Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488
Rev. Colomb. Entomol. vol.38 no.1 Bogotá Jan./June 2012
Inducers of resistance in potato and its effects on defoliators and predatory insects
Los inductores de resistencia en la papa y sus efectos sobre insectos predadores y defoliadores
FRANSCINELY APARECIDA DE ASSIS1,2, JAIR CAMPOS MORAES1,3, LUÍS CLAUDIO PATERNO SILVEIRA1,4, JONAS FRANCOSO1,5, AMANDA MARIA NASCIMENTO1,6 and CRISTIANA SILVEIRA ANTUNES1,7
1 Universidade Federal de Lavras, CP. 3037, CEP 37200-000, Lavras, MG, Brazil.2 M. Sc., franscinelyagronomia@yahoo.com.br. Corresponding author.
3 Dr., jcmoraes@den.ufla.br
4 Dr., lcpsilveira@den.ufla.br
5 Graduate student of Agronomy, jonasfrancoso@hotmail.com
6 M. Sc., amandanascimen-toagro@yahoo.com.br
7 M. Sc., cristiana.santunes@gmail.com
Received: 24-may-2011 - Accepted: 23-feb-2012
Abstract: The effects of chemical inducers of plant resistance on folivorous beetles and predatory insects in Solarium tuberosum L. were evaluated. The effects of these chemical inducers on crop development and yield were also determined. The experiment was conducted with cv. Emeraude using a randomised complete-block design with four treatments and six replications: 1 - control; 2 - 1% silicic acid; 3 - 0.02% acibenzolar-S-methyl (ASM); and 4 - 1.15% diatomaceous earth (DE). Treatments 2, 3 and 4 were applied by spraying plant foliage every 10 days, total of five applications. The injuries and damages caused by beetles were evaluated. There were no significant differences in the presence of folivorous and predatory beetles in the aerial plant mass and the surrounding soil. However, the plants treated with silica or DE were less preferred by defoliators. Spraying with silicic acid, ASM and DE produced significant increases in plant diameter and height without affecting productivity. The application of silicic acid, ASM or DE increased protection against herbivorous insects and decreased tuber damage.
Key words: Diabrotica speciosa. Silicic acid. Diatomaceous earth. Acibenzolar-S-methyl. Integrated pest management.
Resumen: Se evaluaron los efectos de los inductores químicos de resistencia de plantas a insectos en los escarabajos hebívoros y otros insectos depredadores en Solanum tuberosum. Los efectos de estos inductores químicos también se evaluaron en el desarrollo y en el rendimientos del cultivo. El experimento se llevó a cabo con el cv. Emeraude, utilizando un diseño de bloques al azar con cuatro tratamientos y seis repeticiones: 1 - Control, 2 - 1% de ácido silícico, 3 -0,02% acibenzolar-s-metil (ASM), y 4 - 1,15% tierra de diatomeas (DE). Los tratamientos 2, 3 y 4 se aplicaron por aspersión en el follaje de las plantas cada 10 días, un total de cinco aplicaciones. Se evaluaron las lesiones y los daños causados por Diabrotica speciosa. No hubo diferencias significativas en la presencia de D. speciosa y depredadores en la parte aérea de la planta y en el suelo circundante. Sin embargo, las plantas tratadas con ácido silícico o DE fueron menos preferidas por D. speciosa y el efecto asociado con ácido silícico, ASM y DE produjo un aumento significativo en el diámetro y la altura de la planta sin afectar la productividad. La aplicación de ácido silícico, ASM o DE aumentó la protección contra insectos y disminuyó los daños al tubérculo.
Palabras clave: Diabrotica speciosa. Ácido silícico. Tierra de diatomeas. Acibenzolar-s-metil. Manejo integrado de plagas.
