Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Colombiana de Entomología
versión impresa ISSN 0120-0488versión On-line ISSN 2665-4385
Rev. Colomb. Entomol. v.37 n.2 Bogotá jul./dic. 2011
Seasonal size distribution of Anacroneuria (Plecoptera: Perlidae) in an andean tropical river
Distribución estacional del tamaño de Anacroneuria (Plecoptera: Perlidae) en un río tropical andino
HUgo BoHórqUeZ1, gLADyS reINoSo2, and gIovANy gUevArA3*
1 Biólogo. grupo de Investigación en Zoología, Facultad de Ciencias, Universidad del Tolima, Ibagué-Tolima-Colombia. boris3100@yahoo.es.
2 M.Sc. grupo de Investigación en Zoología, Facultad de Ciencias, Universidad del Tolima, Ibagué-Tolima-Colombia. greinoso@ut.edu.co.
3* Ph.D. Grupo de Investigación en Zoología, Facultad de Ciencias, Universidad del Tolima, Ibagué-Colombia. Facultad de Ciencias, Universidad Austral de Chile, Campus Isla-Teja, valdivia-Chile. Present address: Departamento de Desarrollo rural y recursos Naturales, Facultad de Ciencias Agropecuarias, Universidad de Caldas, Manizales-Colombia. ggcolombia@gmail.com. Corresponding author.
Recibido: 28-feb-2011 - Aceptado: 25-oct-2011Abstract: Total body length and head capsule width (mm) were used as biometric characteristics in discriminating between size-frequency estimates of three Anacroneuria morphospecies, from 28 sampling sites along a tropical river subject to bimodal variations in water level in Central Colombia. A total of 344 specimens grouped into four size classes were collected. The highest abundance of Anacroneuria larvae was observed during the dry season and there was a higher abundance of small-sized individuals (body length <12mm). overall, the highest abundance of stonefy larvae was recorded between 1000 to 1600m.a.s.l. Conductivity and pH showed a significant correlation with the total abundance of preimaginal stonefies. Plecoptera size distribution patterns could be useful in assessing which stonefy size-spectra are susceptible to disturbance in tropical rivers and in evaluating their population dynamics.
Key words: Neotropics. Andes. Biometry. Body Size. Tropics.
Resumen: Se utilizaron el largo total y el ancho de la capsula cefálica como rasgos biométricos para estimar la frecuencia de tamaño de tres morfo-especies de Anacroneuria (Insecta: Plecoptera) registrados en 28 estaciones de muestreo ubicadas longitudinalmente en un río tropical del centro de Colombia sometido a un régimen de precipitación bimodal. Se coleccionaron 344 especímenes que fueron agrupados en cuatro clases de tamaño. La mayor abundancia de larvas se registró durante la estación seca con una mayor presencia de individuos pequeños (tamaño corporal <12mm). Considerando ambas épocas hidrológicas, la mayor abundancia de plecópteros ocurrió entre 1000 y 1600m.s.n.m. La conductividad y el pH del agua mostraron una correlación significativa con la abundancia de plecópteros pre-emergentes. Los patrones de distribución de tamaño de larvas de Plecoptera constituyen una herramienta valiosa para establecer la vulnerabilidad de los estadios larvales frente al efecto de la perturbación en ríos tropicales y para evaluar su dinámica poblacional.
Palabras clave: Neotrópico. Andes. Biometría. Tamaño Corporal. Trópico.
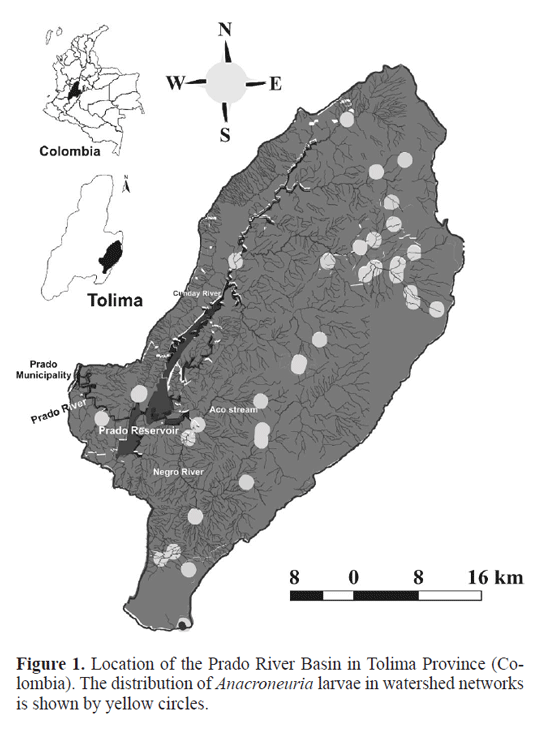
Introduction The order Plecoptera, is a group of hemimetabolous insects with more than 3497 species described worldwide, of which 474 species are Neotropical (Fochetti and Tierno de Figueroa 2008), but recently 32 out of 508 valid names were considered questionable by Froehlich (2010). This order is an important component in lotic systems, both in terms of abundance and in terms of ecological significance (Zwick 2004; McLellan and Zwick 2007; Stark et al. 2009). In comparison to their temperate counterparts, tropical stonefilies are poorly understood (Sheldon and Theischinger 2009), and regional and local species lists are extremely incomplete. One reason for the paucity of systematics and