Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488
Rev. Colomb. Entomol. vol.38 no.1 Bogotá Jan./June 2012
Larvicidal activity of Piper tuberculatum on Spodoptera frugiperda (Lepidoptera: Noctuidae) under laboratory conditions
GLADYS V. SOBERÓN RISCO12, CONSUELO ROJAS IDROGO1,3, MASSUO J. KATO4, JORGE SAAVEDRA DÍAZ1,5, JOSÉ ARMANDO-JR.6 and GUILLERMO E. DELGADO PAREDES1,7
1 Universidad Nacional Pedro Ruiz Gallo, Ciudad Universitaria, Juan XXIII No 391, Lambayeque-Perú.
2 M. Sc. Facultad de Ciencias Biológicas. glavisor@hotmail.com.
3 M. Sc. Facultad de Ciencias Biológicas. crojasi2002@yahoo.es.
4 Ph. D. Instituto de Química, Universidade de Sao Paulo, CP 26077, 05508900 Sao Paulo, SP, Brazil. majokato@iq.usp.br.
5 Ph. D. Facultad de Agronomía. jsaadiaz@gmail.com.
6 Ph. D. Faculdad de Medicina do ABC, Av. Príncipe de Gales, 821, Santo André, Sao Paulo, SP, Brazil. ajrj@uol.com.br.
7 Ph. D. Facultad de Ciencias Biológicas. guidelg2001@yahoo.es. Corresponding author.
Received: 5-apr-2011 - Accepted: 18-may-2012
Abstract: The larvicidal activity of the neotropical "matico" Piper tuberculatum was evaluated. The secondary compounds were extracted of leaves, stems and mature spikes with fruits and seeds from wild plants and in vitro plants of Piper tuberculatum. The acute toxicities to the fall armyworm, Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae), of extracts of spikes with fruits and seeds and in vitro plants of P. tuberculatum were evaluated by means of contact bioassays. Only CH2Cl2:MeOH (2:1) and EtOH extracts of mature spikes and CH2Cl2:MeOH (2:1) extract from in vitro plants showed significant levels of larval mortality. The CH2Cl2:MeOH (2:1) and EtOH extracts of mature spikes caused 90% mortality when doses of 0.1850 mg/µL were applied to the S. frugiperda in 24 and 48 h of exposure, respectively. The CH2Cl2:MeOH (2:1) extract from in vitro plants caused 95% mortality when doses of 0.1850 mg/mg/µL were too applied in 48 h of exposure. The mature spikes test best results were: LD50 0.001 mg/µL with EtOH and 0.007 mg/mg/µL with CH2Cl2:MeOH (2:1) and LD90 0.027 mg/µL with EtOH and 0.103 mg/µL with CH2Cl2MeOH (2:1); and, in the case of in vitro plants, only CH2Cl2:MeOH (2:1) extract was: LD50 0.003 mg/µL and LD90 0.060 mg/µL. The potential value of extracts derived from P. tuberculatum as efficient insecticides against S. frugiperda is discussed.
Key words: CH2Cl2:MeOH (2:1) extract. EtOH extract. In vitro propagation. Larval susceptibility. Lethal Dosis.
Resumen: Se evaluó la actividad larvicida del "matico" neotropical Piper tuberculatum. Los compuestos secundarios fueron extraídos de hojas, tallos y espigas maduras (con frutos y semillas) de plantas silvestres y de plantas in vitro de P. tuberculatum. Fue evaluada la toxicidad aguda de extractos de espigas maduras con frutos y semillas y de plantas in vitro de P. tuberculatum sobre el "cogollero" Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae) mediante bioen-sayos de contacto. Solamente extractos Diclorometano:metanol (CH2Cl2:MeOH) (2:1) y etanólicos (EtOH) de espigas maduras y el extracto CH2Cl2:MeOH (2:1) de plantas in vitro mostraron niveles significativos de mortalidad. Los extractos CH2Cl2:MeOH (2:1) y EtOH de espigas maduras causaron 90% de mortalidad cuando dosis de 0,1850 mg/µL se aplicaron sobre S. frugiperda en 24 y 48 h de exposición, respectivamente. El extracto CH2Cl2:MeOH (2:1) de plantas in vitro causó 95% de mortalidad cuando dosis de 0,1850 mg/µL también se aplicaron en 48 h de exposición. Los mejores resultados para las espigas maduras fueron: CL50 0,001 mg/µL con EtOH y 0,007 mg/µL con CH2Cl2:MeOH (2:1) y CL90 0,027 mg/µL con EtOH y 0,103 mg/µL con CH2Cl2:MeOH (2:1); y en el caso de las plantas in vitro, solamente el extracto CH2Cl2:MeOH (2:1) fue: CL50 0,003 mg/µL y CL90 0,060 mg/µL. Se discute el valor potencial de los extractos de P. tuberculatum como un eficiente insecticida sobre S. frugiperda.
Palabras clave: Extracto CH2Cl2:MeOH (2:1). Extracto EtOH. Propagación in vitro. Susceptibilidad larval. Concentración letal.
Introduction
Spodoptera frugiperda (J. E. Smith, 1797) (Lepidoptera: Noctuidae), the fall armyworm, is a polyphagous insect of enormous agricultural importance, not only because of the damage it provokes, but also because of control difficulties (Santos et al. 2003). The species is a migratory pest endemic to the Western Hemisphere that occurs from Southern Canada to Argentina and causes considerable economic losses in several important crops such as maize, sorghum, rice, cotton, alfalfa, forage grasses, and occasionally other crops in most of the countries of its range (Clark et al. 2007). In its distribution area, two genetically distinct strains are found that differed in their plant host distribution (Pashley 1986); one strain feeds primarily on maize and sorghum (corn strain), and the other strain feeds on rice and bermuda grass (rice strain) (Pashley et al. 1985; Pashley 1986).