Introduction
The potato (Solanum tuberosum L.) is an important food source worldwide. However, this cultivated Solanaceae species is one of the crops that suffer the most from feeding by insect herbivores. The beetle Diabrotica speciosa (Germar, 1824) (Coleoptera: Chrysomelidae) is commonly found in South America, where it feeds on plant species such as soy, beans, corn, and potatoes (Ávila and Parra 2002; Lara et al. 2004). The damage caused by D. speciosa varies depending on the developmental stage of the insect. The larvae, which are subterranean, damage the plant roots or create holes in the potato tubers, whereas the adults consume the leaves (Ávila and Parra 2003; Azeredo et al. 2004).
Insecticides from the carbamates, organophosphates, pyrethroids and, more recently, neonicotinoids are used to control D. speciosa. However, chemical control may raise the cost of crop production in addition to harming the environment and non target organisms. Consequently, the adoption of nonpolluting methods such as biological control is necessary even for the conservation of beneficial insects in crops (Hagen 1962).
Several natural enemies of D. speciosa are found in Brazil. These include Lebia concinna (Brulle, 1837) (Coleoptera: Carabidae); Doru lineare (Eschsholtz, 1822) (Dermaptera: Forficulidae); Nabis sp. (Heteroptera: Nabidae); Cycloneda sanguinea (Linnaeus, 1763); Scymnus sp. (Coleoptera: Coccinelidae) and the braconid Centistes gasseni (Shaw, 1995) (Hymenoptera: Braconidae) (Walsh et al. 2003). The heteropteran Cosmoclopius nigroannulatus (Stal, 1860) (Hemiptera: Reduviidae) may also control D. speciosa and Epitrix sp. (Coleoptera: Chrysomelidae) (Azevedo and Nascimento 2009; Jahnke et al. 2002). In addition to biological control, the use of induced resistance in plants can help control insect pests with low environmental impact. Induced resistance increases plant resistance by using external agents requiring changes in the plant genomes (Van Loon et al. 1998).
Prior research has proven the efficiency of silicates as a plant resistance inducer against sucking insects (Pereira et al. 2010; Costa et al. 2011) and chewing insects (Keeping and Meyer 2006; Kvedaras et al. 2007). Although the beneficial use of acibenzolar-S-methyl (ASM) against fungal plant pathogens (Duarte et al. 2009; Cabral et al. 2010) has been shown. Little is known about the effectiveness of ASM against insect pests (Moraes et al. 2009; Alcantra et al. 2010).
There have been few field and laboratory studies on the use of diatomaceous earth (DE) against folivorous beetles (Assis et al. 2011), however, the use of DE has been successful in controlling beetles in stored grains (Mvumi et al. 2006; Ziaee and Khashaveh 2007; Wakil et al. 2010).
The aim of this study was to evaluate the effects of chemical inducers of plant resistance on folivorous beetles and predatory insects in Solanum tuberosum. The effects of these chemical inducers on crop development and yield were also determined.
Materials and Methods
Study Area. The study was conducted during the dry season in a non-irrigated area at the Universidade Federal de Lavras (UFLA) in Lavras, MG (21°45'S 45°00'W and 918 masl), Brazil, between February and June 2009.
The soil was prepared by disking, and the planting was done in rows with 0.8 to 0.25 m between plants. Each plot was composed of four 4-m-long rows; only the two central rows were used, leaving 0.5 m unplanted at the borders. The combined plots comprised a total area of 115.2 m2. The plots were fertilised with the organic product Geneplus® (Genfértil, Mogi Mirim, Brazil) (1% total N, 15% C and pH 6.0) at a dosage rate of 15 t/ha (modified from Gomes et al. 2009), and it was seeded with tubers (cultivar Emeraude). The first weeding and piling were performed 30 days post-seeding.
