Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488
Rev. Colomb. Entomol. vol.38 no.1 Bogotá Jan./June 2012
Scientific note
Reproduction of Trichospilus diatraeae (Hymenoptera: Eulophidae) in pupae of two lepidopterans defoliators of eucalypt
Reproducción de Trichospilus diatraeae (Hymenoptera: Eulophidae) en pupas de dos lepidópteros defoliadores de eucalipto
PATRIK LUIZ PASTORI1, FABRICIO FAGUNDES PEREIRA2, GILBERTO SANTOS ANDRADE3, ROBSON OLIVEIRA SILVA4, JOSÉ COLA ZANUNCIO5 and ALEXANDRE ÍGOR AZEVEDO PEREIRA6
1 Ph. D. Engenheiro Agrónomo. Professor, Departamento de Fitotecnia, Universidade Federal do Ceará 60.356-000, Fortaleza, Ceará Brasil. plpastori@yahoo.com.br. Corresponding author.2 Ph. D. Engenheiro Agrónomo. Professor, Faculdade de Ciencias Biológicas e Ambientais, Universidade Federal da Grande Dourados, 79.804-970, Dourados, Mato Grosso do Sul, Brasil.
3 Ph. D. Engenheiro Agrónomo. Professor, Departamento de Agronomia, Universidade Federal de Rondónia Campus Rolim de Moura, 76.940-000, Rolim de Moura Rondónia, Brasil.
4 Engenheiro Agrónomo, Departamento de Biologia Animal, Universidade Federal de Viçosa, 36.570-000, Viçosa, Minas Gerais, Brasil.
5 Ph. D. Engenheiro Florestal. Professor, Departamento de Biologia Animal, Universidade Federal de Viçosa, 36.570-000, Viçosa, Minas Gerais, Brasil.
6 M. Sc. Engenheiro Agrónomo, Professor, Instituto Federal Goiano, Campus Urutaí, 75.790-000, Urutaí, Goiás, Brasil.
Received: 23-mar-2011 - Accepted: 27-sep-2011
Abstract: Biological control of lepidopteran defoliators using parasitoids is a promising alternative. The objective of this work was to evaluate the reproduction of Trichospilus diatraeae (Hymenoptera: Eulophidae) in pupae of the eucalypt defoliators Thyrinteina arnobia (Lepidoptera: Geometridae) and Hylesia paulex (Lepidoptera: Saturniidae). Host pupae were individualized in glass tubes (14 x 2.2 cm) with six parasitoid females for 24 h under controlled conditions [25 ± 2°C; 70 ± 10% (RH) and; 14 h photo phase]. T. diatraeae parasitized 95.8 ± 2.85% pupae of T. arnobia and 79.2 ± 6.72% of H. paulex, with an emergence rate of 89.6 ± 5.03% and 69.8 ± 6.13%, respectively. However, H. paulex pupae yielded large parasitoid progenies. No difference in the parasitoid sex ratio, adult size and longevity were observed between both hosts. The successful parasitism and development of T. diatraeae in pupae of T. arnobia and H. paulex suggest that this parasitoid can be an alternative for the biological control of these defoliators in eucalyptus plantations.
Key words: Biological control. Hylesia paulex. Lepidoptera. Parasitoids. Thyrinteina arnobia.
Resumen: El control biológico con parasitoides de los lepidópteros defoliadores con parasitoides es una alternativa prometedora. El objetivo de este trabajo fue evaluar la reproducción de Trichospilus diatraeae (Hymenoptera: Eulophidae) en pupas de los defoliadores del eucalipto Thyrinteina arnobia (Lepidoptera: Geometridae) e Hylesia paulex (Lepidoptera: Saturniidae). Pupas del anfitrión fueron individualizadas en tubos de vidrio (14 x 2,2 cm) con seis hembras del parasitoide durante 24 h en condiciones controladas [25 ± 2°C, 70 ± 10% (HR) y foto-período de 14 horas]. T. diatraeae parasita 95,8 ± 2,85% de las pupas de T. arnobia y 79,2 ± 6,72% de H. paulex, con un índice de emergencia de 89,6 ± 5,03% y 69,8% ± 6,13, respectivamente. Sin embargo, las pupas de H. paulex produjeron grandes progenies del parasitoide. No se observaron diferencias en la proporción de sexos, tamaño de adulto y longevidad de los parasitoides en las progenies. El éxito del parasitismo y desarrollo de T. diatraeae en las pupas de T. arnobia y H. paulex sugieren que este parasitoide puede ser una alternativa para el control biológico de estos defoliadores en plantaciones de eucalipto.