The indiscriminate use of synthetic insecticides has caused environmental contaminations and toxicity to living organisms (Nakata et al. 2005), indicating the need for the development of products that not hazardous to the environment, target-specific and biodegradable. Thus, the development of new insecticides from plant extracts sources can be an alternative for the control of Spodoptera bugs.
Species of different plant families and their derived products have received increased attention from scientists and more than 2000 plant species are already known to have insecticide properties (Sukamar et al. 1991). Among these families the Piperaceae family has been investigated, especially the species of the genus Piper (Marquis 1991; Bernard et al. 1995). More than 15 species of Piper have been reported in the literature to have insecticidal activity (Bernard et al. 1995; Parmar et al. 1997). For example, the Amazonian species, Piper rotundistipulum (Trel. and Yunck., 1950), is locally used as insecticide and fish poison (Schultes and Raffauf 1990). Piper guineense (Schumach., 1827) and Piper nigrum (Linnaeus, 1753) are used as insecticide and molluski-cide in several parts of Africa (Ivbijaro and Bolaji 1990). The Indian species Piper longum (Linnaeus, 1753), Piper betle (Linnaeus, 1753), Piper peepuloides (Roxb., 1820) and Piper cubeba (Linnaeus, 1753) have demonstrated insecticidal activity against mosquitos and flies (Miyakado et al. 1989).
The benzene extract of the leaves of Piper futokatsura (Sieb., 1900) from Taiwan and Japan are known as a feeding deterrent to Spodoptera litura (Fabricius, 1775) larvae (Matsui and Munskata 1975), and the leaves of Piper umbellatum (Linnaeus, 1753), Piper hispidum (Sw., 1788), Piper auritum (Kunth, 1816), and others Piper spp., which are native to Central America and the Northwest Amazonian basin, are used by indigenous peoples to prevent malaria and removing head lice (Schultes 1980). Likewise, the leaf and stem petrol and dichloromethane extracts of Piper falconeri (C.DC., 1925) have shown insecticidal activity against Musca domestica (Linnaeus, 1758) (flies) and Aedes aegypti (Linnaeus, 1762) (mosquitoes); the Piper acutisleginum (DC., 1869) dichloromethane extract has also been reported to show insecticidal activity against M. domestica and A. aegypti (Parmar et al. 1997) and the dichloromethane and ethanolic extracts of spikes and in vitro plants of Piper tuberculatum (Jacq., 1795) have shown insecticidal activity against Diatraea saccharalis (Fabricius, 1794) (Soberón et al. 2006).
In addition, natural insecticides such as pyrethrum, rote-none and nicotine, among others, have been extensively used until recently; these substances can affect the feeding behavior and growth regulators, disrupting the insect hormonal balances (Balandrin et al. 1985). An example is azadirachtin, a biopesticide obtained from the neem tree Azadirachta indica (A. Juss., 1830), which could be readily biodegradable, selective, non-mutagenic, with low toxicity to mammals, and causes minimal effects on the environment (Gupta 2004); however, this substance is very expensive, it cannot be synthesized chemically and has to be purified, using costly and sophisticated methods, from large quantities of a seasonally produced seed (Allan et al. 1999). An alternative are the plant tissue culture methods. In effect, plant cell cultures have been actively studied as a potential source of high-value biological compounds (Edahiro and Seki 2006). The major advantages of a cell culture system over the conventional cultivation of whole plants are: useful compounds can be produced under controlled conditions independent of climatic changes or soil conditions; cultured cells would be free of microbes and insects; the cells of any plants could easily be multiplied to yield their specific metabolites; automated control of cell growth and rational regulation of metabolite processes would reduce of labor costs and improve productivity (Vanisree et al. 2004).
Chemical studies carried out on Brazilian Piperaceae species have revealed the occurrence of pyrones, lignoids and chromenes besides various amides bearing isobutyl, pyrro-lidine, dihydropyridone and piperidine moieties (Parmar et al. 1997; Baldoqui et al. 1999; Navickiene et al. 2000; da Silva et al. 2002). These amides have generated interest as a result of their potent insecticidal and antifungal properties (Miyakado et al. 1989; Bernard et al. 1995; Navickiene et al. 2000; da Silva et al. 2002). P. tuberculatum known as "matico" or "cordoncillo", a species quite abundant in the West Indies and it is widely distributed from Brazil to Mexico, has been described seven isolated amide structures from seedswith CH2Cl2:MeOH (2:1) (Navickiene et al. 2000). These amides, are active against the fungus Cladosporium clado-sporioides (Fres., de Vries, 1952) ranged from 5.0 to 10.0 ug (da Silva et al. 2002) and Cladosporium sphaerospermum (Penz., 1882) ranged from 0.1 to 5.0 ug (Navickiene et al. 2000).
The objective of this research was to investigate the insecticidal activity of extracts of leaves, stems and mature spikes, with fruits and seeds, of wild plants and in vitro plants of P. tuberculatum on third instar larval of S. frugiperda.
Materials and Methods
Plant material. Spikes with mature seeds, leaves and stems of P. tuberculatum were collected in November 2003 from 'Cumbil' river (Lambayeque, Peru). Botanical identification was performed by Doctor Guillermo E. Delgado from Universidad Nacional Pedro Ruiz Gallo (UNPRG) based on taxonomic description realized by Yuncker (1973). The botanic specimen vouchers were deposited at same herbarium of the institution (HPR).
