Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488
Rev. Colomb. Entomol. vol.38 no.1 Bogotá Jan./June 2012
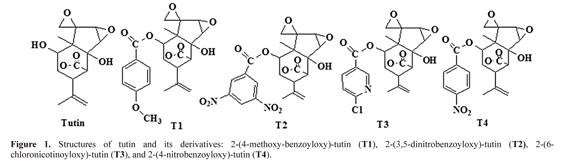
Effects of tutin and its derivatives on GAD and GABA-T in Pseudelatia separata (Lepidoptera: Noctuidae)
Efectos de tutin y sus derivados en el GAD y GABA-T en Pseudelatia separata (Lepidoptera: Noctuidae)
WEIWEI DU1, XIAONING NAN2 and MENGLOU LI3
1 M. Sc. Entomology of Forest Protection. College of Forestry, Northwest A & F University, No.3 Taicheng Road, Yangling, Shaanxi, 712100, China.2 M. Sc. Entomology of Forest Protection. College of Forestry, Northwest A & F University, No. 3 Taicheng Road, Yangling, Shaanxi, 712100, China.
3 B. Sc. Entomology of Forest Protection. College of Forestry, Northwest A & F University, No. 3 Taicheng Road, Yangling, Shaanxi, 712100, China. Telephone: +86 29 87082125; Fax: +86 29 87082125.limenglou@hotmail.com. Corresponding author.
Received: 19-sep-2011 - Accepted: 9-may-2012
Abstract: Tutin inhibits the receptors of the neurotransmitter γ-aminobutyric acid (GABA). In this work, we compared tutin with its derivatives, 2-(4-methoxybenzoyloxy)-tutin (T1), 2-(3,5-dinitrobenzoyloxy)-tutin (T2), 2-(6-chloronicotinoyloxy)-tutin (T3), and 2-(4-nitrobenzoyloxy)-tutin (T4), in terms of their effects on feeding and the feedback regulation mechanism of GABA metabolism. Spectrophotometry was used to determine the glutamic acid decarboxylase (GAD) and γ-aminobutyric acid transaminase (GABA-T) activities of these compounds in 3rd instar larvae of Pseudelatia separata. Clear antifeedant activities were manifested by T1 and especially T3, whereas T4 stimulated feeding in P. separata. GAD and GABA-T activities in larvae treated with all five toxins differed significantly from the control and from one other (P < 0.05) in terms of treatment types and exposure times. The effects of the toxins on GAD and GABA-T were time-dependent for 48 h. GAD activities were inhibited by tutin, T2, T3, and T4, and were enhanced by T1. GABA-T activities were increased by all the toxins in varying degrees. Variation of GABA content in P. separata larvae resulted from the disturbance of GAD and GABA-T by tutin and its derivatives. Results suggest that T3, with its nicotinoyl group, is the most promising novel active ingredient for pest control.
Key words: Antifeedant activity. Corlaría sinica. γ-aminobutyric acid (GABA). Spectrophotometry. Metabolism.
Resumen: La tutina inhibe los receptores del neurotransmisor acido γ-aminobutírico (GABA). En esta publicación se compara la tutina con sus derivados, 2-(4-metoxibenzoiloxi)-tutina (T1), 2-(3,5-dinitrobenzoyloxy)-tutina (T2), 2-(6-chloronicotinoyloxy)-tutina (T3), y 2-(4-nitrobenzoiloxi)-tutina (T4), en términos de sus efectos sobre la alimentación y el mecanismo de regulación de la retroalimentación del metabolismo del GABA. Se usó espectrofotometría para determinar la actividad de descarboxilasa del ácido glutámico (GAD) y γ-aminobutírico ácido transaminasa (GABA-T) en las larvas del tercer estadio de Pseudelatia separata. Actividades antialimentarias se manifiestan en T1 y especialmente en T3, mientras que T4 estimula la alimentación en P. separata. GAD y GABA-T difieren significativamente en las actividades de las larvas tratadas con las cinco toxinas, tanto en el control como ente ellas (P < 0.05) en cuanto a tipos de tratamiento y tiempos de exposición. Los efectos de las toxinas en el GAD y GABA-T dependieron del tiempo durante 48 h. Las actividades de GAD fueron inhibidos por la tutina, T2, T3 y T4, y se mejoraron con T1. Las actividades de GABA-T se incrementaron en todas las toxinas en diferentes grados. Variación del contenido de GABA en larvas de P. separata resultó de la alteración de GAD y GABA-T por tutina y sus derivados. Los resultados sugieren que T3, con su grupo nicotinoíl, es el nuevo ingrediente activo más prometedor para el control de plagas.