Palabras clave: Control biológico. Hylesia paulex. Lepidoptera. Parasitoides. Thyrinteina arnobia.
Introduction
The increase in the area with eucalyptus plantation in Brazil facilitates the adaptation of native Lepidoptera that can cause economical damage to these forests. The abundance of resources and the low occurrence of natural enemies can explain the loss inflicted by these insects in reforestations (Withers 2001). Thyrinteina arnobia (Stoll, 1782) (Lepidoptera: Geometridae) is the main defoliator of eucalypt during population outbreaks. However, species of the genus Hylesia Hubner, 1820 (Lepidoptera: Saturniidae) are receiving more attention due to the damage in several agricultural and forest systems as well as to the health problems caused by the induction of dermatits in humans (Specht et al. 2006).
Between Eulophidae (Hymenoptera: Chalcidoidea) some are gregarious parasitoids, mainly of Lepidoptera (Paron and Berti Filho 2000; Pereira et al. 2008a; Pereira et al. 2008b; Zache et al. 2010). Trichospilus diatraeae Cherian and Margabandhu, 1942 (Hymenoptera: Eulophidae) is a polyphagous endoparasitoid (Paron and Berti Filho 2000) that has received special attention in Brazil as it has been investigated as a potential biological control agent of eucalyptus defoliator pests (Pereira et al. 2008a). However, the use of parasitoids in biological control programs depends on the knowledge of their production in natural (Pastori et al. 2007) or alternative hosts in the laboratory (Pereira et al. 2009) for later release in the field.
The extension of eucalyptus plantations and the necessarily high volume of spraying is a constraint to the chemical control of defoliators in forest systems. Therefore, alternative control methods, such as the use of biological control with natural enemies (Paron e Berti Filho 2000, Pereira et al. 2008a), is required because they are economically and ecologically preferable (Monteiro et al. 2006).
Thus, our objective was to assess the suitability of pupae of T. arnobia and Hylesia paulex Dognin, 1922 (Lepidoptera: Saturniidae) for the development of T. diatraeae knowing their primary potential to control these pest species.
Materials and Methods
Rearing the lepidopterans hosts and the parasitoid: 1) Eggs of T. arnobia were obtained from a laboratory colony reared on branches of Eucalyptus cloeziana plants into organza fabric bags (0.70 x 0.40 cm). Larvae were removed and new food was offered every three days. Pupae were collected, sexed, separated in pairs and located in ventilated plastic pots (500 mL) under controlled conditions (25 ± 2°C; 70 ± 10% relative humidity (RH) and 12 h photophase for egg laying. 2) Eggs of H. paulex were obtained from a laboratory colony reared in ventilated plastic pots (500 mL) whit leaves of Eucalyptus cloeziana as food, which were replaced daily. Adult pairs were obtained, fed a 10% honey solution and placed into screened cages (30 x 30 x 30 cm) containing branches of E. cloeziana for egg laying and food. 3) Adults of T. diatraeae were maintained in glass tubes (14 x 2.2 cm) and fed honey droplets. The tubes were closed with a cotton plug. Twenty-four to 48 h-old pupae of Anticarsia gemmatalis Hubner, 1818 (Lepidoptera: Noctuidae) were exposed to the parasitism for 24 h under the controlled conditions earlier described for parasitoid development and adult emergence.