Experimental design. We used a randomised completeblock formation with four treatments and six replications, comprising a total of 24 experimental plots: T1 - control; T2 - 1% silicic acid (SiO2.XH2O) (Vetec Química Fina, Duque de Caxias, Brazil); T3 - 0.02% ASM (S-methyl benzo[1,2,3] thiadiazole-7-carbothioic) (Bion 500 WG®, Syngenta Crop Protection, Säo Paulo, Brazil); and T4 - 1.15% diatomaceous earth (SiO2) (Insecto®, Bequisa, Säo Vicente, Brazil). Treatments T2, T3 and T4 were applied to the foliage every 10 days as an aqueous solution for a total of five sprayings, using a 1.5-L hand-pressure sprayer with a cone-spray nozzle (Assis et al. 2011).
Insect monitoring and plant evaluation. Monitoring of folivorous and predatory beetles was performed every ten days, one day before each treatment application. Beetles were collected and counted by beating the apical leaflets of five plants per plot, over a white plastic tray (35 x 29 x 5 cm). In addition, we placed two pitfall traps, each made of 9-cm-high x 14-cm-wide plastic containers buried to the rim, at the ends of each row. The traps contained a solution of 300 mL water, 90 g NaCl, and a few drops of liquid neutral detergent. The salt solution was replaced every ten days, and the captured insects were identified in the laboratory. Five evaluations were performed for each collection method.
The height and diameter of the plants were recorded 60 days after planting. Assessments of insect feeding damage were made every 10 days from five random plants per plot. The number of leaflets with holes and the number of holes on the third and fourth leaflets from the apex were counted.
After harvesting, the tubers were weighed and the productivity in kg/plot was determined. The damage caused by insect larvae was classified by the number of holes in 20 medium tubers (diameter measure in average 50 mm) randomly selected from each plot; three damage categories were used to classify the tubers: 1 = 0 to 20 holes; 2 = 21 to 40 holes; and 3 = more than 40 holes (adapted from Salles and Grutzmacher 1999).
Statistical analysis. The data were subjected to analysis of variance, using the System for Statistical Analysis and Genetics (SAEG) (Ribeiro Junior 2001) and the means were compared using the Scott-Knott test (P ≤ 0.05) (Scott and Knott 1974), with the count data transformed into 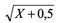 and the percentage data into arcsin
and the percentage data into arcsin 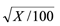 prior to analysis.
prior to analysis.
Results
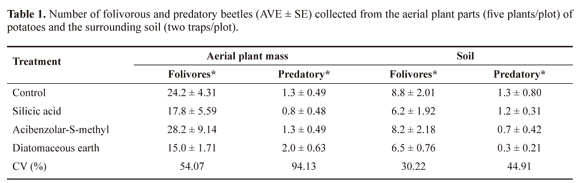
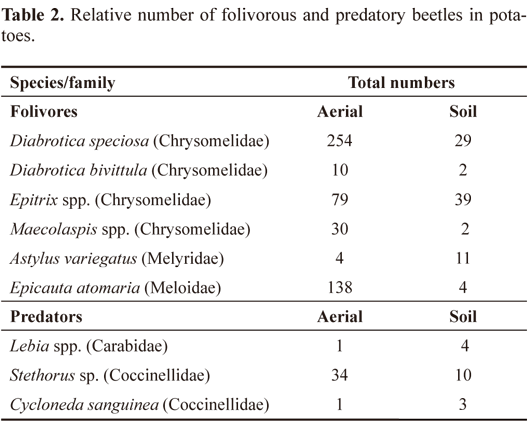
There were no significant differences (P > 0.05) among the treatments regarding the number of folivorous and predatory beetles present on the aerial plant parts or in the root mass (Table 1). Folivorous insects (Chrysomelidae [D. speciosa, D. bivittula (Kirsch, 1883), Epitrix spp., and Maecolaspis spp.], Melyridae [Astylus variegatus (Germar, 1824)], and Meloidae [Epicauta atomaria (Germar, 1821)] were collected from the plant shoots, and 77 beetles of the family Scarabaeidae, from the soil were collected.