ecological studies of tropical river Plecoptera is that identification of tropical species is difficult for nonspecialists (Suhaila and Che Salmah 2011). In Colombia, the order Plecoptera is represented by two families: Perlidae with three genera (Anacroneuria, Macrogynoplax and Klapalekia; TamarisTurizo et al. 2007; Zúñiga et al. 2007) and, Gripopterygidae with one genus (Claudioperla, Barreto et al. 2005; Zúñiga et al. 2009). Anacroneuria Klapálek, 1909 (Plecoptera: Perlidae) is the largest Neotropical genus with more than 300 species (Froehlich 2003; Bispo et al. 2005; Tomanova and Tedesco 2007). Its distribution extends from the southern United States to northern Argentina (Stark 2001; Froehlich 2004). Sixty-one species are reported from Colombia alone (Zúñiga et al. 2007). Nevertheless, there is no published information on stonefilies from the Tolima province, Central Colombia, except for the first record of the Gripopterygidae family in the Páramo ecosystem (Barreto et al. 2005), a special biogeographic zone at the highest altitudes of the Andean region in northern South America (Hofistede 1995). Despite their importance as water quality indicators, stonefilies have received little attention in Colombia and the peer-reviewed literature is scarce (Stark et al. 2009). The life histories and trophic interactions of stonefily larvae are not well-known even though they play an important ecological role in the freshwater ecosystems (Bohórquez et al. 2006; Tamaris-Turizo et al. 2007; Gamboa et al. 2009). Biometric studies are important because several biological, physiological and ecological properties can be extracted from specific body dimensions (body length or head width; Beer-Stiller and Zwick 1995; Krasnov et al. 1996; Klingenberg and Spence 1997; Zwick 2003). The morphological characteristics of aquatic insect larvae can be infiluenced by biotic and abiotic factors (Crosa and Buffagni 2002; Tomanova and Tedesco 2007). Altitude, temperature, seasonality, competition and predation are key factors regulating insect abundances in aquatic environments (Brittain 1983; Saltveit et al. 1994; Céréghino and Lavandier 1998; Céréghino et al. 2002). Worldwide, biometric research on plecopterans has mainly involved obtaining linear measurements to determine growth in immature stages (larvae) for life-cycle and secondary production analyses (e.g., Sheldon 1969, 1980; Schmidt and Tarter 1985; Lillehamnur et al. 1989; Townsend and Pritchard 1998; Fenoglio et al. 2007; Principe 2008). Simi-larly, studies of morphometric differences (except taxonomic descriptions) in larvae and adults of the Anacroneuria genus and their relationships to environmental factors have been highlighted for some tropical rivers (Tomanova and Tedesco 2007; Fenoglio et al. 2007; Cressa et al. 2008; gamboa and Arrivillaga 2010). In tropical rivers, precipitation and discharge play an important role in structuring the benthic community (Silveira et al. 2006; Wantzen et al. 2006). These rivers and their aquatic communities are conditioned by rainfall during the rainy season (Turcotte and Harper 1982; filecker and Feifarek 1994; Jacobsen and encalada 1998; Buss et al. 2004; Jacobsen 2004; Bispo et al. 2006). rivers in the Andean region of Colombia are also highly infuenced by seasonal rainfall, normally occurring during two wet seasons, with sporadic spates even during the dry season (restrepo and Kjerfve 2000; Arias et al. 2007). The present study was carried out in the Prado River Basin, which is a valuable tributary of the Magdalena River, the most important river system in Colombia (galvis and Mojica 2007). The objectives of this study were to measure the variation in body size of Anacroneuria larvae across sampling sites of varying in altitude during two contrasting seasons (dry and wet); to identify morphological differences caused by these temporal and spatial parameters; and to determine whether this life-history characteristic varies with elevation and season.