Experimental work. T. diatraeae were reared for one generation in pupae of both of the hosts in order to eliminate a likely pre-imaginal conditioning by creating the alternative host, A. gemmatalis. Each pupae of host, T. arnobia (187.4 ± 10.14 mg) and H. paulex (468.7 ± 24.30 mg), with up to 24 hours, were individualized in glass tubes (14 x 2.2 cm) with six females of T. diatraeae for 24 hours under controlled conditions [25 ± 2°C; 70 ± 10% relative humidity (RH) and 14 h photo phase]. Females of this parasitoid were removed from the tubes after 24 hours and the parasitized pupae returned to the acclimatized chamber at described conditions where they were maintained until the emergence of the parasitoids.
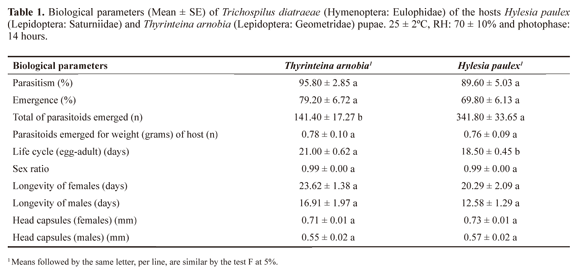
Parasitism rate, emergence rate, number and size of the parasitoids emerged per pupa, duration of the cycle (egg-adult), sex ratio (based on the characteristics of the antennas and calculated with the equation SR= number of females/ number of adults) and longevity were recorded.
The experimental design was fully randomized with 16 replications each with a group of tree host pupae for determine of parasitism rate, emergence rate, number and size of the parasitoids emerged per pupa, duration of the cycle and sex ratio and 12 and 24 replications constituted by 12 males and 24 females, selected on the descendants of each treatment for longevity and head capsule width of males and females, respectively. The averages were compared by ANOVA test with the test F at 5% probability level.
Results
The parasitism (F = 1.1710; df = 30.0; P = 0.2878) of T. arnobia and H. paulex pupae by T. diatraeae was 95.8 ± 2.85% and 89.6 ± 5.03% with emergence (F = 1.0630; df = 30.0; P = 0.3107) of 79.2 ± 6.72% and 69.8 ± 6.13%, respectively, without differences (Table 1). The total of individuals of this parasitoid emerged was 2.4 times larger from pupae of H. paulex (341.8 ± 33.65) than from those of T. arnobia (141.4 ± 17.27) (F = 28.070; df = 30.0; P ≤ 0.00001), but the average number of parasitoids emerged per gram of the host was similar (F = 0.0152; df = 30.0; P ≤ 0.9028) (Table 1).
The duration of the life cycle (egg-adult) of T. diatraeae was longer with pupae of T. arnobia (F = 10.6450; df = 30.0; P ≤ 0.0027) (Table 1). The sex ratio of this progeny was 0.99 ± 0.00 from both hosts (F = 0.0000; df = 30.0; P = 0.6117) (Table 1)
The longevity of females (F = 1.7723; df = 46.0; P = 0.1896) and males (F = 3.3777; df = 22.0; P = 0.0796) of T. diatraeae from pupae of T. arnobia and the size of head capsule of its females (F = 2.2530; df = 46.0; P = 0.1401) and males (F = 0.4940; df = 22.0; P = 0.4896) were similar to those emerged from H. paulex pupae (Table 1).
Discussion
Parasitism and emergence of T. diatraeae from the natural hosts T. arnobia and H. paulex suggest that this natural enemy can be present in the field when populations of these hosts are even at low numbers (Pereira et al. 2008b). Parasitism rate near 90.0% was also found for T. diatraeae in Diatraea saccharalis (Fabricius, 1794) (Lepidoptera: Crambidae); A. gemmatalis; Spodoptera frugiperda (J. E. Smith, 1797) and Heliothis virescens (Fabricius, 1781) (Lepidoptera: Noctuidae) in the laboratory (Paron and Berti Filho 2000). This indicates that some parasitoid of the Eulophidae family presents capacity to adaption to different hosts (Pereira et al. 2008b). The largest number of individuals of T. diatraeae emerged from pupae of H. paulex shows that its adult can produce more numerous progeny with the same initial individuals, such as observations with pupae of D. saccharalis, A. gemmatalis, S. frugiperda and H. virescens (Paron e Berti Filho 2000). This occurs because females of this parasitoid can determine the size of its host (Zaviezo and Mills 2000) and, for this reason, females of T. diatraeae probably laid lower number of eggs on pupae of the host T. arnobia. On the other hand, the larger reproductive success with H. paulex than with T. arnobia can be due to the largest nutritional resource of the first host.