Predatory insects of the families Carabidae (Lebia spp.) and Coccinellidae (Stethorus sp. and Cycloneda sanguinea (Linnaeus, 1763) were found in both the aerial plant parts and the soil (Table 2).
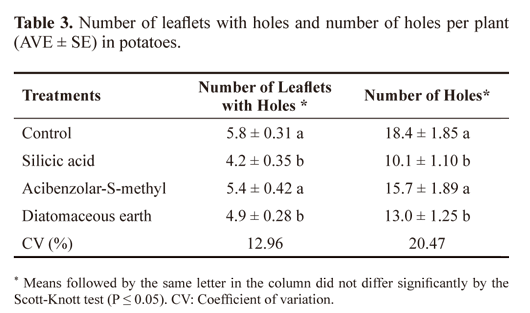
Control plants and the plants treated with ASM were preferred by adult folivorous beetles as they had the highest number of leaflets with holes (5.8 ± 0.31 and 5.4 ± 0.42) and the highest number of holes (18.4 ± 1.85 and 15.7 ± 1.89), respectively (Table 3). Sprays containing either silicic acid or DE had significantly reduced (P ≤ 0.05) leaf damage (Table 3), specifically Diabrotica spp. and Epitrix spp.
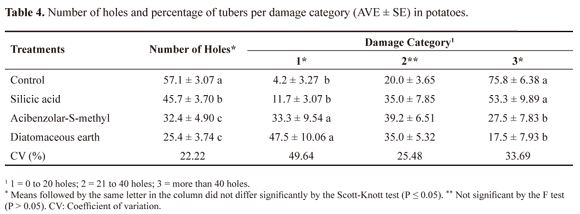
Applications of silicic acid, ASM and DE generally provided protection to the tubers (Table 4). We observed significant differences ( P ≤ 0.05) in damage categories one and three, with a high percentage of tubers from the plants treated with ASM (33.3 ± 9.54%) and DE (47.5 ± 10.06%) presenting between 0 and 20 holes, whereas a low percentage of tubers had over 40 holes (27.5 ± 7.83% and 17.5 ± 7.93%, respectively) (Table 4).
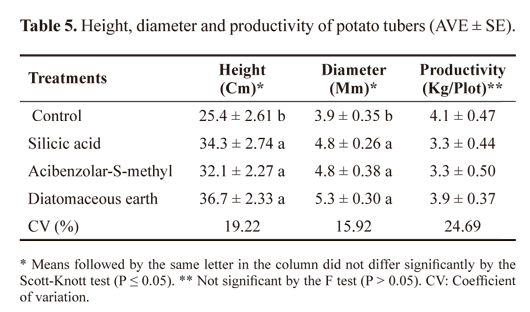
In terms of plant development, there were significant increases in the heights and diameters of all the plants in treatments groups compared to the control plants (P ≤ 0.05); however, tuber productivity was not affected by treatment (Table 5).
Discussion
The foliar applications of silicic acid, ASM, and DE did not have a repellent effect on insect pests in the potato crops as there was no change in host selection (Table 1). Thus, the number of folivorous beetles in the aerial parts of the plants and in the soil did not differ among treatments. These results are consistent with those of Silva et al. (2010) who also found no effect of silicic acid on the intensity of infestation of adult D. speciosa. However, populations of Chlosyne lacinia saundersii (Doubleday and Hewitson, 1849) (Lepidoptera: Nymphalidae) were reduced fourfold or more in plants treated with silicic acid compared to the untreated plants (Antunes et al. 2010). On the other hand, applications of DE on onions did not significantly affect the population of Thrips tabaci (Lindeman, 1888) (Thysanoptera: Thripidae) (Goncalves 2007).