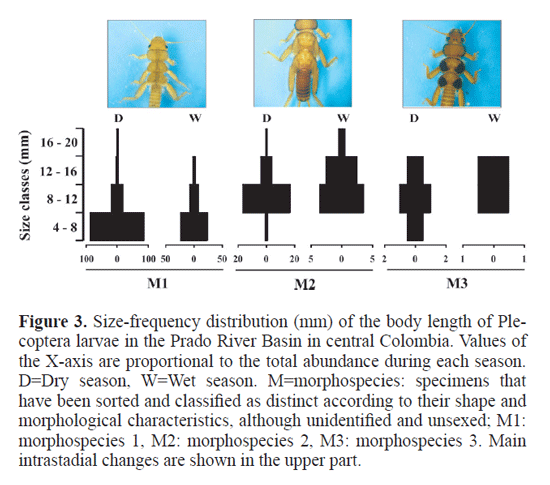
Materials and Methods Study Area. The Prado river Basin is located in central Colombia, in the Department of Tolima (3°45N - 74°56W; Fig. 1), in the upper Magdalena valley, between the Central range and the eastern range of the Colombian Andes. The basin covers 170.000ha, with an annual mean temperature of 23.4°C and annual mean precipitation of 1896mm associated with two rainy seasons, one from March to May and the second between october and December (see Canosa and Pinilla 2001; Bohórquez et al. 2006; guevara et al. 2009). Selected sampling sites ranged from 280 to 2260m.a.s.l. Sampling of stonefy larvae. Sampling of Anacroneuria larvae was carried out over ten consecutive days at 28 sampling sites, during two contrasting seasons, August-September (dry season) and November 2005 (wet season). Stonefy larvae were collected using a hand-net (250µm mesh) and a Surber net (30 x 30cm, 250µm mesh) and then stored in 75% ethanol. All samples were pooled for each sampling site. In the laboratory, body length (BL; from the front of the head to the tip of the abdomen), head capsule width (HCW; across the compound eyes), and mesothoracic wing pad length (WL; from the tip of the wing pad to the paramedial contact point with the mesonotum) of each specimen (sensu Nesterovitch and Zwick 2003) were measured to the nearest 0.01mm using a stereomicroscope (Olympus, 10X). We sorted the collected larvae into three different Anacroneuria morphospecies (M1, M2, M3; see Bohórquez et al. 2006) related to variations in body size and pigmentation, among other morphological traits. Intrastadial sizes characterized by differences in the shape of the margins, the orientation and the pigmented pattern of hind wingpads, i.e., rounded, pronounced and darkish, were traced by assigning larvae to one of four arbitrary size classes (obtained from frequency histograms), given that estimation of larval instars is difficult for stonefies because of their molting times (otsuki and Iwakuma 2008). Therefore, body length measurements for each morphospecies were used to construct size-frequency distributions pooled into four size classes (mm). Selected physical, chemical and microbiological parameters were recorded at each sampling site and later analyzed following methodologies proposed by APHA-AWWA-WeF (Clesceri et al. 1999).
The relationships between total larval abundance and elevation or physicochemical variables were analyzed by means of Spearman rank Correlation test, due to lack of normality. We used linear regressions to test the relationships be-tween the body length and head capsule width measurements. Since morphospecies 3 (M3) abundance was low during each sampling period (4 during the dry season, 2 during the wet season), we combined the data from both seasons when fitting linear regressions. All analyses were performed using STATISTICA version 7 (StatSoft 2004) considering a significance of 5% (α=0.05).
Results
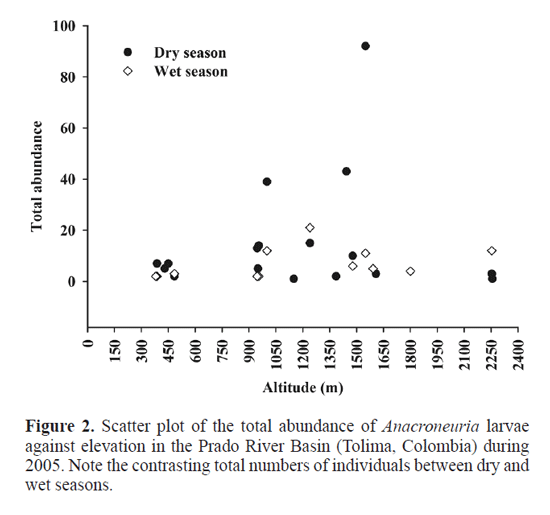
Abundance and physicochemical variables. During the dry season, there were three-fold more plecopteran larvae than during the rainy season. The total abundance was significantly correlated with elevation during the wet season («=12; r=0.66, P=0.018), but not during the dry season («=17; r=0.08, P>0.05). The highest larval abundance was regis-tered between 1000 to 1600m.a.s.l. (Fig. 2). The physicochemical variables, water temperature, air temperature, pH, conductivity, total hardness, and total and fecal coliforms, were highest during the dry season. On the contrary, conductivity, dissolved oxygen and 02 saturation percentage displayed higher valúes during high water level conditions (Table 1). The pH and conductivity variables showed a significant correlation with total abundance during the dry season («=17; r=-0.58, P=0.014; r=-0.57, P=0.015, respectively), while only conductivity was marginally significant during the wet season («=12; r=-0.57, P=0.047). Measurements of body size. We measured 344 Anacroneuria larvae, of which 262 were collected during the dry season and 82 during the wet season. Larvae varied in size, representing a wide range of growth phases (Fig. 3). Body size ranged from 4 to 20mm. In all cases, larval stages were more abundant during the dry season, with a great abundance of the smallest larvae, i.e., <12mm in body length (Fig. 3). However, we did not find a significant relationship between total body length and altitude, or any physical and chemical parameter. The smallest head capsule width was 1.08mm, and the máximum was 3.95mm. We found a relationship between total body length and head capsule width in every morphospecies and according to season. This relationship was strongly isometric (Fig. 4) and linear, with the following equations being highly significant for all morphospecies: MI (dry and wet season; r2=0.98, PO.001 and ^=0.91, PO.001); M2 (^=0.81, PO.001 and r2=0.69, PO.001); M3 (^=0.80, PO.016). Larvae of all morphospecies exhibited continuous variation of both HCW and WL and could not be grouped precisely into instars based on either one of these variables.