In vitro micropropagated plants. The culture was initiated from axenic seedlings explants. A total of 50 seeds per flask were surface-sterilized by 70% ethanol (v/v) 1 min followed by 2-2.5% sodium hypochlorite (w/v) for 20 min and then washed three times with sterile water. Floating seeds were discarded; about 3-10 of them were transferred to glass test tubes containing 20 mL of MS medium (Murashige and Skoog 1962) and 2% sucrose. Shoot-tip and nodal segments, 1 cm length containing a lateral bud, taken from three-month-olds in vitro seedlings, were used as explant source. MS medium, supplemented with 0.02 mg/L indoleacetic acid (IAA), 0.02 mg/L gibberellic acid (GA3) and 3% sucrose was used to initiate cultures, and were maintained by subculturing every six months on a fresh medium containing the same formulation. In all cultures, the same MS medium was supplemented with 100 mg/L m-inositol and 1 mg/L thiamine.HCl, adjusted to pH 5.7 ± 0.1, solidified with "Phytagel" 0.3% prior to autoclaving, dispensed into tubes (150 x 25 mm) containing 20 mL MS medium and covered with polypropylene caps. All cultures were incubated at 24-28°C in a 16-h light, 8 h dark photoperiod provided by cool white fluorescent tubes, with 5 umol m-2 s-1, for seed germination and 30 umol m-2 s-1 for clonal propagation.
Insects. Eggs of S. frugiperda were collected from a corn crop during its vegetative to early reproductive stage, in the Fundo La Peña, UNPRG - Lambayeque, Peru, and were reared in the Laboratorio de Entomología of the Facultad de Agronomía (UNPRG), under laboratory conditions. Insects were maintained in Petri dishes lined with damp filter paper (one fall armyworm per dish to avoid cannibalism) under a controlled environment (26 ± 2oC, 80 ± 5% relative humidity, 16:8 h light:dark photoperiod). Third instar larvae of S. frugiperda were fed to repletion with fresh leaves of maize.
Extraction of the constituents. Spikes, leaves and stems (45 g, respectively) of wild plants of P. tuberculatum were oven dried at 40°C, milled and submerged three times in CH2Cl2:MeOH (2:1) and EtOH 96%, respectively, at room temperature, yielding between 1.6 to 11.8% (0.72 to 5.31 g) of extract; likewise, in vitro micropropagated plants (9 g) yielding 6.3% (0.57 g) of extract with CH2Cl2:MeOH (2:1). In the case of extraction with boiling water, 10 g of dried spikes, leaves and stems were supplemented with 100 mL of destillated water and submitted to boiling (up to 100°C) by 10 min; the extracts obtained were evaporated at reduced pressure (45°C).
Topical test. Bioassays were carried out at in the Laboratorio de Entomologia of UNPRG. The stock solutions of extracts were prepared by dissolving 100 mg of dry extract in 1 mL of MeOH-water to obtain a concentration of 100 mg/mL. After 24 h, and using and Eppendorf® 0-10 µL pipette, 6.5 µL of the solution, containing an aliquot of each one of the treatments, was applied directly on the larval mesothorax of S. fru-giperda. The plant extract was tested at doses of 0.0, 0.0007, 0.0014, 0.0029, 0.0057, 0.0115, 0.0230, 0.0460, 0.920 and 0.1850 mg/µL. Twenty larvae were tested per treatment and the experiment was carried out twice. The control insects received a topic application with MeOH-water alone. Larval mortality was recorded at 24, 48 and 72 hour post-treatments, under the same conditions of temperature and humidity described above. The larvae were considered dead if they displayed no observable response to a mechanical stimulus, i.e. short-term pressure applied with a spatula.
A dose - response correlation was obtained using a linear regression model to fit the probit data to the log of the dose of each extract applied. LD50 and LD90 values were determined used the software US. EPA Probit Program Version 1.5 (2003).
Results
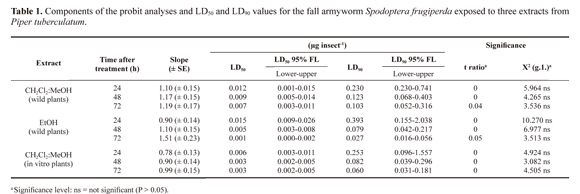
CH2Cl2:MeOH (2:1) and EtOH extracts of leaves and stems, and boiling water extracts of leaves, stems and spikes from wild plants of P. tuberculatum did not show larvicidal activity against the third instar larval of S. frugiperda tested at dose ranging from 0.0007 to 0.1850 mg/µL (data not shown).The response of fall armyworm to the topical applications of CH2Cl2:MeOH (2:1) and EtOH extracts from mature spikes of wild plants and CH2Cl2:MeOH (2:1) extract from in vitro plants of P. tuberculatum showed a positive relationship between dose and mortality. The responses varied with the time of exposure.
The resultant regression lines for all the extracts appeared to be very similar, showing a relatively fast intoxication process on the insects exposed to P. tuberculatum extracts. In a general way, the LD50 and LD90 values decreased when the time between application and evaluation increased (Table 1). The data presented confirm that mature spikes and in vitro plants extracts from P. tuberculatum presented potential insecticide activities.
The larval mortality at 90% was reached after 24 h when using 0.1850 mg/µL of CH2Cl2:MeOH (2:1) extract from mature spikes; and a mortality of 100% was reached with 0.1850 mg/µL EtOH extract from mature spikes in 72 h. In reference to the in vitro plants, the extract obtained with CH2Cl2:MeOH (2:1) alone generated a 95% larval mortality with 0.1850 mg/ µL in 48 h.The mortality of the control group was 0%.
