Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488On-line version ISSN 2665-4385
Rev. Colomb. Entomol. vol.37 no.2 Bogotá July/Dec. 2011
Within tree distribution of a discoid gall on Caryocar brasiliense (Caryocaraceae)
Distribución de una agalla discoide en el dosel de Caryocar brasiliense (Caryocaraceae)
GERMANO LEÃO DEMOLIN LEITE1, ALINE FONSECA DO NASCIMENTO2, FABIENE MARIA DE JESUS1, SÉRGIO MONTEZE ALVES1, and GERALDO WILSON FERNANDES2
1 Insetário G.W.G. de Moraes, ICA/UFMG, CP: 135. Montes Claros, MG, Brasil. Endereço eletrônico: gldleite@ig.com.br. Autor para correspondencia. D.Sc, M.Sc., D.Sc., and M.Sc., respectively. 2 Ecologia Evolutiva & Biodiversidade/DBG/UFMG, Belo Horizonte, MG, Brasil. PhD.
Received: 23-dec-2010 - Accepted: 19-jul-2011
Resumen: Se evaluó la distribución de agallas discoide en el dosel de Caryocar brasiliense en dos áreas de pastos y cerrado. El mayor número de agallas discoide se observo en el cerrado. Más agallas discoide se encuentran en la cara noreste, mientras que menor número se observaron en el follaje hacia el sur en la sabana. Sin embargo, en un potrero, el menor número de agallas discoide se observo en la cara este. Más agallas discoide se encuentran en las hojas de interior de la copa (hojas uno y dos) que en el borde (hojas tres y cuatro), especialmente en la posición de la hoja tres en el cerrado. Hojas de posición tres y cuatro reportan 83,6% de todas las agallas en la muestra. Este insecto cambia su comportamiento en potreros, atacando más en la segunda hoja y en este caso, las hojas de posición tres y cuatro llevaban el 32,8% de todas las agallas en la muestra. En sabana y pastizales se encontraron más agallas discoide en la línea media en comparación con la porción distal y proximal al eje longitudinal de las láminas en una. La agalla discoide prefere colonizar la parte central de la hoja o cerca de la vena principal de las hojas en toda la región en una pradera. En el cerrado, este insecto sigue la misma tendencia, pero quizás debido a una mayor población, se extendió sobre la hoja.
Palabras clave: Sabana. Insectos agalla. Pequi. Hymenoptera. Competencia.
Abstract. We recorded the discoid gall spatial distribution within Caryocar brasiliense trees in two areas of pasture and one of savanna. A higher number of discoid gall/leafet was observed in savanna than in the pastures. More discoid galls were found on the eastern slope trees followed by the northern slope trees while fewer galls were found on the foliage in the southern slope trees in savanna. However, in the pasture one, the lower discoid galls were observed on the eastern slope. More discoid galls were found on the leaves in the interior (leaves one and two) of the tree crown than at the border (leaves three and four), principally in the leaf position three in savanna. Leaf positions three and four supported 83.6% of all galls sampled. On the other hand, this insect changed its behavior in pasture one, attacking more the second leaf and, in this case, leaf positions three and four supported 32.8% of all galls sampled. More discoid galls were found on the median region compared to the distal and proximal longitudinal regions on leafet in savanna and in pasture one. The discoid gall insect preferred colonized the central portion than the border portion or near mid vein on transversal regions on a leafet in the pasture one. In the savanna, followed the same trend, but perhaps due to greater population, the discoid galls have spread more evenly by the leaf of the tree.
Key words: Cerrado. Insect galls. Pequi. Hymenoptera. Competition.