Discussion
Larvae occurrence. Our results suggest that the greatest abundances of larger larvae from M2 and M3 of Anacroneuria spp. during the dry season corresponded to individuals near emergence (pre-emergent), although it has been suggested that some Colombian Anacroneuria emerge throughout year, thereby displaying possible non-seasonality (Tamaris-Turizo et al. 2007). In other regions of the country emergence peaks are related to the transition between dry and rainy seasons (Zúñiga et al. 2003). Also, some mature Anacroneuria larvae (last instar) reaching the adult stage have been found to be of smaller size (M. del C. Zúñiga, pers. com.). various insects, hemi as well as holometabolous, emerge at successively smaller sizes throughout the emergence period (Nesterovitch and Zwick 2003). Although a frequency distribution of measurements was constructed to visualize the number of larval instars, the limits of each instar were defined by lower frequencies ftted to one of four size classes. We associated the instars of each morphospecies with the development, shape and coloration of wing pads (e.g., roessler and Zamora 1997). Our results indicate the existence of at least four larval instars for these morphospecies. However, we strongly recommend carrying out thorough periodical sampling over a year and ftting the WL : HCW ratio against the HCW, as suggested by Beer-Stiller and Zwick (1995) to determine voltinism and identify real instars.
Zwick (2003) argued that in Plecoptera, the frst small rudiments of wingpads appear in the antepenultimate instar, and the definite shape is reached during subsequent molts. We sorted morphs according to biometric characters (mainly wingpads) and color similarities, and identifed and grouped them according to stonefy congeneric specie, which unquestionably under-represents the actual species richness for the river and the region. For instance, we report the presence of at least three species which are probably correlated to the three morphs, but a minimum of 58 species have been described in Colombia based on adult collections (Zúñiga et al. 2007). Since most ecological and biological studies ideally involve aquatic insects in the adult stage (mainly males), it is neces sary to rear larvae in order to associate them with the adults (Hamada and Couceiro 2003). Therefore, more detailed studies are required in the region, beginning with adult collections, species identification and adultlarvae associations (rearing). These aspects are important for future life-cycle studies, nutrient fuxes, biodiversity and secondary production analyses in tropical rivers where the limnology remain understudied (Allan et al. 2006).
Alternation between dry and wet seasons. The entire Prado river Basin and network of streams experience two pronounced dry seasons (from January through March, and July through September) alternating with two rainy seasons from April to June and october to December, exhibiting notable seasonal precipitation, fow and discharge (Salazar et al. 2002; guevara et al. 2009). During this bimodal water level variation, changes occur in the richness and abundance of benthos (Caupaz et al. 2006; guevara et al. 2007a) and fish communities (Castroroa et al. 2007). This discharge in Andean rivers has been observed to be strongly seasonal (Allan et al. 2006). For instance, in rivers of the Colombian Andes subjected to high water-level conditions, a reduction in the abundance of invertebrates has been reported (Arias et al. 2007; guevara et al. 2007b), but in northern Colombia, in the Sierra Nevada de Santa Marta Natural National Park, Anacroneuria larvae show similar distribution patterns (Tamaris-Turizo et al. 2007). Additionally, in tropical rivers the effects of predation by insectivorous fish on the population dynamics of Anacroneuria larvae are important (Tomanova and Tedesco 2007).
In general, tropical freshwater bodies are characterized by drastic variations in benthic community abundance during high water level conditions (Winemiller and Jepsen 1998; Jacobsen and encalada 1998; rincon and Cressa 2000; Want-zen 2003). Turcotte and Harper (1982) suggested that rain-fall spates are the major factor regulating benthic densities in non-seasonal environments (Amazon drainage basin, ecuador). The stonefy larvae abundance found during the wet season (November) was markedly different from that found during the dry season (August-September). The high precipitation registered during the second sampling period, November (wet season), increased discharge and consequently stream and river instability, altering the community structure by dislodging substrate and forcing drifting (Céréghino and Lavandier 1998; Céréghino et al. 2002). In a tropical river of venezuela, Anacroneuria larvae were most abundant during the period of least precipitation (Pérez and Segnini 2005). These results are concomitant with a study of caddisfy larvae in the Coello river watershed, Central Colombia, where lowest abundances were recorded during the rainy season (Guevara et al. 2005, 2007b).
Physicochemical variables and altitude. Several physico-chemical parameters varied in relation to seasonal hydrological variation in tropical rivers (Winemiller and Jepsen 1998). During extended wet periods, water temperature and conductivity tend to be lower, and dissolved oxygen concentrations tend to be higher. This tendency was observed in the majority of the variables measured in our study, which were related to seasonal changes in water level. However, only pH and conductivity were significantly correlated with abundance under low water level conditions suggesting a possible cause-and-effect relationship between these variables. During rising water events, conductivity appears to affect the total abundance of Anacroneuria larvae (Bücker et al. 2010). Throughout the drainage networks of the Prado river Basin, physicochemical attributes also are affected by human activities (e.g., agriculture, livestock, and cobble and sand extraction) mainly in lower altitude areas, generating different population responses in stonefies to variations in altitude. Water temperature and dissolved oxygen decreased with altitude, while other measured variables were uncorrelated with elevation, as was reported for Ecuadorian streams (Jacobsen 2008). It is there fore necessary to explore with more detail the relationships among physicochemical variables, Anacroneuria species richness, abundance, emergence patterns, and altitude from headwater streams to the rivers mouth.