The small variations in LD50 and LD90 values of both extracts with respect to time of exposure suggest a rapid toxic action. Similar to what happens with larvae of Anticarsia gemmatalis (Hubner, 1818) (Navickiene et al. 2007), almost immediately following the application of doses of each treatment, larval movement decreased and feeding practically ceased. Also, typical intoxication symptoms, as described by Marchini et al. (1992), such as spasmodic movements, regurgitation and faecal elimination, were observed, thus confirming the acute toxicity of these extracts to fall armyworm.
Discussion
Preliminary tests have demonstrated that CH2Cl2:MeOH (2:1) and EtOH extracts of leaves and stems, and boiling water extracts of leaves, stems and spikes from wild plants of P. tuberculatum did not show larvicidal activity against the third instar larval of S. frugiperda tested at dose ranging from 0.0007 to 0.1850 mg/µL. Results agree with dose reported in the control of third instar larval of D. saccharalis (Soberón et al. 2006) and second and third instar larval and adult stage of Aedes aegypti and Anopheles pseudopunctipennis Theobald, 1901(Bazán-Calderón et al. 2011); however, disagree with the results reported for extracts of leaves and stems of P. tuberculatum used in control of Aedes atropalpus (Coquillett, 1902) (Bernard et al. 1995) and A. gemmatalis (Navickiene et al. 2007). According to Scott et al. (2002; 2003) interplant differences related to the efficacy of extracts may be due to the large variability observed with the individual piperamide concentrations, especially 4,5-dihydropiperlonguminine, in leaves. It is also important to decide where and when plants should be collected to obtain material with the highest biological activity; in this case, the geographical region may not matter, but site-specific properties could affect piperamide levels: soils nutrients, microclimate and levels of herbivory. Thus, as is the case with Piper species, there can be a selective advantage in producing different compounds and, in addition, compounds that interfere with detoxification but are not metabolized, even if they themselves are not toxic (Navickiene et al. 2007).
The results confirm that CH2Cl2:MeOH (2:1) and EtOH extracts of mature spikes from wild plants and QLC^MeOH (2:1) extracts from in vitro plants of P. tuberculatum showed a potent insecticidal activity on third instar larval of this Lep-idoptera species. The CH2Cl2:MeOH (2:1) and EtOH extracts of mature spikes showed higher toxicity than CH2Cl2:MeOH (2:1) extract from in vitro plants and also EtOH extract was more effective than CH2Cl2:MeOH (2:1) extract.
The results suggest two possibilities for the direct use: insecticidal activity of mature spike EtOH extracts from P. tuberculatum that allows rural people to freely use the extract obtained with traditional alcoholic drinks as "aguardiente, yonque or cañazo". The second possibility, even thought the in vitro plant extracts showed lower toxicity than mature spike extracts, may be an active compound biosynthesis at large scale using the establishment of cellular suspensions (Danelutte et al. 2005).
Several studies have shown insecticidal activity of plants extracts against S. frugiperda. For instance, the antifeedant activity of Citrus-derived limonoids limonin, nomlin, and obacunone and their semisynthetic detrivaties, obtained from seeds of Citrus limonoides = C. limon (L., Burrm. F., 1768) (Ruberto et al. 2002); the insecticidal activity of crude etha-nolic seed extracts of Annona muricata (Linnaeus, 1753), A. squamosa (Linnaeus, 1753) (Annonaceae), Lansium domesticum (Correa, 1807) and Sandoricum koetjape (Merr., 1912) (Meliaceae) (Leatemia and Isman 2004a); and the potential use of Asteracae extracts to control S. frugiperda and selectivity to their parasitoids Trichogramma pretiosum (Riley, 1879) and Telenomus remus (Nixon, 1937) (de Souza Tavares et al. 2009). On the other hand, when added to the diet, Melia azedarach (Linnaeus, 1753) (Meliaceae) SLE (senescent leaves extracts) showed lower toxicity than Jatropha gossypifolia (Linnaeus, 1753) (Euphorbiaceae) SLE; however, after two weeks on the diet, the M. azedarach SLE proved lethal to 100 percent of the larval population; likewise, acute toxicity after topical application in a dipping assay was relatively low for both J. gossypifolia or M. azedarach SLEs (LC50 of 2.6 and 1.4 g L-1, respectively, after 24 h) (Bullangpoti et al. 2012). These results were lightly similar with the results obtained in our work. The insecticidal and insectistatic activities of the seed extract and the three main constituents, oleic, palmitic and stearic acids of Carica papaya (Linnaeus, 1753) (Caricaceae), were tested; larval viability values were 0%, 29.2%, and 50% when the seed extract was applied at 24,000, 16,000, and 9,600 ppm, respectively, and the larval viability of the main compounds was 33.3%, 48.5%, and 62.5% when exposed to 1,600 ppm of palmitic acid, oleic acid, or stearic acid, respectively (Pérez-Gutiérrez et al. 2011). In our study were obtained similar results with same doses. To determinate the insecticidal and insectistatic activities of methano, hexane and ethyl acetate extracts of the seeds and leaves of Ricinus communis (Linnaeus, 1753) (Euphorbiaceae), castor oil and ricinine were tested at different concentrations against S. frugiperda; the half maximum larvae viability concentration (LVC50) were 0.38 x 103 ppm for the ricinine, 0.75 x 103 ppm for a methanol extract of seeds, 1.97 x 103 ppm for an ethyl acetate seed extract and 2.69 x 103 ppm for the castor oil (Ramos-López et al. 2010), doses too very similar with the used in our study. In other work, of the 20 species tested, 7 showed mortality for caterpillars S. frugiperda: Petiveria alliacea (Linnaeus, 1753) (98%), Malva sylvestris (Linnaeus, 1753) (90%), Artemisia verlotorum (Lamotte, 1876) (90%), Baccharis genistelloides (Lam., Pers., 1807) (80%), Zengiber officinale (Rosc., 1807) (70%), Cymbopogon citratus (DC., Stapf, 1906) (60%) and Ruta graveolens (Linnaeus, 1753) (58%) (Tagliari et al. 2010); however, not were indicated the doses applied. Likewise, using fruits of Moringa oleifera (Lam., 1785) (Moringaceae), the highest total correct mortality percentage was recorded with the highest concentration of moringa oil (100%) and unsaponifiable matter (80.7%); it was concluded that moringa oils at 10% concentration could be applied as botanical insecticide to prevent the plants from S. frugiperda attack (Kamel 2010). These results not could be comparable with our work.