Palabras clave: Actividad antialimentaria. Coriaria sinica. y-Ácido aminobutírico (GABA). Espectrofotometría. Metabolismo.
Introduction
Tutin, a toxic compound of sesquiterpene extracted from Coriaria sinica Maxim (Coriariaceae: Coriaria), has neurotoxic, gastrotoxic, and antifeedant effects in many insects, and inhibits pathogenic microorganisms, such as Botryosphaeria berengeriana Notaris. f. sp. piricola (Nose, 1933) Kogane-zawa and Sakuma (1984), Alternaria macrospora Zimm., 1904, Phytophthora infestans (Mont.) de Bary (1845), and Pseudomonas solanacearum (Smith, 1896) (Zhang et al. 2006; Zhou et al. 2006). We therefore hypothesized that the use of tutin or its derivatives as a new pesticide warrants further study. We previously synthesized three derivatives (Li et al. 2007) that had higher antifeedant activities than tutin; however, these were still insufficient for adequate pest control. In the present study, we synthesized four new tutin derivatives based on our previous laboratory studies. Previous research has shown tutin to be an inhibitor of γ-aminobutyric acid (GABA) receptors, which are nerve-muscle synapse inhibitory neurotransmitters in crustaceans and insects (Li et al. 2000a, 2000b, 2003).
When tutin acts on GABA receptors, the biosynthesis of GABA is affected (Nistri et al. 1997). The molecular structure of GABA is transformed in rats treated with tutin, leading to the accumulation of GABA because it cannot normally combine with its receptors. Thus, GABA biosynthesis and metabolism are affected (Schousboe et al. 1973; Erecinska et al. 1996; Sze 1997). These findings indicate that tutin can affect GABA biosynthesis in the physiological metabolism of organisms via feedback regulation. The research of Deewat-thanawong et al. (2010) on GABA contents in CO2-treated tomatoes showed that GABA influences enzymatic activities during GABA biosynthesis.
Feedback regulation generally exists in the physiological metabolism of organisms. Shi et al. (2011) studied breast cancer cells and proved that the (32-adrenergic receptor and human epidermal growth factor receptor 2 comprise a positive feedback loop. Many enzymatic reactions in the process of cholesterol biosynthesis feature feedback regulation; an increase in cholesterol concentration causes a decrease in hydroxymethylglutaryl coenzyme A concentration, and vice versa (Sever et al. 2003).
Given the findings above, we hypothesize that the inhibition of GABA receptors by tutin will influence GABA contents and even GABA biosynthesis enzymes because of feedback regulation. This has not yet been confirmed in any previous study. GABA is involved in many metabolic activities, and its metabolic bypass is a branch of the citric acid cycle. Glutamic acid decarboxylase (GAD) and γ-aminobutyric acid transaminase (GABA-T) are the GABA synthesizing and metabolizing enzymes, respectively (Sherif and Ahmed 1995). In the metabolic process, glutamate generates GABA by the catalysis of GAD, and GABA generates succinic semialdehyde by the catalysis of GABA-T (Zhu et al. 2007). Therefore, GABA content is influenced by GAD and GABA-T activities. The object of this study was to assayed GAD and GABA-T activities in Pseudelatia separata (Walkers, 1865) (Lepidoptera: Noctuidae) to compare tutin with its derivatives in terms of their effects on GABA metabolism. P. separata is one of the most important crop pests in China and other countries in Asia. It can cause great threat on Agricultural production and food security. Cui et al. (2007) studied the toxicity of tutin with P. separata in the former researches too. We then used the results of these assays as a basis for selecting an optimal pesticide amongst rutin's derivatives.