Introduction
Insect galls are known to distribute differentially within host organs. Many galling larva achieve higher density, size, and even higher ftness at the proximal portion compared to more distal portion of the leaf due to be the more rapid and efficient interception of plant photosynthates at the leaf base (Auslander et al. 2003; Leite et al. 2009). In a similar way, galls are also more abundant and larger at the basal portion of the stems of its host plant perhaps due to stronger sinks in this region of the plant (De Souza et al. 2001). On the other hand, more galls of Eurytoma sp. (Hymenoptera: Eurytomidae) were found on the median region of the leaf compared to the distal and proximal areas of Caryocar brasiliense Cambess. (Caryocaraceae) leaves maybe due to the lower density of trichomes in this portion (Leite et al. 2009). Leaf trichomes are known to have a strong infuence on the behavior, selection, and ftness of insect herbivores (Agrawal 2004; Leite et al. 2009).
Galling larva achieve higher density at leaf margin compared to more central portion of the leaf, like observed for Eurytoma sp. galls in leaves of C. brasiliense (Leite et al. 2009), although the density of trichomes are higher on the leaf margin than on the other transversal leaf portions (Leite et al. 2009), extra foral nectaries were also mostly frequent on the leaf margins (Rezende 1998). The role of extra foral nectaries on the host plant interaction with galling insects is not well known (Lach and Hoffmann 2011). Otherwise, Leite et al. (2009) postulated that they might infuence the Eurytoma sp. female preference as they may have an indirect positive role in protecting the galling insects against natural enemies by attracting ants. Ants were reported to be associated with extra foral nectaries of Caryocar (Oliveira 1997) and in the studied plants they were commonly seen associated with the leaf extra foral nectaries.
The position of the leaves in the stems can also affect the colonization of gall insects. More Eurytoma sp. galls were found on the leaves of C. brasiliense in the interior of the tree crown than at the border, i.e. leaf positions three and four supported 66.7% of all galls sampled (Leite et al. 2009). Several mechanisms may infuence the trend found. First, differential mortality caused by parasitoids, predators, and even differential plant resistance could diminish the galling success on the more proximal region of the stems (Leite et al. 2009). Even if females prefer leaves at the border, stronger selective pressures would impair their success at such habitat (Leite et al. 2009). An alternative hypothesis is that the most distal leaves (leaves one and two) were too young to be found by the galling insects while leaves three and four were exposed to galling for a longer period (Leite et al. 2009). The last hypotheses can explain this trend is the microclimatic differences between the interior of the tree crown and the border.
Insect galls may also distribute differentially between habitats as well as within the host crown. Plants in sunny habitats are known to support higher density of galls compared to plants in shaded habitats (Fernandes and Price 1992; Auslander et al. 2003; Leite et al. 2009) due to sun exposure may infuence the quality of the host plant tissue as well as the gall tissue or even the plant ability to find and elicit induced responses to the invading organism (Fernandes et al. 2000, 2005). Furthermore, natural enemies could also be strong selective forces against galling on more favorable, shaded habitats (Fernandes and Price 1992). Therefore, differential selection could lead to female preference and larval ftness for foliage in sunny habitats. More Eurytoma sp. galls were found on the eastern slope tree compared to the other slopes tree while fewer galls were found on the foliage in the northern slope of C. brasiliense tree maybe due to the dispersion by winds (Leite et al. 2009). These authors postulated that the foliage on the eastern slope of the host trees are the most exposed to higher winds and strong sunlight in the South Hemisphere.
Caryocar brasiliense Camb. (Caryocaraceae) is one of the most common and important plant species in the dry and harsh semi arid vegetation of Brazil, called cerrado (Leite et al. 2006). It is host to a large number of invertebrate and vertebrate herbivores (Oliveira 1997; Leite et al. 2011) as the gall insects (Oliveira 1997; Leite et al. 2009), showing four species of leaf galls in the cerrado of northern Minas Gerais State, Brazil.
In an effort to initiate ecological studies in this system, we obtained the frst data on spatial distribution of a discoid galling insect (Hymenoptera) (Fig. 1) at the tree level aimed at sampling because this plant has been domesticated for future commercial plantations. This gall is discoid with 3.70 mm2 of diameter and 0.57 mm of height, without hairs, its color is light green, and located between the leaf veins (Fig. 1). As C. brasiliense has three leafet compound leaves we frstly asked whether gall abundance would differ among the leafets on a single leaf. Second, we studied the effect of foliage slope orientation on gall abundance. A third analysis addressed the distribution of the galls on the leaves along the host stem (within tree). Fourthly, because the grouping of galls on a given leaf portion may indicate site preference, we analyze the within leaf (longitudinal) distribution of galls, and addressed the transversal zonation of discoid galls within leaves. Finally we studied the differences among areas of pasture and savanna.
