected to polynomial logistic regression (P=0.05) (PROC GENMOD, SAS Institute 2006). The determination of the harmfulness of the products was based on the guidelines for the insecticides under laboratory conditions by the IOBC/ WPRS (Amano and Haseeb 2001): survival >70%, non-harmful, survival >30% and <70%, slightly harmful; survival> 1% and <30%, moderately harmful and survival <1% severely harmful.
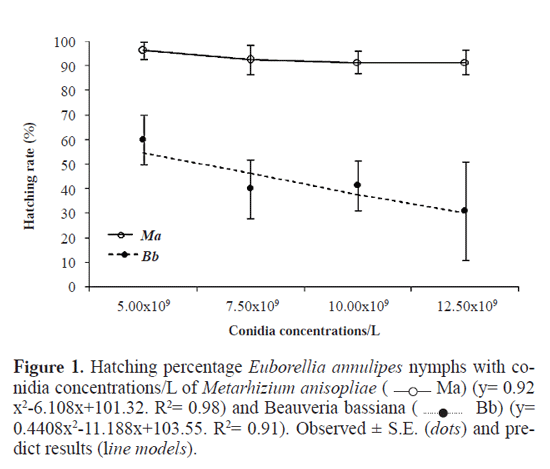
Results and Discussion In our test with C. capitata, it has been recorded a mortality rate approximately 98% and 97% caused by fungi M. aniso-pliae and B. bassiana, respectively. Therefore, the survival of E. annulipes was not because the lack of fungi virulence. High rates of hatching nymphs of E. annulipes have been recorded in clutches that received applications of M. aniso-pliae. The hatching percentage nymphs confrmed was 96.23, 92.47, 91.58 and 91.50% in the concentrations of 5.00x109, 7.50x109, 10.00x109 and 12.50x109 conidia/L of M. aniso-pliae, respectively. Moreover, B. bassiana affected the hatch-ing rate of nymphs of E. annulipes, the survival ranged from 31.13 (12.50x109) to 60.06% (5.00x109) (Fig. 1). Results obtained by Duso et al. (2008) with the predatory mite Phytoseiulus persimilis (Athias-Henriot, 1957) (Acari: Phytosei-idae) have shown high hatching rate of this predator species after application of B. bassiana, although this researchers have reported mortality of up to 43% in females. In the other hand, M. anisopliae and B. bassiana did not affect the survival of nymphs. There was overall survival (100%) of nymphs of 1st and 2nd instars in all tested concentrations of both the fungi B. bassiana and M. anisopliae (data not shown in fgures). Results obtained by Kohno et al. (2007) are in agreement with those obtained in this study. These authors verifed no effect of B. bassiana to nymphs of 2nd instar Labidura riparia (Pallas, 1773) (Dermaptera, Labiduridae). The fungi M. anisopliae and B. bassiana did not affect the survival of adults of E. annulipes. In all tested concentrations of M. anisopliae in female E. annulipes, survival was 100%. The females which received topical application of B. bassiana have been shown a survival rate ranging from 80.3% (12.10x109 conidia) up to 100% (5.00x109 conidia); whereas, for males of E. annulipes, the survival rate in insects treated with B. bassiana ranged from 95% (12.10x109 conidia) to 100% (5.00x109 conidia) and from 96.02% (12.10x109 conidia) to 100% (5.00x109 conidia) after receiving applications of M. anisopliae (Figs. 2a and 2b). Efficient selectivity is based on physiological differences between pests and their natural enemies, being that pest killed at a concentration of product that does not affect individual benefts. A slightly harmful effect (survival> 30% and <70%) has been observed in all the concentrations of conidia B. bassiana in clutches of E. annulipes (Fig. 1). The microbial insecticides tested were not harmful (survival> 70%) for adults (Figs. 2A and 2B) and nymphs of frst and second instar. Therefore, the two mycoinsecticides can be used to control pests without significantly affecting nymphs or adults of E. annulipes. The greater susceptibility of eggs of E. annulipes in relation to nymphs or adults should be the interaction that occurs due to pathophysiologic effects on the cuticle of nymphs or adults of the predator, which hindered the process of infection and lessens the potential for invasion of microorganisms for these stages of E. annulipes. More-over, the ability of this insect to survive in areas of land, soil inhabitant galleries can to be an important factor to provide a greater hardening of their cuticle. Other factors such as intestinal pH, biochemical or immunological