In general terms, a decline in species richness with increasing altitude was observed (Von Ellenrieder 2007), as well as a decline in frequency of occurrence in certain species towards higher altitudes, without disappearing altogether (Jacobsen et al. 2003; Jacobsen and Brodersen 2008). other species can disappear completely at different points along the altitude gradient, which affects their local and regional abundance (romero-Alcaraz and Avila 2000).
Although the size of stonefies in temperate regions seems to be correlated with environmental factors such as photoperiod, water temperature and season (Nesterovitch and Zwick 2003), our results suggest that environmental factors in the study area do not infuence size or that these physicochemical parameters are not sufficiently different to affect larval size. Also, we did not find a significant relationship between body length and elevation, but it is possible that a further evaluation of pre-emergent Anacroneuria larvae may reveal significant correlations with altitude and/or any other physico-chemical variable (e.g., Tomanova and Tedesco 2007; Cressa et al. 2008).
Acknowledgements The authors would like thank to the anonymous reviewers for the very helpful suggestions and criticisms that improved the manuscript draft. All remaining errors and omissions are our responsibility. Thanks also to CorToLIMA (regional environmental authority) and the University of Tolima for supporting this research. g. guevara thanks the Austral University of Chile for the doctoral fellowships (MeCeSUP UCo0214 - AUS0703) and the Subcommittee of the Permanent Committee for Mayfies and Stonefies Conferences for travel scholarships to attend the International Joint Meeting on ephemeroptera and Plecoptera held in Stuttgart in June 2008.
Cited Literature ALLAN, J. D.; FLeCKer, A. S.; SegNINI, S.; TAPHorN, D. C.; SoKoL, E.; KLINg, g. W. 2006. Limnology of Andean piedmont rivers of venezuela. Journal of the North American Benthological Society 25 (1): 66-81. [ Links ]
ARIAS, D. M.; reINoSo, g.; gUevArA, g.; vILLA, F. A. 2007. Distribucion espacial y temporal de los coleopteros acuaticos en la cuenca del rio Coello (Tolima, Colombia). Caldasia 29 (1): 177-194. [ Links ]
BARRETO, g.; reINoSo, g.; gUevArA, g.; vILLA, F. A. 2005. Primer registro de Gripopterygidae (Insecta: Plecoptera) para Colombia. Caldasia 27 (2): 243-246. [ Links ]
Beer-STILLer, A.; ZWICK, P. 1995. Biometric studies of some stonefies and a mayfy (Plecoptera and ephemeroptera). Hydrobiologia 299 (2): 169-178. [ Links ]
BISPo, P. C.; oLIveIrA, L. g.; BINI, L. M.; SoUSA, K. g. 2006. Ephemeroptera, Plecoptera and Trichoptera assemblages from riffes in mountain streams of Central Brazil: environmental factors infuencing the distribution and abundance of immatures. Brazilian Journal of Biology 66 (2b): 611-622. [ Links ]
BISPo, P. C.; NeveS, C. o.; FroeHLICH, C. g. 2005. Two new species of Perlidae (Plecoptera) from Mato grosso State, western Brazil. Zootaxa 795: 1-6. [ Links ]
BoHórqUeZ, H. F.; reINoSo, g.; gUevArA, g.; vILLA, F. A. 2006. estudio de la abundancia, distribucion y estadios de las ninfas de plecopteros en la cuenca del rio Prado, Departamento del Tolima. Asociacion Colombiana de Limnologia Neoliminos 1: 117-122. [ Links ]
BrITTAIN, J. e. 1983. The infuence of temperature on nymphal growth rates in mountain stonefies (Plecoptera). ecology 64: 440-446. [ Links ]
BÜCKer, A.; SoNDerMANN, M.; FreDe, H.-g.; BreUer, L. 2010. The infuence of landuse on macroinvertebrate communities in montane tropical streams a case study from Ecuador. Fundamental and Applied Limnology 177 (4): 267-282. [ Links ]
BUSS, D. F.; BAPTISTA, D. F.; NeSSIMIAN, J. L.; egLer, M. 2004. Substrate specificity, environmental degradation and disturbance structuring macroinvertebrate assemblages in neotropical streams. Hydrobiologia 518 (1): 179-188. [ Links ]
CANoSA, A.; PINILLA, g. 2001. Total bacterial populations in three lentic water bodies of the Colombian Andes using the epifuorescence technique. Lakes & reservoirs: research and Management 6 (2): 169-174. [ Links ]