Material and Methods
This work was developed in one savanna and two pastures (prior savanna) of the Municipality of Montes Claros, Minas Gerais State, Brazil in April, June, August and October 2006, where the average temperatures were 22.5, 19.0, 22.3 and 23.5°C; air relative humidity 80.0, 68.0, 54.0 and 78.0%, month round accumulated precipitation of 211.5, 0.3, 3.8 and 279.0mm; winds prevailed from the northeast at an average speed of 1.60, 1.70, 2.10 and 2.0m/s; insolation 7.21, 8.3, 9.4 and 4.7h, respectively, obtained from the main climate station of Montes Claros of the Estação Nacional de Metereologia (see Leite et al. 2006). The north of Minas Gerais State has a climate Aw: tropical of savanna, according to the classification of Köppen, with dry winter and rainy summer (Vianello & Alves 2000). The areas studied were: savannah vegetation stricto sensu, pasture 1 (in activity) and pasture 2 (abandoned pasture = savanna in recovery). These areas show different characteristics of soil and foristic diversity. The geographical coordinates, altitude, soil type, physiochemical characteristics of the soil, foristic density, height and width of the crown as well as diameter at breast height of C. brasiliense were published in Leite et al. (2011).
As the discoid gall density on C. brasiliense leaves is highly variable among trees - (data not published), we chose 10 trees per area that had the discoid gall infestation. To evaluate the distribution of discoid galls within the tree crown we recorded the number of galls on the compound leaves of C. brasiliense of four randomly selected stems positioned on north, south, east, and west slope trees of ten adult trees per area. To evaluate the distribution of discoid galls among the leafets we recorded: i) gall abundance according to foliage orientation (slope); ii) the differential abundance of galls on the right, central and left leafets ( Leite et al. 2009); iii) gall abundance on the distal, median, and proximal region of a leafet (see Leite et al. 2009); iv) gall abundance on the leafet border, central area, and adjacent to the mid leaf vein of the leafet ( Leite et al. 2009), and v) difference among areas. 1.269 leafets of 423 leaves in savanna, 3.030 leafets of 1010 leaves in pasture 1 and 2.613 leafets of 432 leaves in pasture 2 were censured for discoid galls.
All data were transformed to √x + 0.5 and were analyzed by analysis of variance and followed by the Tukey test at the significance level P< 0.05.
Results and Discussion
The higher number of discoid galls was observed in savanna (10.86±1.17 A - Tukey test), followed by pasture one (3.17 ± 0.31 B) and pasture two (0.23±0.08 C) (F=76.955; df=1563; P<<0.001). The pasture one (in activity) has less foristic diversity (< trees, groves, shrubs and herbs) and more soil covering by grass than pasture two - savanna in recovery - and savanna, without differences between last two areas (Leite et al. 2011). Savanna has less C. brasiliense per hectare than the pastures and these trees are higher in pasture one than other areas (Leite et al. 2011). In addition, the soils in the pasture one and savanna are more acid, pasture one is richer in aluminum and sum of bases and less sandy than the other areas (Leite et al. 2011). Perhaps these variables can explain the difference in density of the discoid gall among savanna and pastures studied (Fernandes and Price 1988, 1992; Gonçalves-Alvim and Fernandes 2001; Fernades et al. 2005). With the increase in the foristic diversity increases the diversity of leaf galls species (total four species) on C. brasiliense as well as increased the density of other galls while decreases that of Eurytoma sp. (Hymenoptera: Eurytomidae), the most abundant species (unpublished data). Moreover, in the pasture one had more galls of Eurytoma sp. (~3/leafet) than the savanna (~0.8/leafet) (unpublished data) and pasture two ~0.3/leafet, (Leite et al. 2009). Maybe this fact is due to a competition between discoid gall and Eurytoma sp. explaining why we observed more discoid gall in savanna than in the pastures.