defense-physiological may be related to the tolerance of nymphs or adults of E. annulipes concentrations of mycoinseticidas used in this study. According Kiselek (1975), B. bassiana was harmless to adults Cryptolaemus montrouzieri Mulsant, 1853 (Coc-cinellidae: Coleoptera) while the effect was lethal to larvae (50%) that consumed insects infected by this fungus. In this study, nymphs and adults of E. annulipes were not affected by concentrations tested in this study adopted of B. bassiana and M. anisopliae. In other words, the results of this study have revealed that B. bassiana caused a reduction in the rates of hatched nymphs, unlike M. anisopliae. More-over, insecticide applications that are not directly to the soil may enable the adoption of that microorganism in programs of integrated pest management in agroecosystems in which E. annulipes is present, because this kind of predator tends to deposit their eggs in soil galleries built by adults (Bha-radwaj 1966). Other studies have also demonstrated the selectivity of entomopathogenic organisms to natural enemies, like B. bassiana with Orius insidiosus (Say, 1832) (Hemiptera: An-thocoridae) (Cavalcanti 2006); M. anisopliae and B. bassiana with parasitoid Oomyzus sokolowskii (Kurdjumov, 1912) (Hymenoptera: Eulophidae) (Santos Jr. et al. 2006). The con-centrations adopted in this study of entomopathogenic fungi B. bassiana and M. anisopliae did not affect the mortality of nymphs and adults of E. annulipes. Neither we found ovicid-al activity of M. anisopliae to E. annulipes. However, care-ful measures should be adopted in applications from 5.0x109 conidia/L of B. bassiana directly into clutches of E. annuli-pes, because from this concentration we found a significant reduction in hatching rate this predator. We also suggest the implementation of other studies, as the effects these bio-insecticides on the ftness of E. annulipes. Acknowledgements The authors thank the Brazilian agency Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) to concession scholarship to frst author. The authors would like to thank Robério de Oliveira for his help in the laboratory. We especially thank Tricia Hornsby (Ohio State University) for her valuable help with an important review of English in this manuscript. Literature Cited ABBOTT, W. S. 1925. A method of computing the effectiveness of on insecticide. Journal of Economic Entomology 18: 265-267. ALVES, S. B. 1992. Perspectivas para utilização de fungos entomopatogênicos no controle de pragas no Brasil. Pesquisa Agropecuária Brasileira 27: 77-86. [ Links ] [ Links ] ALVES, S. B.; ALMEIDA, J. E. M.; ALMEIDA, A.; MOINO JR., L. F. ; ALVES, A. 1998. Técnicas de laboratório. In: ALVES, S. B. (ed.). Controle microbiano de insetos, 637-711. Piracicaba, Fealq. [ Links ] AMANO, H.; HASEEB, M. 2001. Recently-proposed methods and concepts of testing the effects of pesticides on the beneficial mite and insect species: study limitations and implications in IPM. Applied Entomology and Zoology 36: 1-11. [ Links ] BHARADWAJ, R. K. 1966. Observation on the bionomics of Euboriellia annulipes (Dermaptera: Labiduridae). Annals of the Entomological Society of America 59: 441-450. [ Links ] BUSTILLO, A. E.; BERNAL, M. G.; BENAVIDES, P.; HAVES, B. 1999. Dynamics of Beauveria bassiana and Metarhizium anisopliae infecting Hypothenemus hampei (Coleoptera: Scolytidae) populations emerging from fallen coffee berries. Florida Entomologist 82: 491-498. [ Links ] CAVALCANTI, R. S. 2006. Associação Beauveria bassiana (Bals.) Vuill. - nematóides entomopatogênicos (Rhabditida) - Orius insidiosus (Say) no controle de tripes (Thysanoptera) em cultivo protegido. Tese (Doutorado). Universidade Federal de Lavras, 132p. [ Links ] DUSO, C. V.; MALAGNINI, A.; POZZEBON, M.; CASTAGNOLI, M. ; LIGUORIB, S. S. 