Only some species of Piper, of the flora of Peru, as Piper aduncum (Linnaeus, 1753), Piper aequale (Vahl., 1797), P. hispidum, Piper reticulatum (Linnaeus, 1753) and P. tuberculatum showed significative activity against the mosquito A. atropalpus (Bernard et al. 1995). In this paper was reported that 100 mg/L (0.1 mg/mL or 0.0001 mg/µL) hexanic crude extract of P. tuberculatum leaves presented most intense activity with 54% mortality in second instar larval of A. atropalpus, after 24 h of exposure; this mortality was attributed to isobutylamide 4,5-dihydropiperlonguminine (pure substance isolated from the active fraction) because caused 47% mortality of mosquito larvae at 0.01 mg/L in the same time of exposure. Comparing these results, in our work was showed that intermediate doses of extract, from mature spikes as 0.0115 mg/µL and 0.0230 mg/µL extract, from in vitro plants, produced a mortality exceeds 50% in S. frugiperda at 24 h of exposure.
In previous studies with seed extracts of P. tuberculatum were isolated several amides, mainly bearing isobutyl, pyrrolidine, dihydropyridone and piperidine moieties (Navickiene et al. 2000; da Silva et al. 2002). The antifungal activity of each amide was determined by direct bioautography against C. sphaerospermum and C. cladosporioides with 1- 5 µg and 5 - 10 µg, respectively, as the minimum quantity of compounds, specially piperine and 5,6-dihydropiperlongu-minine, necessary to inhibit growth of the fungus (Navickie-ne et al. 2000; da Silva et al. 2002). Likewise, extracts of P. tuberculatum were used in control of dermatophyte fungi Microsporum canis (Bodin, 1902), Microsporum gypseum (Bodin, Guiart & Grigorakis, 1907) and Trichopyton rubrum (Malmsten, 1845) (Palacios et al. 2009) therefore, these amides have shown to have a potent fungicidal activity as well as a inseciticidal activity as reported in the control of the third instar larval of D. saccharalis (Soberón et al. 2006) and control of II and III instar larval and adults of A. aegypti and A. pseudopunctipennis (Bazán-Calderón et al. 2010).
Recently, was evaluated the toxicity of extracts and two isobutyl amides (pellitorine and 4,5-dihydropiperlongumi-nine) from P. tuberculatum in velvetbean caterpillar, A. gem-matalis; the extracts caused 80% of mortality when doses higher than 800.00 µg insect-1 of extract of seeds, leaves and stems were administered; pellitorine and 4,5-dihydro-piperlonguminine showed 100% mortality at doses of 200 and 700 µg insect-1 respectively (Navickiene et al. 2007). In our work, the extracts caused 90 - 100% of mortality when doses of 600.00 - 1200.00 ug insect-1 (0.6 - 1.2 mg/6.5 uL) of CH2Cl2:MeOH (2:1) and EtOH extracts of mature spikes from wild plants and CH2Cl2:MeOH (2:1) extract from in vitro plants of P. tuberculatum were administered in 72 h of exposure.
This fact has a profound ecological significance since it presupposes an advantage to using plant extracts as a source of complex molecules that exhibit various bioactivities, raising the levels of toxicity in relation to chemically pure individual compounds, in addition to the risk of induce resistance (Bobadilla et al. 2005). It is knew, chemical studies on Piperaceae species have revelled the occurrence of various compounds as alkaloids, amides, propenylphenols, lignans, neolignans, terpenes, steroids, kawapyrones, piperolides, chalcones, dihydrochalcones, flavones, flavanones and miscellaneous compounds (Parmar et al. 1997).
Several of these compounds are known to synergize natural and synthetic insecticides (Bernard et al. 1995), for instance, the phenylpropanoid dillapiol, related to piperonyl butoxide, synergizes not only pyrethrins but also several carbamates and organochlorates (Parmar and Tomar 1983). Recently, has been proposed as work strategy the use of heterogeneous extracts of total plant biomass to induce a synergistic effect over some specific organism (Leatemia and Isman 2004b).
In this context Scott et al. (2002) demonstrated that the amides presents in P. tuberculatum plants has higher toxicity when combined in binary, tertiary and quaternary mixtures compared to single compounds or binary mixtures; one of the four amide compounds, 4,5-dihydropiperlonguminine, was the most toxic in mosquito larvae bioassays. Navick-iene et al. (2007) reported that seed extracts of P. tuberculatum may be more powerful than the pellitorine isolated, therefore, would be advisable the preferential use of crude extracts. There are no simple explanations for the observed differences in the efficacy of the whole extract from different parts of the plant and the isolated piperamides. Variations in the concentration of the insecticide compounds among the plant tissues suggest that varied selective pressures operate in the plants, and a great number of combinations of compositions can arise inside individuals in certain species (Jones and Firn 1991), which can provide a higher protection level to the plant against herbivores (Berenbaum and Zangerl 1996). Our results make it possible to conclude that Piper extracts may be good candidates for use in crop protection.