Materials and Methods
Test insects. P. separata, were reared on fresh wheat leaves at the Pesticide Research Center, Northwest Agriculture and Forestry University, Yangling, Shaanxi, China. As normally done, two days after molting, third instar larvae with similar body sizes were selected for the experiments.
Toxins. Tutin was extracted with 95% alcohol by ultrasonic extraction method from dry seeds of Coriaria sinica Maxim in Integrated Pest Control Laboratory which locates on College of Forestry, Northwest A & F University. Then the extract was concentrated by rotary evaporation instrument and pure tutin was separated by chromatography (Cui 2007). The benzoyl derivatives of tutin 2-(4-methoxybenzoyloxy)- tutin (T1), 2-(3,5-dinitrobenzoyloxy)-tutin (T2), 2-(6-chloronicotinoyloxy)- tutin (T3), and 2-(4-nitrobenzoyloxy)-tutin (T4) were respectly synthesized by change 2-hydroxy group of tutin with 4 - methoxy benzoyl chloride, 3,5-dinitrobenzoyl chloride, 6 - chlorine chloride and 4-nitrobenzoyl chloride (Fig. 1).
Chemicals. Analytically pure acetone, p-mercaptoethanol, phosphate buffer (NaH2PO4.2H2O-Na2HPO4.12H2O), borate buffer (H3BO3-NaOH), phenol, and sodium hypochlorite solution were used. GABA, L-glutamic acid, 5-pyridoxal phosphate, NAD, and α-ketoglutarate were purchased from WOLSEN.
Determination of antifeedant activities. For the toxin sample solutions, tutin and its derivatives were dissolved in acetone to a final concentration of 2.0 mg/mL following Guo et al. (2009). Acetone was used as the control.
The nonrestrictive leaf-disc method was used to determine the antifeedant activities of the compounds (Mu 1997). Fresh young wheat leaves were cut into round leaf-discs 10 mm in diameter, and each leaf-disc was evenly coated with 5 µL of the sample solution on one side. Once the acetone had evaporated, the infected leaf-discs were ready for testing. A piece of qualitative filter paper with diameter 9 cm was then laid at the bottom of 9 cm-diameter Petri dishes and tap water was sprayed onto it to maintain the relative humidity at 60-90%. We used a total of 30 Petri dishes and 180 test larvae. Every five Petri dishes were grouped as one, and each dish placed six 3rd instar larvae starved for 12 h and thirty infected leaf-discs were placed.
Each of the groups of five dishes in a group was separately treated with the infected leaf-discs of control, tutin, Tl, T2, T3, or T4 for 4, 8, 12, 24, and 48 h. The eaten areas of the leaves were measured with quadrille paper (Feng and Shi 2006) after 4, 8, 12, 24, and 48h of incubation at 18-22°C and light-dark cycling with L:D = 12:12h. Each experiment was repeated three times. Water and the corresponding infected leaf-discs were replenished and recorded in the process. Anti-feedant activity was measured by calculating the antifeedant rate: Antifeedant rate = [(eaten area of control - eaten area of toxin) / eaten area of control] x 100%.
Preparation of crude enzymes. Every group of six test larvae was weighed after 4, 8, 12, 24, and 48 h of treatment. The larvae, approximately 20mg quartz sand, and phosphate buffer (1 mL, pH 7.0, 0.04 M) were then homogenized in an ice bath using a pestle. The homogenate was transferred to a centrifuge tube (2.0 mL), along with the washing liquor from and phosphate buffer to a volume of 2.0 mL. The tube was centrifuged at 10000 rpm for 15 min at 4°C (Eppendorf centrifuge, 5415R, Germany) (Jayakumar et al. 1999). The supernatant was then recovered as crude enzyme and stored at 4°C until use.