Here, the species D. speciosa stood out among the folivorous beetles, similarly to the results reported by Grutzmacher and Link (2000) in a dry-season potato culture. The inducers of resistance did not influence the occurrence of predatory beetles found on the potato crops. In other words, these substances did not attract natural enemies. Antunes et al. (2010) observed similar results for C. sanguinea on corn sprayed with silicic acid. However, Kvedaras et al. (2010) found that in cucumber (Cucumis sativus L.) plants treated with potassium silicate and attacked by larvae of Helicoverpa armigera (Hubner, 1805) (Lepidoptera: Noctuidae) had higher populations of the predator Dicranolaius bellulus (Guérin-Méneville, 1830) (Coleoptera: Melyridae) compared to plants treated with silicate but not attacked by insect pests. This suggests that the use of silicate to plants with herbivore pre-infestations increases the attractiveness of these plants to natural enemies, thus increasing biological control.
The applications of silicic acid and DE to the potato plants increased resistance to damage by adults folivorous insects. These results agree with those obtained by Gomes et al. (2009) showing that D. speciosa caused twice as many lesions in control plants than in those treated with silicic acid. This result may be explained by the deposition of silicic acid in the form of amorphous hydrated silica (SiO2.nH2O) on the cell wall. The deposition provokes the formation of a double layer of cuticle silicates, making it harder for the insect herbivores to penetrate and masticate due to the hardening of the plant cell walls (Yoshida et al. 1962; Datnoff et al. 1991). The formation of a mechanical barrier observed particularly in silicon-accumulating plants, especially in Poaceae (Ma and Takahashi 2002). The action of silicic acid is not restricted to mechanical resistance, constitutive or induced, and may be associated with secondary compounds in plant defense (Pereira et al. 2010; Costa et al. 2011).
The feeding behaviour of the larvae was affected by the use of inducers of plant resistance, as evidenced by the lower number of tuber holes in treated plants (Table 4). ASM and DE treatments yielded a higher percentage of tubers classified in the lowest damage category. This reduction in tuber attack by insect pests correlates with the reduced leaf damage in the plants treated with silicic acid or DE. ASM possibly negatively affected the oviposition of the pest insects, based on the small amount of damage to the tuber for the same population as compared to control.
The use of ASM on cotton (Gossypium hirsutum L.) yielded greater protection to plants against aphids and whiteflies. The protection may be due to deterrent substances produced by the action of this compound in the plant (Inbar et al. 2001; Alcantra et al. 2010).
Although the present study focused on a different species of beetle in a different environment, the results of the application of DE are consistent with those found by Bavaresco (2007); no damage was found by Acanthoscelides obtectus (Say, 1831) (Coleoptera: Bruchidae) in stored beans treated with DE.
The use of silicic acid and ASM resulted in increases in the heights and diameters of the potato plants. However, the plant productivity did not increase, which may have been due to the occurrence of a prolonged drought during the period of tuber production.
The height and diameter results found in this study differ from those found by Gomes et al. (2009), who grew potatoes during the rainy season and did not observe any effects of silicic acid application on plants. It is possible that the silicic acid is utilised more efficiently in plants during stressful conditions (Korndorfer et al. 2004; Kvedaras et al. 2007). However, the positive effects of silicic acid support observations by Neri et al. (2009) in corn, which accumulates this mineral.
The use of ASM on tomatoes and melons has not proven the beneficial effects of this inducer on the height and dry-shoot mass of these plants (Araujo and Menezes 2009; Cabral et al. 2010). This effect can be attributed to the transfer cost of metabolic processes involved in growth for the synthesis of plant-defence compounds. For productivity, the results of the ASM application were unsatisfactory, as the activation of resistance by the use of inducers results in a higher energy cost for the plant, which does not always result in increased production (Dietrich et al. 2005; Duarte et al. 2009). However, based on the results, we can conclude that the application of silicic acid, ASM or DE generally increased the protection of potatoes and reduced tuber damage.
Acknowledgements
The authors wish to thank Conselho Nacional de Desen-volvimento Científico e Tecnológico (CNPq) and Fundacäo de Amparo à Pesquisa de Minas Gerais (FAPEMIG), Brazil, for their financial support and other resources supporting this project.