The action mechanism of the pellitorine, 4,5-dihidrop-iperlonguminina and other related compounds (piperamides) found in Piper species on third instar larval of S. frugiperda is not well known, however could be attributed the toxicity at the presence of methylenedioxyphenyl ring (MDP) in the molecular structure (Bernard et al. 1995; Scott et al. 2003), like just as reported to other compounds of similar structure at pipercida, guineensinamida, guineensina, pellitorine and kalecida isolated from P. guineense and very actives in the control of adults of M. domestica (Gbewonyo et al. 1993).
The first three amides were the most active against adults of M. domestica and each of these contain a MDP structural moiety. The 5,6-dihydropiperlonguminine, isolated from P. tuberculatum, also has an MDP ring and is the main component of the active fraction of the spikes extract (Navickiene et al. 2000). Greger (1988) pointed out that these types of amides, including olefinic and alkyl isobutylamides, are common to a restricted number of related plant families, namely the Piperaceae, Asteraceae, and Rutaceae. In all these families are abundant in the tropics particularly in the humid tropics in the case of the Piperaceae, where herbivory is a selective potent force.
In addition, the piperamides presents dual biological activities, being neurotoxic, affecting the activity of the central nervous system, and also as inhibitors of cytochrome P450 enzymes; these characteristics are too useful to plants of Piper genus as a defence strategy against herbivores (Navickiene et al. 2007).
In conclusion, P. tuberculatum is an abundant species and is semidomesticated in Peru where is used as a hedge plant. It is for the reasons that the potential use of this species as a source of botanical insect control material looks promising.
Acknowledgements
The authors are grateful to the Prof. Jorge Chanamé-Céspedes for the statistical analyses.
Literature cited
ALLAN, E. J.; STUCHBURY, T; MORDUE (LUNTZ), A. J. 1999. Azadirachta indica A. Juss. (Neem Tree): In vitro culture, micropropagation, and the production of azadirachtin and other secondary metabolites. pp. 11-41. In: Bajaj, Y. P. S. (Ed.). Biotechnology in Agriculture and Forestry 43. Medicinal and Aromatic Plants XI. Springer-Verlag, Berlin. [ Links ]
BALANDRIN, M. F.; KLOCKE, J. A.; WURTELE, E. S.; BOLLINGER, W. H. 1985. Natural plant chemicals: Source of industrial and medicinal materials. Science 228: 1154-1160. [ Links ]
BALDOQUI, D. C.; KATO, M. J.; CAVALHEIRO, A. J.; BOLZANI, V. DA S.; YOUNG, M. C. M.; FURLAN, M. 1999. A chromene and prenylated benzoic acid from Piper aduncum. Phytochemistry 51 (7): 899-902. [ Links ]
BAZÁN-CALDERÓN, J.; VENTURA-FLORES, R.; KATO, M. J.; ROJAS-IDROGO, C.; DELGADO-PAREDES, G. E. 2011. Actividad insecticida de Piper tuberculatum Jacq. sobre Aedes aegypti L. (Diptera: Culicidae) y Anophelespseudopunctipennis Tehobal (Diptera: Culicidae). Anales de Biología 33: 135-147. [ Links ]
BERENBAUM, M .R.; ZANGERL, A. R. 1996. Phytochemical diversity. Adaptation or random variation?. pp. 1-24. In: Beren-baum, M.R. (Ed.). Phytochemical Diversity and Redundancy in Ecological Interaction; Recent Advances in Phytochemistry. Plenum Press, New York. [ Links ]
BERNARD, C. B.; KRISHNAMURTY, H. G.; CHAURET, D.;DURST, T.; PHILOGÉNE, B. J. R.; SÁNCHEZ-VINDAS, I.; HASBURN, C.; POVEDA, L.; SAN ROMÁN, L.; ARNASON. J. T. 1995. Insecticidal defenses of Piperaceae from the neotropics. Journal of Chemical Ecology 21: 801-814. [ Links ]
BOBADILLA, M.; ZAVALA, F.; SISNIEGAS, M.; ZAVALETA,G.; MOSTACERO, J.; TARAMONA, L. 2005. Evaluación larvicida de suspensiones acuosas de Annona muricata Linnaeus "guanabana" sobre Aedes aegypti Linnaeus (Diptera, Culicidae). Revista Peruana de Biología 12: 145-152. [ Links ]
BULLANGPOTI, V.; WAJNBERG, E.; AUDANT, P.; FEYEREI-SEN, R. 2012. Antifeedant activity of Jatropha gossipyfolia and Melia azedarach senescent leaf extracts on Spodoptera frugiperda (Lepidoptera: Noctuidae) and their potential use as synergists. Pest Management Science, in press. [ Links ]
CLARK, P. L.; MOLINA-OCHOA, J.; MARTINELLI, S.; SKODA, S. R.; ISENHOUR, D. J.; LEE, D. J.; KRUMM, J. T.; FOSTER, J. E. 2007. Population variation of the fall armyworm, Spodoptera frugiperda, in the Western Hemisphere. Journal of Insect Science 7: 1-10. [ Links ]
DANELUTTE, A. P.; COSTANTIN, M. B.; DELGADO, G. E.; BRAZ-FILHO, R.; KATO, M. J. 2005. Divergence of secondary metabolism in cell suspension cultures and differentiated plants of Piper cernuum and P. crassinervium. Journal of the Brazilian Chemical Society 16 (6B): 1425-1430. [ Links ]