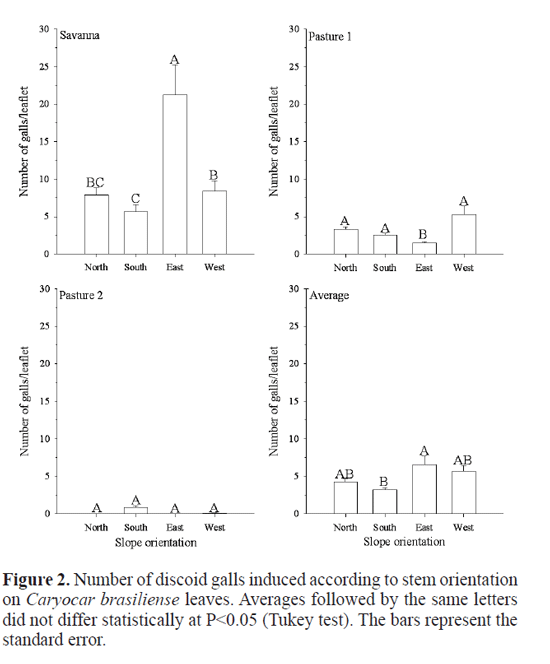
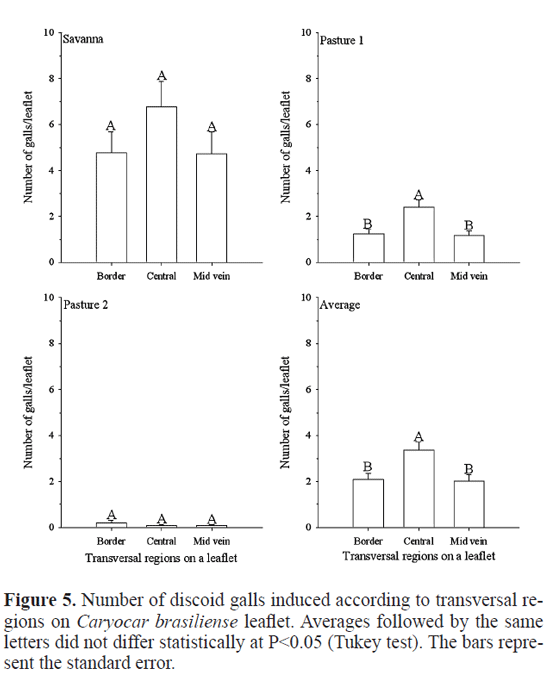
We did not find significant differences in the distribution of discoid galls in pasture two (P>0.05) according to slope orientation of the tree crown, leaf position in the branches, longitudinal and transversal regions on a leafet on C. brasil-iense (Figs. 2-6) probably due to the low density of this gall in this area. On the other hand, more discoid galls were found on the eastern slope tree followed the northern slope tree while fewer galls were found on the foliage in the southern slope tree in savanna (F=8.477; df=408; P<<0.001), and in the pasture one, the lower discoid galls were observed on the eastern slope tree (F=8.076; df=1004; P<< 0.001) (Fig. 2). Leite et al. (2009) observed more Eurytoma sp. galls on the eastern slope tree compared to the other slopes tree while fewer galls were found on the foliage in the northern slope of C. brasiliense tree maybe due to the dispersion by winds. Leite et al. (2009) postulated that the foliage on the eastern slope of the host trees are the most exposed to higher winds and strong sunlight, since prevalent winds in the region are northeaster/eastern (Leite et al. 2006), corroborating previous findings in which galling insects are most abundant on sunlight-exposed foliage (Fernandes and Price 1988). Why did the discoid gall show different behaviors in the savanna and pasture one? Perhaps the answer is to avoid competition with the major galling insect on C. brasiliense leaves, the Eurytoma sp.