Determination of GAD activity. Determination of GAD activity was based on the principles of the reaction of Berthelot (Xu et al. 2004; Tao et al. 2006). The crude enzyme (Vo = 0.2 mL) and solution I composed by 0.2 mL, pH 7.0 phosphate buffer containing 10 mM L-glutamic acid and 0.1 mM 5-pyridoxal phosphate were placed into a test tube (10 mL, polypropylene) and incubated at 30°C for 30 min. The incubated mixture was placed in an ice bath, after which borate buffer (0.4 mL, 0.2 M) was added to terminate the reaction. Phenol (2 mL, 6%) and sodium hypochlorite solution (0.4 mL, containing 10% active chlorine) were also added to finish the color reaction. The mixture was mixed thoroughly by hand, placed in a hot water bath (100°C) for 10 min, and then rapidly cooled in an ice bath for 20 min. The absorbance of the solution at 630 nm was then measured and recorded using a 752N type UV-VIS spectrophotometer (Shanghai Precision and Scientific Instrument CO., LTD). GAD measurements were performed in triplicates for each crude enzyme extract. GAD activity was expressed as absorbance per unit insect weight, as indicated in the formula: GAD activity (U/g) = A630 /(FW x Vo/V), where FW = larvae weight (g), Vo = the determining volume of crude enzyme (mL), and V = total volume of crude enzyme (mL). The GAD relative activity, which shows the relative effect between the toxins and the control, was calculated using the formula: GAD relative activity = [(average GAD activity of toxin - average GAD activity of control) / average GAD activity of control] x 100% (Xu et al. 2004; Tao et al. 2006).
Determination of GABA-T activity. We followed the procedure outlined by Niladri et al. to determine GABA-T activity (Basu et al. 2010). Crude enzyme (Vo = 0.3 mL) was added to phosphate buffer (3.5 mL, 100 mM, pH 8.75, containing 1mM NAD and 3.5 x 10-2 mM β-mercaptoethanol). The mixture was shaken and solution II (0.2 mL, containing 4.8 mM α-ketoglutarate and 18mM GABA, pH 7.0) was added. The mixture was incubated at 30°C for 30 min, after which its absorbance at 340 nm was measured and recorded using a 752N type UV-VIS spectrophotometer (de Graaf et al. 2006; Suzuki et al. 2009; Li et al. 2007). GABA-T measurements were performed in triplicates for each crude enzyme extract. GABA-T activity was presented as absorbance per unit insect weight, as indicated in the formula: GABA-T activity (U/g) = A340/(FW x Vo /V). GABA-T relative activity was calculated in the same manner as the GAD relative activity.
Data analysis. Analyses of variance were used to assess the effects of toxins and time (independent variables) on GABA shunt enzymes (dependent variables), and Duncan's multiple range tests (SSR method) were used to determine pair-wise differences. The critical level of significance for all statistical analyses was set at α = 0.05 using SPSS (Statistical Product and Service Solutions) version 13.0. Graphs were drawn using Originlab version 8.0 software.
Results
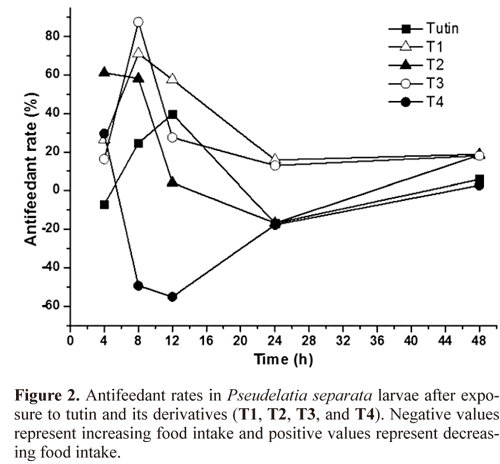
Antifeedant activity. All the antifeedant activities of P. separata treated with actone and toxins were similar in the 48 h. The antifeedant activities of P. separata treated with T1, T2, and T3 were higher than that treated with tutin, whereas T4 had the lowest antifeedant activity and even stimulated the appetite of P. separata. The highest antifeedant rate was observed after 8 h in T3 (88%), while the second highest rate was observed in Tl (Fig. 2).