Literature cited
ALCANTRA, E.; MORAES, J. C.; ANTONIO, A. 2010. Efeito de indutores da resistencia e cultivares de algodào no comportamento de Aphis gossypii. Revista Ciencia Agronòmica (Brasil, Ceará) 41 (4): 619-624. [ Links ]
ANTUNES, C. S.; MORAES, J. C.; ANTONIO, A.; SILVA,V F. 2010. Influencia da aplicacào de silicio na ocorrència de lagartas (Lepidoptera) e de seus inimigos naturais chaves em milho (Zea mays L.) e em girassol (Helianthus annuus L.). Bioscience Journal (Brasil, Uberländia) 26 (4): 619-625. [ Links ]
ARAÚJO, F. F.; MENEZES, D. 2009. Inducäo de resistencia a doencas foliares em tomateiro por indutores biòtico (Bacillus subtilis) e abiòtico (Acibenzolar-S-metil). Summa Phytopathologica (Brasil, Botucatu) 35 (3): 169-172. [ Links ]
ASSIS, F. A.; MORAES, J. C.; NASCIMENTO, A. M.; FRANCOSO, J. 2011. Efeitos da terra diatomácea sobre Diabrotica speciosa (Germar, 1824) (Coleoptera: Chrysomelidae) em batata inglesa. Ciencia e Agrotecnologia (Brasil, Lavras) 35 (3): 482486. [ Links ]
ÁVILA, C.J.; PARRA, J. R. P. 2002. Desenvolvimento da Diabrotica speciosa (Germar) (Coleoptera: Chrysomelidae) em diferentes hospedeiros. Ciencia Rural (Brasil, Santa Maria) 32 (5):739-743. [ Links ]
ÁVILA, C. J.; PARRA, J. R. P. 2003. Leaf consumption by Diabrotica speciosa (Coleoptera: Chrysomelidae) adults on different host plants. Scientia Agricola (Brasil, Piracicaba) 60 (4):789-792. [ Links ]
AZEREDO, E. H.; LIMA, E.; CASSINO, P. C. R. 2004. Impacto dos nutrientes N e K e de acucares solúveis sobre populacöes de Diabrotica speciosa (Germar) (Coleoptera, Chrysomelidae) e Agrotis ipsilon (Hufnagel) (Lepidoptera, Noctuidae) na cultura da batata, Solanum tuberosum L. (Solanaceae). Revista Brasileira de Entomologia 48 (1): 105-113. [ Links ]
AZEVEDO, R. L.; NASCIMENTO, A. S. 2009. Observacòes sobre o comportamento predatòrio de Cosmoclopius nigroannulatus (Stàl, 1860) (Hemiptera: Reduviidae) em plantas de feijào Guandu. EntomoBrasilis (Brasil, Vassouras) 2 (1): 25-26. [ Links ]
BAVARESCO, A. 2007. Avaliacào de tratamientos alternativos para o controle do Acanthoscelides obtectus (Say) (Coleoptera: Bruchidae). Revista de Ciencias Agroveterinárias (Brasil, Lages) 6 (2): 125-133. [ Links ]
CABRAL, C. P.; GAMA, M. A. S.; ALEXANDRE, E. R.; MARIANO, R. L. R.; SILVEIRA, E. B. 2010. Efeito de acibenzolar-S-metil, mananoligossacarideo e bioflavonóides cítricos no controle da mancha-aquosa e no crescimento do meloeiro. Tropical Plant Pathology (Brasil, Brasilia) 35 (2): 119-123. [ Links ]
COSTA, R. R.; MORAES, J. C.; COSTA, R.R. DA . 2011. Feeding behaviour of the greenbug Schizaphis graminum on wheat plants treated with imidacloprid and/or silicon. Journal of Applied Entomology 135 (1/2): 115-120. [ Links ]
DATNOFF, L. E.; RAID, R. N.; SNYDER, G. H.; JONES, D. B. 1991. Effect of calcium silicate on blast and brown spot intensities and yields of rice. Plant Disease 75 (7): 729-732. [ Links ]