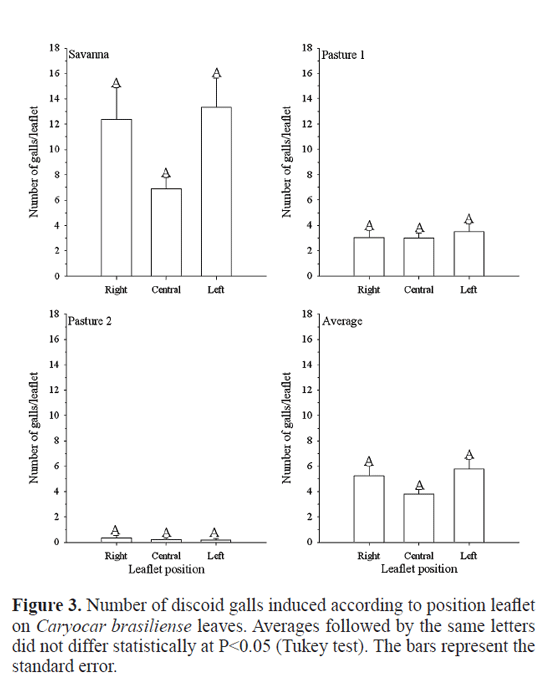
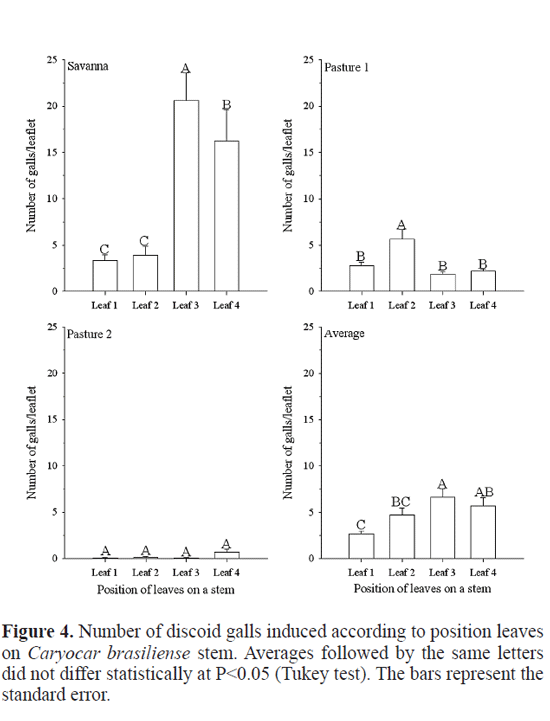
The average number of discoid galls did not differ statistically among the three leafets of C. brasiliense in the three areas studied (P>0.05) (Fig. 3). The same result was obtained by Leite et al. (2009) with Eurytoma sp.. More discoid galls were found (F=45.508; df=408; P<<0.001) on the leaves in the interior of the tree crown than at the border, principally in the leaf position three in savanna. Leaf positions three and four supported 83.6% of all galls sampled (Fig. 4). On the other hand, this insect changed its behavior in the pasture one, attacking more (F=7.679; df=1004; P=<< 0.001) the second leaf and, in this case, leaf positions three and four supported 32.8% of all galls sampled (Fig. 4), maybe avoiding or reducing the competition with Eurytoma sp., since this last gall insect were found on the leaves of C. brasiliense in the interior (leaf three and leaf four) of the tree crown than at the border (Leite et al. 2009), being in higher population in pasture one than in savanna (unpublished data). Several mechanisms may infuence the trend found in the case of Eurytoma sp.. First, differential mortality caused by parasitoids, predators, and even differential plant resistance could diminish the galling success on the more proximal region of the stems (Leite et al. 2009). Even if females prefer leaves at the border, stronger selective pressures would impair their success at such habitat (Leite et al. 2009). An alternative hypothesis is that the most distal leaves (leaves one and two) were too young to be found by the galling insects while leaves three and four were exposed to galling for a longer period (Leite et al. 2009). The last hypotheses can explain this trend is the microclimatic differences between the interior of the tree crown and the border.