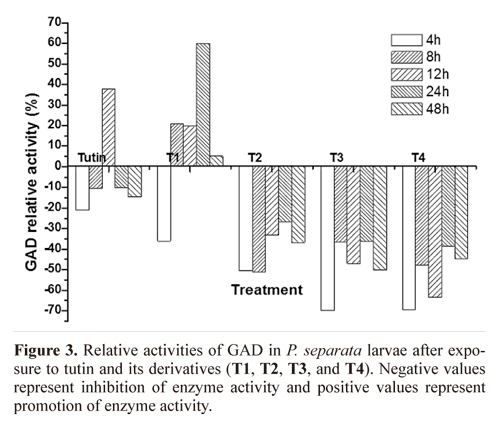
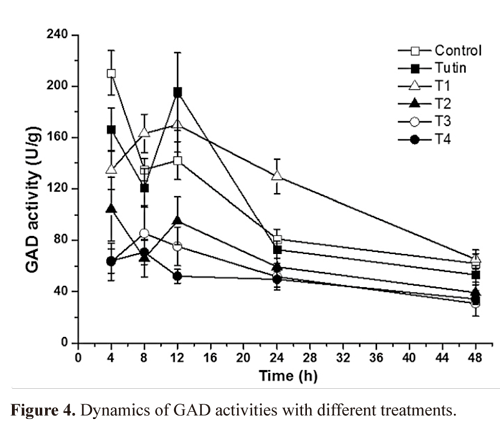
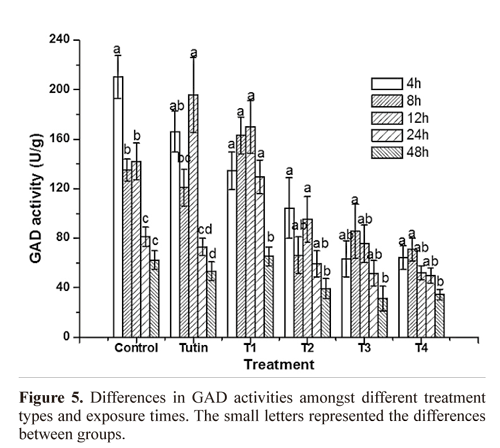
GAD activity. Except for T1, all toxins inhibited GAD activities, with T3 and T4 inhibiting to a greater degree (Fig. 3). Inhibition of GAD activity was higher in treatments with the control, tutin, and T1 than in those with T2, T3, and T4 (Fig.4). GAD activities for all six groups (the control and the five toxins) showed a decreasing trend with time.
GAD activities in P. separata treated with toxins were significantly different from the control (Fig. 5). Using Duncan's multiple range tests, we found that insects treated with tutin, T2, and T3 showed significant differences in inhibition of GAD activity after different lengths of exposure time. Generally, changes in GAD activities were similar between the 4 and 8 h treatments, and both were significantly different from the 12, 24, and 48h treatments. The best inhibited effect was observed at 48h, and the lowest GAD activity was in the sample treated with T3.