DA SILVA, R. V.; NAVICKIENE, H. M. D.; KATO, M. J.; BOLZANI, V. DA S.; MÉDA, C. I.; YOUNG, M. C. M.; FURLÁN, M. 2002. Antifungal amides from Piper arboreum and Piper tuberculatum. Phytochemistry 59: 521-527. [ Links ]
DE SOUZA TAVARES, W.; CRUZ, I.; PETACCI, F.; DE ASSIS Jr., S. L.; DE SOUZA FREITAS, S.; ZANUNCIO, S. C.; SERRAO, J. E. 2009. Potential use of Asteraceae extracts to control Spodoptera frugiperda (Lepidoptera: Noctuida) and selectivity to their parasitoids Trichogramma pretiosum (Hymenoptera: Trichogrammidae) and Telenomus remus (Hymenoptera: Scelionidae). Industrial Crops and Products 30: 384-388. [ Links ]
EDAHIRO, J-I.; SEKI, M. 2006. Phenylpropanoid metabolite supports cell aggregate formation in strawberry cell suspension culture. Journal of Bioscience and Bioengineering 102: 8-13. [ Links ]
GBEWONYO, W. S. K.; CANDY, D. J.; ANDERSON, M. 1993. Structure-activity relationships of insecticidal amides from Piper guineense root. Pesticide Science 37: 57-66. [ Links ]
GREGER, H. 1988. Comparative phytochemistry of the alkamides. pp. 159-178. In: Lam, J.; Breteler, H.; Arnason H.; Hansen, I. (Eds.). Chemistry and Biology of Naturally Occurring Acetylenes and Related Compounds, Bioactive Molecules. Vol. 7. Elsevier, Amsterdam. [ Links ]
GUPTA, P. K. 2004. Pesticide exposure - Indian scene. Toxicology 198: 83-90. [ Links ]
IVBIJARO, M. F.; BOLAJI, O. O. 1990. Effects of cypermethrin + dimethoate and extracts of P. guineense and Azadirachta indica on the pests and yield of cowpea, Vigna unguiculata. Journal Agriculture Science 115: 227-231. [ Links ]
JONES, C. G.; FIRN, R. D. 1991. Evolution of plant secondary chemical diversity. The Philosophical Transactions of the Royal Society 333: 273-280. [ Links ]
KAMEL, A. M. 2010. Can we use the moringa oil as botanical insecticide against Spodoptera frugiperda? Academic Journal of Entomology 3 (2): 59-64. [ Links ]
LEATEMIA, J. A.; ISMAN, M. B. 2004a. Insecticidal activity of crude seed extracts of Annona spp., Lansium domesticum and Sandoricum koetjape against lepidopteran larvae. Phytoparasitica 32: 30-37. [ Links ]
LEATEMIA, J.; ISMAN, B. 2004b. Toxicity and antifeedant activity of crude seed extracts of Annona squamosa (Annonaceae) against lepidopteran pests and natural enemies. International Journal Tropical Insect Science 24: 150-158. [ Links ]
MARCHINI, L. C.; ALVES, S. B.; NAKANO, O. 1992. Sericultura. Curso de Entomología Aplicada à Agricultura. FEALQ, Piracicaba, Brazil, pp. 705-736. [ Links ]
MARQUIS, R. J. 1991. Herbivore fauna of Piper (Piperaceae) in a Costa Rican wet forest: Diversity, specificity, and impact. pp. 179-208. In: Price, P. W.; Lewinsohn, T. M.; Fernandes, G. W.; Benson, W. W. (Eds.). Plant-Animal interactions: Evolutionary ecology in tropical and temperate regions. John Wiley and Sons, New York. [ Links ]
MATSUI, K.; MUNSKATA, K. 1975. The structure of piperenone, a new insect antifeeding substance from Piperfutokadzura. Tetrahedron Letters 10: 1905-1908. [ Links ]
MIYAKADO, M.; NAKAYAMA, I.; OHNO, N. 1989. Insecticidal unsaturated isobutylamides: From natural products to agro-chemical leads. pp. 183-187. In: Arnason, J. T.; Philogène, B. J. R.; Morand, P. (Eds.). Insecticides of plant origin. ACS Symposium Series 387, American Chemical Society, New York. [ Links ]
MURASHIGE, T.; SKOOG, F. 1962. A revised medium for rapid growth an bioassays with tissue culture. Physiology Plantarum 15: 473-497. [ Links ]
NAKATA, H.; HIRAKAWA, Y.; KAWAZO, M.; NAKABO, T.;ARIZONO, K.; ABE, S. I.; KITANO, T.; SHIMADA, H.; WATANABE, I.; LI, W.; DING, X. 2005. Concentrations and compositions of organochlorine contaminants in sediments, soils, crustaceans, fishes and birds collected from Lake Tai, Hangzhou Bay and Shangai city region. China Environmental Poly 133: 415-429. [ Links ]
NAVICKIENE, H. M. D.; ALÉCIO, A. C.; KATO, M. J.; BOL-ZANI, V. DA S.; YOUNG, M. C. M.; CAVALHEIRO, A. J.; FURLAN, M. 2000. Antifungal amides from Piper hispidum and Piper tuberculatum. Phytochemistry 55: 621-626. [ Links ]
NAVICKIENE, H. M. D.; MIRANDA, J. E.; BORTOLI, S. A.; KATO, M. J.; BOLZANI, V. DA S.; FURLAN, M. 2007. Toxicity of extracts and isobutyl amides from Piper tuberculatum: potent compounds with potential for the control of the velvetbean caterpillar, Anticarsia gemmatalis. Pest Management Science 63: 399-403. [ Links ]