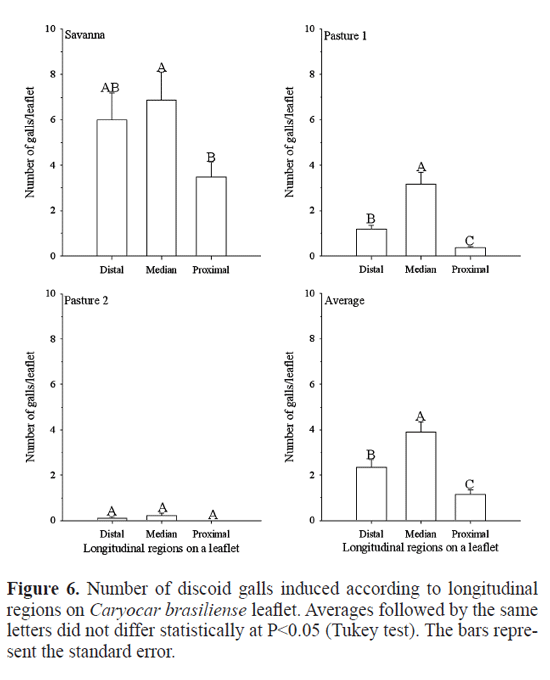
More discoid galls were found on the median region compared to the distal and proximal longitudinal regions on leaflet in savanna (F=5.155; df=414; P<<0.001) and in pasture one (F=58.831; df=1006; P<<0.001) (Fig. 5). Similar results were observed for galls of Eurytoma sp. maybe due to the lower density of trichomes in this portion (Leite et al. 2009). Leaf trichomes are known to have a strong infuence on the behavior, selection, and ftness of insect herbivores (Agrawal 2004). Many galling larva achieve higher density, size, and even higher ftness at the proximal portion compared to more d
istal portion of the leaf or stems because in these areas are more rapid and efficient interceptation of plant photosyn-thates, being stronger sinks (De Souza et al. 2001; Auslander et al. 2003).
The discoid gall insect preferred colonized (F=31.855; df=1005; P<<0.001) the central portion than the border portion or near mid vein on transversal regions on a leafet in the pasture one (Fig. 6). In the savanna, followed the same trend (F=3.498; df=409; P=0.03), but perhaps due to greater population, the discoid galls have spread more evenly by the leaf of the tree (Fig. 6). Leite et al. (2009) postulated that extra foral nectaries, mostly frequent on the leaf margins (Rezende 1998), might infuence the Eurytoma sp. female preference as they may have an indirect positive role in protecting the galling insects against natural enemies by attracting ants. Ants were reported to be associated with the leaf extra fora nectaries of C. brasiliense (Oliveira 1997) and in the studied plants they were commonly seen associated with the leaf extra foral nectaries (Lach and Hoffmann 2011). The preference of the discoid gall insect attacks the central portion of leafet than the border portion, where has extra foral nectaries and ants (less natural enemies) maybe due to the higher number of Eurytoma sp. galls, principally in pasture one (unpublished data), in the border portion of leafets (Leite et al. 2009), avoiding or reducing the competition with Eurytoma sp.
Conclusions
Some mechanisms may infuence the ftness of discoid gall in C. brasiliense trees. Discoid galls are most abundant in the foliages most exposed to higher winds and strong sunlight (stressed micro area). This galling insect prefers to attack the leaves in the interior of the tree crown than at the border maybe due to natural enemies, differential plant resistance, time of colonization, and microclimatic differences between these parts of the tree crown. Trichomes and competition with others galling insects can effect the colonization of discoid gall because it occurs more on the median region of leafet where less density of trichomes and Eurytoma sp. has.