DIETRICH, R.; PLOSS, K.; HEIL, M. 2005. Growth responses and fitness cost after induction of pathogen resistance deped on environmental condition. Plant, Cell and Environment 28: 211-222. [ Links ]
DUARTE, H. S. S.; ZAMBOLIM, L.; RODRIGUES, F.A.; RIOS, J. A.; LOPES, U. P. 2009. Silicato de potássio, acibenzolar-S- metil e fungicidas no controle da ferrugem da soja. Ciencia Rural (Brasil, Santa Maria) 39 (8): 2271-2277. [ Links ]
GOMES, F. B.; MORAES, J. C.; NERI, D. K. P. 2009. Adubacao com silicio como fator de resistencia a insetos-praga e promotor de produtividade em cultura de batata inglesa em sistema orgänico. Ciencia e Agrotecnologia (Brasil, Lavras) 33 (1):18-23. [ Links ]
GONCALVES, P. A. S. 2007. Manejo de Thrips tabaci em cebola orgänica com terra de diatomáceas. Revista Brasileira de Agroecologia (Brasil, Pelotas) 2 (3): 69-74. [ Links ]
GRUTZMACHER, A. D.; LINK, D. 2000. Levantamento da entomofauna associada a cultivares de batata em duas épocas de cultivo. Pesquisa Agropecuária Brasileira (Brasil, Brasilia) 35 (3): 653-659. [ Links ]
HAGEN, K. S. 1962. Biology and ecology of predaceous coccinelidae. Annual Review Entomology 7 (35): 289-326. [ Links ]
INBAR, M.; DOOSTDAR, H.; GERLING, D.; MAYER, R. T. 2001. Induction of systemic acquired resistance in cotton by BTH has a negligible effect on phytophagous insects. Entomologia Experimentalis et Applicata 99 (1): 65-70. [ Links ]
JAHNKE, S. M.; REDAELLI, L. R.; DIEFENBACH, L. M. G. 2002. Population dynamics of Cosmoclopius nigroannulatus Stal (Hemiptera, Reduviidae) in tobacco culture. Brazilian Journal of Biology 62 (4): 819-826. [ Links ]
KEEPING, M. G.; MEYER, J. H. 2006. Silicon-mediated resistance of sugarcane to Eldana saccharina Walker (Lepidoptera: Pyralidae): effects of silicon source and cultivar. Journal of Applied Entomology 130 (8): 410-420. [ Links ]
KORNDÖRFER, G. H.; PEREIRA, H. S.; CAMARGO, M. S. 2004. Silicatos de càlcio e magnèsio na agricultura. 3.ed. Uberlândia: UFU/ICIAG, 23p. (Boletim Técnico, 1). [ Links ]
KVEDARAS, O. L.; KEEPING, M. G.; GOEBEL, F. R.; BYRNE, M. J. 2007. Water stress augments silicon-mediated resistance of susceptible sugarcane cultivars to the stalk borer Eldana saccharina (Lepidoptera: Pyralidae). Bulletin of Entomological Research 97 (2): 175-183. [ Links ]
KVEDARAS, O. L.; AN, M.; CHOI, Y. S.; GURR, G. M. 2010. Silicon enhances natural enemy attraction and biological control through induced plant defences. Bulletin of Entomological Research 100 (3): 367-371. [ Links ]
LARA, F. M.; SCARANELLO, A. L.; BALDIN, E. L. L.; BOICA JÚNIOR, A. L.; LOURENCÄO, A. L. 2004. Resistencia de genòtipos de batata a larvas e adultos de Diabrotica speciosa. Horticultura Brasileira (Brasil, Brasilia) 22 (4): 761-765. [ Links ]
MA, J. F.; TAKAHASHI, E. 2002. Soil, fertilizer, and plant silicon research in Japan. 1 ed. Elsevier Science, Amsterdam. 503 p. [ Links ]