2008. Comparative toxicity of botanical and reduced-risk insecticides to mediterranean populations of Tetranychus urticae and Phytoseiulus persimilis (Acari Tetranychidae, Phytoseiidae). Biological Control 47: 16-21. [ Links ] FLEXNER, J. L.; LIGHTHART, B.; CROFT, B. A. 1986. The effects of microbial pesticides on nontarget, beneficial arthropods. Agriculture, Ecosystems & Environment 16: 203-254. [ Links ] FRANÇA, I. W. B.; MARQUES, E. J.; TORRES, J. B.; OLIVEIRA, J. V. 2006. Efeitos de Metarhizium anisopliae (Metsch.) Sorok. e Beauveria bassiana (Bals.) Vuill. sobre o percevejo predador Podisus nigrispinus (Dallas) (Hemiptera: Pentatomidae). Neotropical Entomology 35: 349-356. [ Links ] GENEROSO, A. R. 2002. Compatibilidade de Beauveria bassiana e Paecilomyces fumosoroseus com Chrysoperla externa (Neuroptera: Chrysopidae) e metodologia para avaliação da seletividade. Dissertação (Mestrado) - Universidade Estadual Paulista, Jaboticabal. 63p. [ Links ] GINSBERG, H. S.; LEBRUN, R. A.; HEYER, K.; ZHIOUA, E. 2002. Potential nontarget effects of Metarhizium anisopliae (Deuteromycetes) used for biological control of ticks (Acari: Ixodidae). Environmental Entomology 31: 1191-1196. [ Links ] HESKETH, H.; ROY, H. E.; EILENBERG, J.; PELL, J. K. HAILS, R. S. 2010. Challenges in modeling complexity of fungal entomopathogens in semi-natural populations of insects. Biocontrol 55: 55-73. [ Links ] KISELEK, E. V. 1975. The effect of biopreparations on insect enemies. Zash Rast, 23p. [ Links ] KOHNO, K.; TAKEDA, M.; HAMAMURA, T. 2007. Insecticide susceptibility of a generalist predator Labidura riparia (Dermaptera: Labiduridae) Applied Entomology and Zoology 42: 501–505. [ Links ] LEMOS, W. P.; MEDEIROS, R. S.; RAMALHO, F. S. 1998. Infuência da temperatura no desenvolvimento de Euborellia annulipes (Lucas) (Dermaptera: Anisolabilidae) predador de bicu-do-doalgodoeiro. Anais da Sociedade Entomológica do Brasil 27: 67-76. [ Links ] LEMOS, W. P.; MEDEIROS, R. S.; RAMALHO, F. S. 1999. Exigências térmicas de Euborellia annulipes (Lucas) (Dermaptera, Anisolabididae) e sua relação com a presa: bicudodo-algo-doeiro. Revista Brasileira de Entomologia 43: 61-68. [ Links ] LUDWIG, S. W.; OETTING, R. D. 2001. Susceptibility of natural enemies to infection by Beauveria bassiana and impact of insecticides on Ipheseius degenerans (Acari: Phytoseiidae), Journal of Agricultural and Urban Entomology 18 (3): 169-178. [ Links ] OLIVEIRA, F. Q. 2008. Eficiência dos fungos Beauveria bassiana e Metarhizium anisopliae no controle de moscas das - frutas Ceratitis capitata (Diptera: Tephritidae). BSc em Agronomia. Universidade Federal da Paraíba. 37p. [ Links ] OLIVEIRA, F. Q.; BATISTA, L. J; MALAQUIAS, J. B.; ALMEIDA, D. M; OLIVEIRA, R. 2010a. Determination of the median lethal concentration (LC50) of mycoinsecticides for the control of Ceratitis capitata (Diptera: Tephritidae). Revista Colombiana de Entomología 36 (2): 213-216. [ Links ] ROY, H. E.; HAILS, R. S.; HESKETH, H.; ROY, D. B.; PELL, J. K. 2009. Beyond biological control: non-pest insects and their pathogens in a changing world. Insect Conservation and Biodiversity 2: 65-72. [ Links ] SANTOS JR., R.; MARQUES, H. J. G.; BARROS, E. J. R.; GONDIM JR., M. G. C.; ZAGO, H. B.; SILVA, C. M. 2006. Efeito de Metarhizium anisopliae (Metsch.) Sorok. e Beauveria bassiana (Bals.) Vuill. sobre adultos de Oomyzus sokolowskii (Kurdjumov) (Hymenoptera: Eulophidae). Acta Scientiarum. Agronomy 28: 241-245. [ Links ] SAS. 2006. SAS Users Guide: Statistics. SAS Institute, Cary, NC, USA. [ Links ] TOMKINS, J. L.; SIMMONS, L.W. 1998. Female choice and manipulations of forceps size and symmetry in the earwig Forficula auricularia L. Animal Behavior 56: 347-356. [ Links ]