CASTro-roA, D.; VILLA-NAvArro, F. A.; gArCÍA-MeLo, J. e.; gArCIA-MeLo, L. J.; HerrADA-yArA, M. e.; reINoSo-FloreZ, g. 2007. Distribucion y aspectos ecologicos de Bryconamericus tolimae en la cuenca del rio Prado, Colombia. Dahlia 9: 77-86. [ Links ]
CAUPAZ, F. J.; reINoSo, g.; gUevArA, g.; vILLA, F. A. 2006. Diversidad y distribucion de la familia elmidae (Insecta: Coleoptera) en la cuenca del rio Prado (Tolima, Colombia). Asociacion Colombiana de Limnologia Neolimnos 1 (1): 106-116. [ Links ]
CÉrÉgHINo, r.; CUgNy, P.; LAvANDIer, P. 2002. Infuence of Intermittent Hydropeaking on the Longitudinal Zonation Patterns of Benthic Invertebrates in a Mountain Stream. International Review of Hydrobiology 87 (1): 47-60. [ Links ]
CÉrÉgHINo, r.; LAvANDIer, P. 1998. Infuence of hydropeaking on the distribution and larval development of the Plecoptera from a mountain stream. regulated rivers: research & Management 14 (3): 297-309. [ Links ]
CLeSCerI, L. S.; greeNBerg, A. e.; eAToN, A. D. 1999. Standard Methods for the Examination of Water and Wastewater, American Public Health Association, American Water Work Association, Water environment Federation, Washington, D. C. [ Links ]
CreSSA, C.; MALDoNADo, v.; SegNINI, S.; CHACoN, M. M. 2008. Size variation with elevation in adults and larvae of some venezuelan stonefies (Insecta: Plecoptera: Perlidae). Aquatic Insects 30 (2): 127-134. [ Links ]
CroSA, g.; BUFFAgNI, A. 2002. Spatial and temporal niche overlap of two mayfy species (ephemeroptera): the role of substratum roughness and body size. Hydrobiologia 474 (1): 107-115. [ Links ]
FeNogLIo, S.; MALACArNe, g.; Bo, T. 2007. Allometric growth in Anacroneuria Klapálek 1909 nymphs (Plecoptera Perlidae). Tropical Zoology 20 (1): 109-114. [ Links ]
FLECKER, A. S.; FeIFAreK, B. 1994. Disturbance and the temporal variability of invertebrate assemblages in two Andean streams. Freshwater Biology 31 (2): 131-142. [ Links ]
FoCHeTTI, r.; TIerNo De FIgUeroA, J. 2008. global diversity of stonefies (Plecoptera; Insecta) in freshwater. Hydrobiologia 595 (1): 365-377. [ Links ]
FroeHLICH, C. g. 2010. Catalogue of Neotropical Plecoptera. Illiesia 6 (12): 118-125. [ Links ]
FroeHLICH, C. g. 2004. Anacroneuria mainly from southern Brazil and northeastern Argentina (Plecoptera: Perlidae). Proceedings of the Biological Society of Washington 115: 75-107. [ Links ]
FroeHLICH, C. g. 2003. Stonefies (Plecoptera: Perlidae) from the Brazilian Amazonia with the description of three new species and a key to Macrogynoplax. Studies on Neotropical Fauna and Environment 38: 129-134. [ Links ]
gALvIS, g.; MoJICA, J. I. 2007. The Magdalena river fresh water fishes and fisheries. Aquatic ecosystem Health & Management 10: 127-139. [ Links ]
gAMBoA, M.; ArrIvILLAgA, J. 2010. Análisis morfométrico de cuatro especies simpatridas del género Anacroneuria (Plecoptera: Perlidae). Limnetica 29 (2): 247-256. [ Links ]
gAMBoA, M.; CHACóN, M. M.; SegNINI, S. 2009. Diet composition of the mature larvae of four Anacroneuria species (Plecoptera: Perlidae) from the venezuelan Andes. Aquatic Insects 31 (sup1): 409-417. [ Links ]
gUevArA, g.; LoZANo, P.; reINoSo, g.; vILLA, F. 2009. Horizontal and seasonal patterns of tropical zooplankton from the eutrophic Prado reservoir (Colombia). Limnologica - ecology and Management of Inland Waters 39 (2): 128-139. [ Links ]
gUevArA, g.; LoPeZ, e. o.; reINoSo, g.; vILLA, F. A. 2007a. Structure and distribution of the Trichoptera fauna in a Colombian Andean river basin (Prado, Tolima) and their relationship to water quality. In: BUeNo-SorIA, J.; BArBA-ALvAreZ, r.; ArMITAge, B. (eds.) Proceedings of the XIIth International Symposium on Trichoptera. The Caddis Press. ohio. [ Links ]
gUevArA, g.; reINoSo, g.; vILLA, F. A. 2007b. Caddisfy larvae (Insecta: Trichoptera) of the Coello river Basin in Tolima (Colombia): spatial and temporal patterns and bioecological aspects. In: BUeNo-SorIA, J.; BArBA-ALvAreZ, r.; ArMITAge, B. (eds.) Proceedings of the XIIth International Symposium on Trichoptera. The Caddis Press. ohio. [ Links ]
gUevArA, g.; reINoSo, g.; vILLA, F. A. 2005. estudio del orden Trichoptera en su estado larval en la cuenca del rio Coello Departamento del Tolima. revista de la Asociacion Colombiana de Ciencias Biológicas 17: 59-70. [ Links ]