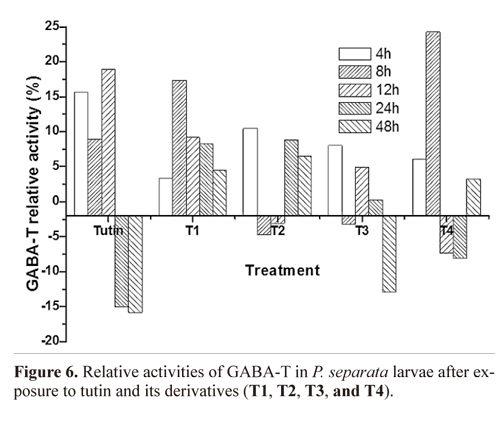
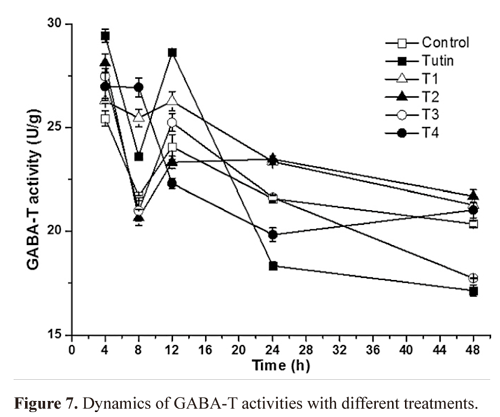
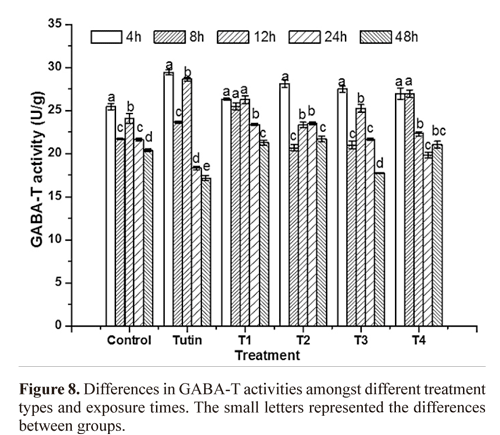
GABA-T activity. TI treatment completely promoted GABA-T activities, whereas T2, T3, and T4 yielded fluctuating effects on GABA-T activities within 48 h of exposure (Fig. 6). Before 12 h, tutin promoted GABA-T activities, but inhibited them after 24 h. Except for T1, all the other derivatives disturbed GABA-T activities in test larvae, particularly the T3 treatment. GABA-T activities decreased with increasing exposure time (Fig. 7). At 4 h, although all toxins showed fluctuating effects on GABA-T activity, they all promoted it. Significant differences in GABA-T activities were observed amongst the five toxins and the control and amongst different assessment times (Fig. 8).
Discussion
Li et al. (2007) found that tutin had antifeedant effects on P. separata, and 2-iso-butenoyltutin, one of the derivatives synthesized from tutin, significantly increased antifeedant activity compared with tutin. In the present study, the 2-hydroxy group of tutin was replaced into four other groups, resulting in four novel derivatives (T1 to T4). We fed 3rd instar larvae of P. separata with wheat leaves inoculated with tutin and these new derivatives, and found that the antifeedant rate of T3 was higher than that of 2-iso-butenoyltutin, making T3 a promising substance for pest control.
We further studied the relationship between antifeed-ant activity and GABA shunt enzymes. The content of the neurotransmitter GABA in the central nervous system of the mammalian brain is related to the available amounts of GAD, GABA-T, and other enzymes (Sherif and Ahmed 1995). The inhibition of GAD and GABA-T activities showed that GABA synthesis and metabolism were blocked, and vice versa. The results of the present study show that tutin and its benzoyl derivatives have significant effects on both GAD and GABA-T activities in the test larvae. The tutin derivatives, T2, T3, and T4, stably inhibited GAD activities within 48 h of exposure, whereas T1 promoted them. T3 had the strongest inhibitory effect on GAD activity. Tutin and its derivatives also showed fluctuating effects on GABA-T activities. T2, T3, and T4 disturbed GABA-T activities in test larvae, particularly T3. This led to a variation in GABA content and a disturbance in the metabolic rate, resulting in disorders in nerve conduction and physiological metabolic difficulties in the test larvae. The results also further explain the study that Glu and GABA contents were influenced by tutin in test insects (Li et al. 2000a). Tutin and its derivatives acted on GABA receptors (Fuentealba et al. 2007), however, according to the feedback regulation mechanism, GAD and GABA activities were influenced, which subsequently affects GABA biosynthesis and metabolism (de Graaf et al. 2006).