Cited literature
AGRAWAL, A. A. 2004. Resistance and susceptibility of milkweed: competition, root herbivory, and plant genetic variation. Ecology 85 (8): 2118-2133. [ Links ]
AUSLANDER, M.; NEVO, E.; INBAR, M. 2003. The effects of slope orientation on plant growth, developmental instability and susceptibility to herbivores. Journal of Arid Environments 55 (3): 405-416. [ Links ]
DE SOUZA, A. L. T.; TANAKA, M. O.; FERNANDES, G. W.; FIGUEIRA, J. E. C. 2001. Host plant response and phenotypic plasticity of a galling weevil (Collabismus clitellae: Curculionidae). Austral Ecology 26 (2): 173-178. [ Links ]
FERNANDES, G. W.; PRICE, P. W. 1988. Biogeographical gradients in galling species richness: tests of hypotheses. Oecologia 76 (2): 161-167. [ Links ]
FERNANDES,G. W.; PRICE, P. W. 1992. The adaptive signifcance of insect gall distribution: survivorship of species in xeric and mesic habitats. Oecologia 90 (1): 14-20. [ Links ]
FERNANDES, G. W.; CORNELISSEN, T. G.; ISAIAS, R. M. S.; LARA, A. F. 2000. Plants fight gall formation: hypersensitivity. Ciência e Cultura 52 (1): 49-54. [ Links ]
FERNANDES, G. W.; GONÇALVES-ALVIM, S. J.; CARNEIRO, M. A. A. 2005. Habitat-driven effects on the diversity of gallinducing insects in the Brazilian Cerrado. In: Raman, A.; Schaefer, C.W.; Withers, T. M. (eds) Biology, Ecology, and Evolution of Gallinducing Arthropods. Science Publishers Inc., USA, vol. 2, p. 693-708. [ Links ]
GONÇALVES-ALVIM, S. J.; FERNANDES, G. W. 2001. Biodiversity of galling insects: historical, community and habitat effects in four neotropical savannas. Biodiversity and Conservation 10 (1): 79-98. [ Links ]
LACH, L.; HOFFMANN, B. D. 2011. Are invasive ants better plant-defense mutualists? A comparison of foliage patrolling and herbivory in sites with invasive yellow crazy ants and native weaver ants. Oikos 120 (1): 9-16. [ Links ]
LEITE, G. L. D.; VELOSO, R. V. S; ZANUNCIO, J. C.; FERNANDES, L. A.; ALMEIDA, C. I. M. 2006. Phenology of Caryocar brasiliense in the Brazilian Cerrado Region. Forest Ecology and Management 236 (2-3): 286-294. [ Links ]
LEITE, G. L. D.; VELOSO, R. V. S.; SILVA, F. W. S.; GUANABENS, R. E. M.; FERNANDES, G. W. 2009. Within tree distribution of a gallinducing Eurytoma (Hymenoptera, Eurytomidae) on Caryocar brasiliense (Caryocaraceae). Revista Brasileira de Entomologia 53 (4): 643-648. [ Links ]
LEITE, G. L. D.; VELOSO, R. V. S.; ZANUNCIO, J. C.; ALVES, S. M.; AMORIM, C. A. D.; SOUZA, O. F. F. 2011. Factors affecting Constrictotermes cyphergaster (Isoptera: Termitidae) nesting on Caryocar brasiliense trees in the Brazilian savanna. Sociobiology 57 (1): 1-16. [ Links ]
OLIVEIRA, P. S. 1997. The ecological function of extraforal nectaries: herbivore deterrence by visiting ants and reproductive output in Caryocar brasiliense (Caryocaraceae). Functional Ecology 11 (3): 323-330. [ Links ]
REZENDE, M. H. 1998. Anatomia dos órgãos vegetativos, da for e estruturas secretoras de Caryocar brasiliense Camb. (Caryo-caraceae). São Paulo, 1998. 91p. DSc Dissertation. São Paulo, Universidade de São Paulo. VIANELLO, R. L.; ALVES, A. R. 2000. Meteorologia básica e aplicações. UFV, Viçosa. 467p. [ Links ] [ Links ]