HAMADA, N.; CoUCeIro, S. r. M. 2003. An illustrated key to nymphs of Perlidae (Insecta, Plecoptera) genera in Central Amazonia, Brazil. revista Brasileira de entomologia 47 (3): 477-480. [ Links ]
HofisTeDe, r. g. M. 1995. effects of livestock farming and recommendations for management and conservation of páramo grasslands (Colombia). Land Degradation and Development 6 (3): 133-147. [ Links ]
JACoBSeN, D. 2004. Contrasting patterns in local and zonal family richness of stream invertebrates along an Andean altitudinal gradient. Freshwater Biology 49 (10): 1293-1305. [ Links ]
JACoBSeN, D. 2008. Low oxygen pressure as a driving factor for the altitudinal decline in taxon richness of stream macroinvertebrates. Oecologia 154 (4): 795-807. [ Links ]
JACoBSeN, D.; BroDerSeN, K. P. 2008. Are altitudinal limits of equatorial stream insects refected in their respiratory performance? Freshwater Biology 53: 2295-2308. [ Links ]
JACoBSeN, D.; roSTgAArD, S.; vASCoNeZ, J. J. 2003. Are macroinvertebrates in high altitude streams affected by oxygen deficiency? Freshwater Biology 48 (11): 2025-2032. JACoBSeN, D.; eNCALADA, A. 1998. The macroinvertebrate fauna of Ecuadorian high-land streams in the wet and dry season. Archiv für Hydrobiologie 142: 53-70. [ Links ] [ Links ]
KLINgeNBerg, C. P.; SPeNCe, J. 1997. on the role of body size for life-history evolution. Ecological Entomology 22 (1): 55-68. [ Links ]
KrASNov, B.; WArD, D.; SHeNBroT, g. 1996. Body size and leg length variation in several species of darkling beetles (Coleoptera: Tenebrionidae) along a rainfall and altitudinal gradient in the Negev Desert (Israel). Journal of Arid environments 34 (4): 477-489. [ Links ]
LILLeHAMNUr, A.; BrITTAIN, J. e.; SALTveIT, S. J.; NIeLSeN, P. S. 1989. egg development, nymphal growth and life cycle strategies in Plecoptera. Ecography 12 (2): 173-186. [ Links ]
MCLeLLAN, I. D.; ZWICK, P. 2007. New species of and keys to South American gripopterygidae (Plecoptera). Illiesia 3 (4): 20-42. [ Links ]
NeSTerovITCH, A.; ZWICK, P. 2003. The development of Nemurella pictetii Klapálek (Plecoptera: Nemouridae) in two springstreams in central Europe. Limnologica - Ecology and Management of Inland Waters 33 (4): 231-243. [ Links ]
oTSUKI, A.; IWAKUMA, T. 2008. Life history, growth patterns and feeding habits of two predatory stonefies, Skwala pusilla (Perlodidae) and Kamimuria tibialis (Perlidae) in northern Japan. Aquatic Insects 30 (1): 29-41. [ Links ]
PÉreZ, B.; SegNINI, S. 2005. variación espacial de la composición y diversidad de géneros de ephemeroptera (Insecta) en un río tropical altiandino. Entomotropica 20 (1): 49-57. [ Links ]
PrINCIPe, r. e. 2008. Taxonomic and Size Structures of Aquatic Macroinvertebrate Assemblages in Different Habitats of Tropical Streams, Costa rica. Zoological Studies 47 (5): 525-534. [ Links ]
reSTrePo, J. D.; KJerFve, B. 2000. Magdalena river: interan-nual variability (1975-1995) and revised water discharge and sediment load estimates. Journal of Hydrology 235 (1-2): 137-149. [ Links ]
rINCoN, J.; CreSSA, C. 2000. Temporal variability of macroin-vertebrate assemblages in a Neotropical intermittent stream in Northwestern venezuela. Archiv für Hydrobiologie 148: 421-432. [ Links ]
roeSSLer, e. W.; ZAMorA, H. 1997. Número de estadíos nayadales, ciclo biológico y patrón de crecimiento de Anacroneuria caucana (Insecta-Plecoptera). Unicauca Ciencia 2: 15-24. [ Links ]
roMero-ALCArAZ, e.; AvILA, J. M. 2000. effect of elevation of type of habitat on the abundance and diversity of Scarabaeoid dung beetle (Scarabaeoidea) assemblages in a Mediterranean area from Southern Iberian Peninsula. Zoological Studies 39 (4): 351-359. [ Links ]
SALAZAr, C.; vILLA, F. A.; reINoSo, g. 2002. Distribucion espacial y temporal de las cloroficeas en el embalse de Prado (Tolima). revista de la Asociacion Colombiana de Ciencias Biologicas 14 (1): 57-64. [ Links ]
SALTveIT, S. J.; BreMNeS, T.; BrITTAIN, J. e. 1994. effect of a changed temperature regime on the benthos of a Norwegian regulated river. regulated rivers: research & Management 9 (2): 93-102. [ Links ]