We found that GABA has a feedback effect on GAD and GABA-T, and that T3 [2-(6-chloronicotinoyloxy)-tutin], which is derived from tutin, is the most promising novel active ingredient for pest control. T1 had the lowest inhibitory effect on GAD activities, and was significantly different from the three other derivatives. T3 showed the highest antifeed-ant activity and significant effects on GAD and GABA-T activities. The structures of the derivatives may provide reasons for these differences observed. There exists a methoxy group in T1, a nitro group in T2, a nicotinoyl group in T3, and a double-nitro group in T4. The nicotinoyl group, which results from modification of the 2-hydroxy group of tutin into a benzoyl group, appears to affect enzyme activities more than either the nitro or methoxy groups. From Duncan's multiple range tests, no significant differences between the effects of T2 and T4 were observed on GAD and GABA-T activities. Thus, substituting the 2-hydroxy group of tutin for benzoyl derivatives with one or two nitro groups exerts similar effects on enzymes activities. Such as imidacloprid, thiacloprid, acetamiprid, and nitenpyram, contain nicotinoyl groups that cause contact toxicity, stomach toxicity, absorption toxicity, and so on. Linking a nicotinoyl group to the 2-hydroxy group of tutin not only enhanced its antifeedant activity in test larvae, but also promoted the advantages of nicotinoyl insecticides.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 30872032).
Literature cited
BASU, N.; SCHEUHAMMER, A. M.; R0UVINEN-WATT, K.; EVANS, R. D.; TRUDEAU, V. L.; CHAN, L. H. M. 2010. In vitro and whole animal evidence that methylmercury disrupts GABAergic systems in discrete brain regions in captive mink. Comparative Biochemistry Physiology Part C 151: 379-385. [ Links ]
CUI, J. 2007. Studies on the relationship between the structure and insecticidal activity of tutin. Northwest A&F University, Shaanxi. 20-28. [ Links ]
DE GRAAF, R. A.; PATEL, A.B.; ROTHMAN, D. L.; BEHAR, K. L. 2006. Acute regulation of steady-state GABA levels following GABA-transaminase inhibition in rat cerebral cortex. Neurochemistry International 48: 508-514. [ Links ]
DEEWATTHANAWONG, R.; ROWELL, P.; WATKINS,C. B. 2010. y-aminobutyric acid (GABA) metabolism in C02 treated tomatoes. Postharvest Biology and Technology 57: 97-105. [ Links ]
ERECINSKA, M.; NELSON, D.; DAIKHIN, Y.; YUDK0FF, M. 1996. Regulation of GABA level in rat brain synaptosomes: fluxes through enzymes of the GABA shunt and effects of glutamate, calcium and ketone bodies. Journal of Neurochemistry 67: 2325-2334. [ Links ]
FENG, D. X.; SHI, S. M. 2006. Research on night measurement methods of leaf area. Chinese Agricultural Science Bulletin 21: 150-155. [ Links ]
FUENTEALBA, J.; GUZMÄN, L.; MANRIQUEZ-NAVARR0; P.; PEREZ, C.; SILVA, M.; BECERRA, J.; AGUAYO L. G. 2007. Inhibitory effects of Tutin on Glycine receptors in spinal neurons. European Journal of Pharmacology 559: 61-64. [ Links ]
GU0, X .R.; LI, Y. F.; LI, M. L. 2009. The study on biological activity of Coriaria lactone. Journal of Northwest Forestry University 24: 133-135. [ Links ]
JAYAKUMAR, A. R.; SUJATHA, R.; PAUL, V.; ASOKAN, C.; GOVINDASAMY, S.; JAYAKUMAR, R. 1999. Role of nitric oxide on GABA, glutamic acid, activities of GABA-T and GAD in rat brain cerebral cortex. Brain Research 837: 229-235. [ Links ]