The shorter duration of the life cycle (egg-adult) and the largest number of individuals of T. diatraeae per pupa of H. paulex indicate possible competition between immature of this parasitoid for nutrients, which does not influence the size of head capsule, as found for Melittobia digitata Dahms, 1984 (Hymenoptera: Eulophidae) with pupae of Neobellieria bullata (Parker, 1916) (Diptera: Sarcophagidae) (Silva-Torres and Matthews 2003). Although, differences on nutrient conversion show that the size of the offspring increased with the size of the host, as observed for Hyssopus pallidus (Askew, 1964) (Hymenoptera: Eulophidae) with larvae of Cydia molesta (Busck, 1916) and C. pomonella (L., 1758) (Lepidoptera: Tortricidae) (Häckermann et al. 2007) and Melittobia clavicornis (Cameron, 1908) (Hymenoptera: Eulophidae) with pupae of Trypoxylon politum Say, 1837 (Hymenoptera: Sphecidae), N. bullata and Anthrax sp. (Diptera: Bombyliidae) (González et al. 2004). This demonstrates that host species can affect parasitoid development (Paron and Berti Filho 2000; Jervis et al. 2008, Pereira et al. 2008b).
The similar sex ratio of T. diatraeae between the hosts T. arnobia and H. paulex demonstrates that their pupae are appropriate for the reproduction of this parasitoid, due to the fact that female are responsible for the production of the progeny (Pastori et al. 2007). The number of females produced depends on the host as reported for Cirrospilus coachellae Gates, 2000 (Hymenoptera: Eulophidae) in Marmara gulosa Guillen, Davis and Heraty, 2001 (Lepidoptera: Gracillariidae) and it should be considered when choosing parasitoids for mass rearing (Guillén et al. 2007).
The similar longevity of individuals of T. diatraeae (males and females) from pupae of T. arnobia and H. paulex indicates that nutritional resources of the both host allowed to supply the nutrients required by the parasitoid for reproduction and the energy necessary to maintain these function during life time (Imandeh 2006).
The development of T. diatraeae from H. paulex increases the range of hosts of this parasitoid with a species of the family Saturniidae (Lepidoptera) because this parasitoid was reported in Lepidoptera species of, at least, five families (Crambidae, Noctuidae, Arctiidae, Nymphalidae and Geometridae) (Pereira et al. 2008a).
The reproductive success of T. diatraeae with pupae of T. arnobia and H. paulex supplies basic information of the interaction of this parasitoid with these eucalyptus pests and shows perspectives of using it in biological control of these defoliators in reforestations areas with this plant in Brazil.
Acknowledgements
To "Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenacäo de Aperfeicoamento de Pessoal de Nivel Superior (CAPES)" for financial support.