MORAES, J. C.; FERREIRA, R. S.; COSTA, R. R. 2009. Indutores de resistencia à mosca-branca Bemisia tabaci biòtipo B (Genn, 1889) (Hemiptera: Aleyrodidae) em soja. Ciencia e Agrotecnologia (Brasil, Lavras) 33 (5): 1260-1264. [ Links ]
MVUMI, B. M.; STATHERS, T. E.; GOLOB, P.; GIGA, D. P. 2006. Penetration of Sitophilus zeamais (Coleoptera: Curculionidae) through diatomaceous earth-treated bulk maize grain. International Journal of Tropical Insect Science 26: 28-34. [ Links ]
NERI, D. K. P.; GOMES, F. B.; MORAES, J. C.; GOES, G. B.; MARROCOS, S. T. P. 2009. Influencia do silicio na suscetibilidade de Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) ao inseticida lufenuron e no desenvolvimento de plantas de milho. Ciencia Rural (Brasil, Santa Maria) 39 (6): 1633-1638. [ Links ]
PEREIRA, R. R. C.; MORAES, J. C.; PRADO, E.; COSTA, R. R. 2010. Resistance inducing agents on the biology and probing behaviour of the greenbug in wheat. Scientia Agricola (Brasil,Piracicaba) 67 (4): 430-434. [ Links ]
RIBEIRO JÚNIOR, J. I. 2001. Análises estatisticas no SAEG. Viçosa: UFV, 301 p. [ Links ]SALLES, L. A. B.; GRUTZMACHER, A. D. 1999. Eficiencia do inseticida clorpirifòs no controle de larvas de Diabrotica speciosa (Germ.) (Coleoptera: Chrysomelidae) na cultura da batata. Ciencia Rural (Brasil, Santa Maria) 29 (2): 195-199. [ Links ]
SCOTT, A. J.; KNOTT, M. A. 1974. A cluster analysis method for grouping means in the analysis of variance. Biometrics 30 (3): 507-512. [ Links ]
SILVA, V. F.; MORAES, J. C.; MELO, B. A. 2010. Influence of silicon on the development, productivity and infestation by insect pests in potato crops. Ciencia e Agrotecnologia (Brasil, Lavras) 34 (6): 1465-1469. [ Links ]
VAN LOON, L. C.; BAKKER, P. A. H. M.; PIETERSE, C. M. J. 1998. Systemic resistance induced by rhizosphere bacteria. Annual Review of Phytopathology 36: 453-483. [ Links ]
WAKIL, W.; ASHFAQ, M.; GHAZANFAR, M.U.; RIASAT, T. 2010. Susceptibility of stored-product insects to enhanced diatomaceous earth. Journal of Stored Products Research 46 (4): 248-249. [ Links ]
WALSH, C. G.; ATHANAS, M. M.; SALLES, L. A. B.; SCHRODER, R. F. W. 2003. Distribution, host range, and climatic constraints on Centistes gasseni (Hymenoptera: Braconidae), a South American parasitoid of cucumber beetles, Diabrotica spp. (Coleoptera: Chrysomelidae). Bulletin of Entomological Research 93 (6): 561-567. [ Links ]
YOSHIDA, A. S.; OHNISHI, Y.; KITAGISHI, K. 1962. Histochemistry of silicon in rice plant. Soil Science and Plant Nutrition 8 (2): 107-111. [ Links ]
ZIAEE, M.; KHASHAVEH, A. 2007. Effect of five diatomaceous earth formulations against Tribolium castaneum (Coleoptera: Tenebrionidae), Oryzaephilus surinamensis (Coleoptera: Silvanidae) and Rhyzopertha dominica (Coleoptera: Bostrychidae). Insect Science 14 (5): 359-365. [ Links ]