SCHMIDT, D. A.; TArTer, D. C. 1985. Life history and ecology of Acroneuria carolinensis (Banks) in Panther Creek, Nicholas County, West virginia (Plecoptera: Perlidae). Psyche 92: 393-406. [ Links ]
SHeLDoN, A. L.; THeISCHINger, g. 2009. Stonefies (Plecoptera) in a tropical Australian stream: diversity, distribution and seasonality. Illiesia 5 (6): 40-50. [ Links ]
SHeLDoN, A. L. 1980. Coexistence of perlid stonefies (Plecoptera): Predictions from multivariate morphometrics. Hydrobiologia 71 (1): 99-105. [ Links ]
SHeLDoN, A. L. 1969. Size relationships of Acroneuria californica (Perlidae, Plecoptera) and its prey. Hydrobiologia 34 (1): 85-94. [ Links ]
SILveIrA, M. P.; BUSS, D. F.; NeSSIMIAN, J. L.; BAPTISTA, D. F. 2006. Spatial and temporal distribution of benthic macro-invertebrates in a Southeastern Brazilian river. Brazilian Journal of Biology 66: 623-632. [ Links ]
STArK, B. P.; FroeHLICH, C. g.; ZÚÑIgA, M. C. 2009. South American Stonefies (Plecoptera), Pensoft, Sofa-Moscow. [ Links ]
STArK, B. P. 2001. A synopsis of Neotropical Perlidae (Plecoptera). In: DoMINgUeZ, e. (ed.) Trends in research in Ephemeroptera and Plecoptera. Kluwer Academic/Plenum. New york. [ Links ]
STATSoFT 2004.STATISTICA (data analysis software system). [ Links ]
SUHAILA, A. H.; CHe SALMAH, M. r. 2011. Stonefies (Insecta: Plecoptera) in Malaysian tropical rivers: diversity and seasonality. Journal of Entomology and Nematology 3 (2): 30-36. [ Links ]
TAMArIS-TUrIZo, C. e.; ZÚÑIgA, M. D. C.; TUrIZo-CorreA, r. r. 2007. Distribución espacio-temporal y hábitos alimentarios de ninfas de Anacroneuria (Insecta: Plecoptera: Perlidae) en el río gaira (Sierra Nevada de Santa Marta, Colombia). Caldasia 29 (2): 375-385. [ Links ]
ToMANovA, S.; TeDeSCo, P. A. 2007. Tamaño corporal, tolerancia ecológica y potencial de bioindicación de la calidad del agua de Anacroneuria spp. (Plecoptera: Perlidae) en América del Sur. revista de Biologia Tropical 55 (1): 67-81. [ Links ]
ToWNSeND, g. D.; PrITCHArD, g. 1998. Larval growth and development of the stonefy Pteronarcys californica (Insecta: Plecoptera) in the Crowsnest river, Alberta. Canadian Journal of Zoology 76: 2274-2280. [ Links ]
TUrCoTTe, P.; HArPer, P. P. 1982. The macro-invertebrate fauna of a small Andean stream. Freshwater Biology 12 (5): 411-419. [ Links ]
voN eLLeNrIeDer, N. 2007. Composition and structure of aquatic insect assemblages of yungas mountain cloud forest streams in NW Argentina. revista de la Sociedad entomologica Argentina 66 (3-4): 57-76. [ Links ]
WANTZeN, K. M.; rAMIreZ, A.; WINeMILLer, K. o. 2006. New vistas in Neotropical stream ecology-Preface. Journal of the North American Benthological Society 25 (1): 61-65. [ Links ]
WANTZeN, K. M. 2003. Cerrado streams - characteristics of a threatened freshwater ecosystem type on the Tertiary Shields of Central South America. Amazoniana 14 (3-4): 481-502. [ Links ]
WINeMILLer, K. o.; JePSeN, D. B. 1998. effects of seasonality and fish movement on tropical river food webs. Journal of Fish Biology 53 (sA): 267-296. [ Links ]
ZÚÑIgA, M. C.; DIAS, L.; MArTÍNeZ, D.; ZABALA, g.; BACCA, T. 2009. The frst record of Claudioperla Illies (Plecoptera: gripopterygidae) from Colombia. Aquatic Insects: International Journal of Freshwater Entomology 31 (1, Supp. 1): 743-744. [ Links ]
ZÚÑIgA, M. C.; STArK, B. P.; CArDoNA, W.; TAMArIS-TUrIZo, C.; orTegA, o. e. 2007. Additions to the Colombian Anacroneuria fauna (Plecoptera: Perlidae) with descriptions of seven new species. Illiesia 3 (13): 127-149. [ Links ]
ZÚÑIgA, M. C.; BALLeSTeroS, y. v.; grISALeS, M. 2003. Nocturnal emergence patterns of four species of Anacroneuria (Plecoptera: Perlidae) in a tropical Inter-Andean stream (Colombia, South America). Abstracts of the ZWICK, P. 2004. Key to the west Palaearctic genera of stonefies (Plecoptera) in the larval stage. Limnologica 34 (4): 315-348. [ Links ]
ZWICK, P. 2003. Shapes and patterns of wingpad development in the Plecoptera. In: gAINo, e. (ed.) research update on ephemeroptera and Plecoptera. University of Perugia. Perugia. [ Links ]