PALACIOS, Z. G. F.; DELGADO, G. E.; MORENO, M. C.; KATO, M. J.; ROJAS, C. 2009. Actividad antifúngica in vitro de extractos crudos de Piper tuberculatum. Revista Peruana de Biología 16: 209-214. [ Links ]
PARMAR, B. S.; TOMAR, S. S. 1983. Review of research on insecticide synergists in India - retrospect and prospect. International Journal of Tropical Agricultural 1: 7-17. [ Links ]
PARMAR, V. S.; JAIN, S. C.; BISHT, K. S.; JAIN, R.; TANEJA, P.; JHA, A.; TYAGI, O. D.; PRASAD, A. K.; WENGEL, J.; OL-SENl, C. E.; BOLL, P. M. 1997. Phytochemistry of the genus Piper. Phytochemistry 46: 597-673. [ Links ]
PASHLEY, D. P. 1986. Host associated genetic differentiation in fall armyworm (Lepidoptera: Noctuidae): a sibling species complex?. Annals of the Entomological Society of America 79: 898904. [ Links ]
PASHLEY, D. P.; JOHNSON, S. J.; SPARKS, A. N. 1985. Genetic population structure of migratory moths: the fall armyworm (Lepidoptera: Noctuidae). Annals of the Entomological Society of America 78: 756-762. [ Links ]
PÉREZ-GUTIÉRREZ, S.; ZAVALA-SÁNCHEZ, M. A.; GONZÁLEZ-CHÁVEZ, M. M.; CÁRDENAS-ORTEGA, N. C.; RAMOS-LÓPEZ, M. A. 2011. Bioactivity of Carica papaya (Caricaceae) against Spodoptera frugiperda (Lepidoptera: Noctuidae). Molecules 16: 7502-7509. [ Links ]
RAMOS-LÓPEZ, M. A.; PÉREZ, S.; RODRÍGUEZ-HERNÁNDEZ, C.; GUEVARA-FEFER, P.; ZAVALA-SÁNCHEZ, M. A. 2010. Activity of Ricinus communis (Euphorbiaceae) against Spodoptera frugiperda (Lepidoptera: Noctuidae). African Journal of Biotechnology 9: 1359-1365. [ Links ]
RUBERTO, G.; RENDA, A.; TRINGALI, C.; NAPOLI, E. M.; SIMMONDS, M. S. J. 2002. Citrus limonoides and their semi-synthetic derivatives as antifeedant agents against Spodoptera frugiperda larvae. A structure-activity relationship study. Journal of Agricultural and Food Chemistry 50: 6766-6774. [ Links ]
SANTOS, L. M.; REDAELLI, L. R.; DIEFENBACH, L. M. G.; EFROM, C. F. S. 2003. Larval and pupal stage of Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) in sweet and field corn genotypes. Brazilian Journal of Biology 63: 627-633. [ Links ]
SCHULTES, R. F. 1980. De plantis toxicariis e mundo novo com-mentationes XXVI. Ethnopharmacological notes on the flora of northwestern South America. Botanical Museum Leaflet of Harvard University 28: 1-45. [ Links ]
SCHULTES, R. F.; RAFFAUF, R. F. 1990. Medicinal and toxic plants of the Indians of northwest Amazonia, Piperaceae. pp. 362-368. In: Schultes, R. E.; Raffauf, R. F. (Eds.). The Healing forest: medicinal and toxic plants of the northwest Amazonia. Historical, Ethno- & Economic Botany Series. Vol. 2. Di-oscoride Press, Portland, Oregon. [ Links ]
SCOTT, I. M.; PUNIANI, E.; DURST, T.; PHELPS, D.; MERALI, S.; ASSABGUI, R. A.; SÁNCHEZ-VINDAS, P.; POVEDA, L.; PHILOGÉNE, B. J. R.; ARNASON, J. T. 2002. Insecticidal activity of Piper tuberculatum Jacq. extracts: synergistic interaction of piperamides. Agricultural and Forest Entomology 4: 137-144. [ Links ]
SCOTT, I. M.; JENSEN, H.; SCOTT, J. G.; ISMAN, M. B.; ARNASON, J. T.; PHILOGÉNE, B. J. R. 2003. Botanical insecticides for controlling agricultural pests: piperamides and the Colorado potato beetle Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae). Archives of Insect Biochemistry and Physiology 54: 212-225. [ Links ]
SOBERÓN, G. V.; ROJAS, C.; SAAVEDRA, J.; KATO, M. J.; DELGADO, G. E. 2006. Acción biocida de plantas de Piper tuberculatum Jacq. sobre Diatraea saccharalis (Lepidóptera, Pyralidae). Revista Peruana de Biologia 13: 107-112. [ Links ]
SUKAMAR, K.; PERICH, M. J.; BOOBAR, L. R. 1991. Botanical derivatives in mosquito control: a review. The Journal of the American Mosquito Control Association 7: 210-237. [ Links ]
TAGLIARI, M. S.; KNAAK, N; FIUZA, L. M. 2010. Efeito de extractos de plantas na mortalidade de lagartas de Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae). Arquivos do Instituto Biológico de Säo Paulo. 77: 259-264. [ Links ]
US Environmental Protection Agency (EPA). Probit program version 1.5. 2003. Ecological Monitoring Research Division. Environmental Monitoring Systems Laboratory. U.S. Environmental Protection Agency Cincinnati, OH. USA. [ Links ]
VANISREE, M.; LEE, C. Y.; NALAWADE, S. M.; LIN, C. Y.; TSAY, H. S. 2004. Studies on the production of some important secondary metabolites from medicinal plants by tissue culture. Botanical Bulletin of Academia Sinica 45: 1-22. [ Links ]
YUNCKER, T. G. 1973. The Piperaceae of Brazil II: Piper - Group V; Ottonia, Pothomorphe; Sarcorhachis. Hoehnea 3: 29-284. [ Links ]