Literature cited
GONZÁLEZ, J. M.; ABE, J.; MATTHEWS, R. W. 2004. Offspring production and development in the parasitoid wasp Melittobia clavicornis (Cameron) (Hymenoptera: Eulophidae) from Japan. Entomological Science 7 (1): 15-19. [ Links ]
GUILLEN, M.; HERATY, J. M.; URBANEJA, A. 2007. Fecundity and development of Cirrospilus coachellae (Hymenoptera: Eulophidae), a parasitoid of Marmara gulosa (Lepidoptera: Gracillariidae). Journal of Economic Entomology 100 (3): 664-669. [ Links ]
HÄCKERMANN, J.; ROTT, A. S.; DORN, S. 2007. How two different host species influence the performance of a gregarious parasitoid: Host size is not equal to host quality. Journal of Animal Ecology 76 (2): 376-383. [ Links ]
IMANDEH, N. G. 2006. Effect of the pupal age of Calliphora erythrocephala (Diptera: Calliphoridae) on the reproductive biology of Melittobia acasta (Walker) (Hymenoptera: Chalcidoidea: Eulophidae). Entomological Science 9 (1): 7-11. [ Links ]
JERVIS, M. A.; ELLERS, J.; HARVEY, J. A. 2008. Resource acquisition, allocation, and utilization in parasitoid reproductive strategies. Annual Review of Entomology 53 (1): 361-385. [ Links ]
MONTEIRO, L. B.; SOUZA, A.; PASTORI, P. L. 2006. Comparacäo econòmica entre controle biológico e químico para o manejo de ácarovermelho em macieira. Revista Brasileira de Fruticultura 28 (3): 514517. [ Links ]
PARON, M. R.; BERTI FILHO, E. 2000. Capacidade reprodutiva de Trichospilus diatraeae (Hymenoptera: Eulophidae) em pupas de diferentes hospedeiros (Lepidoptera). Scientia Agricola 57 (2): 355-358. [ Links ]
PASTORI, P. L.; MONTEIRO, L. B.; BOTTON, M.; PRATISSOLI, D. 2007. Capacidade de parasitismo de Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae) em ovos de Bonagota salubricola (Meyrick) (Lepidoptera: Tortricidae) sob diferentes temperaturas.Neotropical Entomology 36 (6): 926-931. [ Links ]
PEREIRA, F. F.; ZANUNCIO, J. C.; TAVARES, M. T.; PASTORI, P. L.;JACQUES, G. C.; VILELA, E. F. 2008a. New record of Trichospilus diatraeae (Hymenoptera: Eulophidae) as a parasitoid of the eucalypt defoliator Thyrinteina arnobia (Lepidoptera: Geometridae) in Brazil.Phytoparasitica 36 (3): 304-306. [ Links ]
PEREIRA, F. F.; ZANUNCIO, T. V.; ZANUNCIO, J. C.; PRATISSOLI, D.; TAVARES, M. T. 2008b. Species of Lepidoptera defoliators of eucalypt as new hosts for the polyphagous parasitoid Palmistichus elaeisis (Hymenoptera: Eulophidae). Brazilian Archives Biology and Technology 51 (2): 259-262. [ Links ]
PEREIRA, F. F.; ZANUNCIO, J. C.; SERRÄO, J. E.; PASTORI, P. L.; RAMALHO, F. S. 2009b. Reproductive performance of Palmistichus elaeisis (Hymenoptera: Eulophidae) with previously refrigerated pupae of Bombyx mori (Lepidoptera: Bombycidae). Brazilian Journal of Biology 69 (3): 865-869. [ Links ]
SILVA-TORRES, C. S. A.; MATTHEWS, R. W. 2003. Development of Melittobia australica Girault and M. digitata Dahms (Parker) (Hymenoptera: Eulophidae) parasiting Neobellieria bullata (Parker) (Diptera: Sarcophagidae) puparia. Neotropical Entomology 32 (4): 645651. [ Links ]
SPECHT, A.; FORMENTINI, A. C.; CORSEUIL, E. 2006. Biologia de Hylesia nigricans (Berg) (Lepidoptera, Saturniidae, Hemileucinae). Revista Brasileira de Zoologia 23 (1): 248-255. [ Links ]
WITHERS, T. M. 2001. Colonization of eucalypts in New Zealand by Australian insects. Austral Ecology 26 (5): 467-476. [ Links ]
ZACHÉ, B.; WILCKEN, C. F.; DACOSTA, R. R.; SOLIMAN, E. P. 2010. Trichospilus diatraeae Cherian & Margabandhu, 1942 (Hymenoptera: Eulophidae), a new parasitoidofMelanolophia consimilaria (Lepidoptera: Geometridae). Phytoparasitica 38 (4): 355-357. [ Links ]
ZAVIEZO, T.; MILLS, N. 2000. Factors influencing the evolution of clutch size in a gregarious insect parasitoid. Journal of Animal Ecology 69 (6): 1047-1057. [ Links ]