LI, M. L.; GU0, X. R.; ZONG, N. 2000a. Chinese insect towards the 21st century. Effect of coriamyrtin on content of GABA and GLU in test insects. Chinese Science and Technology Press, p. 415-416. [ Links ]
LI, M. L.; GU0, X. R.; TANG, G. H. 2000b. Comparing activate reaction of larva esterase isozyme of Parocneria furva caused by Cariaria lactone and 4 insecticides. Acta Agriculturae Borealioccidentalis Sinica 9: 23-27. [ Links ]
LI, M. L.; ZHUANG, S. H.; ZONG, N. 2003. Effect of Tutin on several physiol-biochemical indexes of armyworm. Journal of Northwest Sci-tech University of Agriculture and Forestry (Nat. Sci. Ed.) 31: 54-58. [ Links ]
LI, M. L.; CUI, J.; QIN, R. H.; GAO, J. M.; ZHANG, Y. B.; GUO, X. R.; ZHANG, W. 2007. Semisynthesis and antifeedant activity of new acylated derivatives of tutin, a sesquiterpene lactone from Coriaria sinica. Heterocycles 71: 1155-1162. [ Links ]
LI, Y. F.; CHEN, Q. F.; XU, Y. H.; WANG, H.; ZHOU, Y. F. 2007. Changes of GABA and its catabolic enzymes in the visual cortex of morphine-dependent rats. Journal of Toxicology 21: 95-98. [ Links ]
MU, L. Y. 1997. Research methods of plant chemical protection. China Agricultural Press, Beijing. 56-80. [ Links ]
NISTRI, A.; CONSTANT, A.; QUILLIAM, J. P. 1997. Central inhibition, GABA, and tutin. The Lancet 1: 996-997. [ Links ]
SCHOUSBOE, A.; YU, J. Y.; ROBERTS, E. 1973. Purification and characterization of the 4-aminobyturate-2-ketoglutarate transaminase from mouse brain. Biochemistry 12: 2868-2873. [ Links ]
SEVER, N.; YANG, T.; BROWN, M. S.; GOLDSTEIN, J. L.; DEBOSE-BOYD, R. A. 2003. Accelerated degradation of HMG CoA reductase mediated by binding of Insig-1 to its sterol-sens-ing domain. Molecular Cell 1: 25-33. [ Links ]
SHERIF, F. M.; AHMED, S. S. 1995. Basic aspects of GABA-transaminase in neuropsychiatric disorders. Clinical Biochemistry 28: 145-154. [ Links ]
SHI, M.; LIU D.; DUAN, H. J.; QIAN, L.; WANG, L. N.; NIU, L. J.; ZHANG, H. P.; YONG, Z.; GONG, Z. H.; SONG, L.; YU, M.; HU, M. R.; XIA, Q.; SHEN, B. F.; GUO, N. 2011. The ß2-adrenergic receptor and Her2 comprise a positive feedback loop in human breast cancer cells. Breast Cancer Research Treat 125: 351-362. [ Links ] [ Links ]
SZE, P.Y. 1979. L-Glutamate decarboxylase. Advances Experiment Medicine Biology 123: 59-78. [ Links ]
TAO, Y. H.; YUAN, Z.; TANG, X. Q.; XU, H. B.; YANG, X. L. 2006. Inhibition of GABA shunts enzymes activity by 4-hy-droxybenzaldehyde derivatives. Bioorganic & Medicinal Chemistry Letters 16: 592-595. [ Links ]
XU, J. J.; JIANG, B.; XU, S. Y. 2004. Rapid determination of glutamate decarboxylase activity from lactic acid bacteria by spectrometric method and its applications. Microbiology 31: 66-71. [ Links ]
ZHANG, Y. B.; AI, G. M.; WANG, K. R.; LIU, H. M.; SUN, J. W. 2006. Insecticidal and fungicidal activity of extracts from Coriaria sinica Maxim leaves. Journal of Henan Agriculture Science 1: 60-63. [ Links ]
ZHOU, L. J.; SONG, L. C.; HOU, R. T.; YANG, Z. R.; HE, C. 2006. Preparatory study of the function and the way of the suppression medicament working of Coriaria sinica Maxim's extract. Journal of Sichuan University (Nat. Sci. Ed.) 43: 1165-1169. [ Links ]
ZHU, L.; SONG, F. P.; ZHANG, J.; HUANG, D. F. 2007. Cloning, Expression and Phylogenetic Analysis of Two GABA Shuntrelated Proteins from Bacillus thuringiensis. Microbiology 34: 1031-1036. [ Links ]