Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Colombiana de Entomología
versión impresa ISSN 0120-0488
Rev. Colomb. Entomol. vol.38 no.2 Bogotá jul./dic. 2012
Olfactory responses of Conotrachelus psidii (Coleoptera: Curculionidae) to hosts and conspecific odors
Respuesta olfativa de Conotrachelus psidii (Coleoptera: Curculionidae) a volátiles de guayaba (Psidium guajava) y olores co-específicos
GILSON SILVA-FILHO1, OMAR BAILEZ1 and ANA M. VIANA-BAILEZ1
1 Ph. D. Universidade Estadual do Norte Fluminense, CCTA/LEF, Av. Alberto Lamego 2000, CEP: 28013-602, Campos - RJ, Brasil. obailez@uenf.br, corresponding author.
Received: 14-Apr-2012 - Accepted: 22-Nov-2012
Abstract: Conotrachelus psidii (Coleoptera: Curculionidae) is one of the most important pests of guava plants, Psidium guajava (Myrtaceae). Guava plant is the natural host of this weevil suggesting that volatile compounds from odor of different parts of the plant may be attractive to this insect. The main goal of this research was to determine if host or conspecific odors can act as attractants to guava weevil. The olfactory responses of the guava weevil were tested with a Y-tube olfactometer. The odors included green and ripened fruits, leaves and flower buds of P. guajava, guava weevils and their feces. Stimuli were tested singly and in combinations. Flower buds odors were attractive to C. psidii males suggesting the presence of host kairomones. Males and females weevil feces attracted both male and female weevils, indicating the existence of aggregation pheromones or allelochemicals (undigested plant compounds) in the feces. Further studies should be made to conclude from the nature of these substances. Identification and synthesis of the semiochemicals that attract the guava weevil would allow developing new strategies for monitoring and controlling this pest.
Key words: Psidium guajava. Guava weevil. Semiochemicals. Olfactometer. Attractant.
Resumen: El gorgojo Conotrachelus psidii (Coleoptera: Curculionidae) es una de las más importantes plagas del guayabo (Psidium guajava, Myrtaceae). Como esta planta es hospedera natural de este gorgojo, sustancias volátiles de la planta pueden ser atractivas a este insecto. El principal objetivo de este trabajo fue determinar la respuesta olfativa del gorgojo de la guayaba a aromas de la planta y del propio insecto para determinar si estas sustancias pueden actuar como atrayentes. La respuesta olfativa fue estudiada en olfactómetro de dos vías, modelo en "Y". Las fuentes de aromas utilizadas fueron frutos verdes y maduros, hojas y botones florales del guayabo. También fueron utilizados gorgojos de la guayaba y sus heces. Las fuentes de aromas fueron evaluadas en forma individual o combinada. Las pruebas fueron realizadas con cada estímulo olfativo hasta obtener 50 insectos que optaran por uno de los brazos del olfactómetro. Los aromas de botón floral atrajeron machos de C. psidii indicando una acción cairomonal. Los volátiles de las heces de los insectos atrajeron machos y hembras indicando la existencia de una feromona de agregación o de un aleloquímico de la planta, no digerido en las heces. Se recomienda caracterizar las sustancias responsables de esa atracción y concluir sobre la naturaleza de las mismas. La identificación y síntesis de semioquímicos que atraen al gorgojo de la guayaba podrían contribuir para el desarrollo de nuevas estrategias de monitoreo y control de esta plaga.
Palabras clave: Psidium guajava. Gorgojo de la guayaba. Semioquímicos. Olfactómetro. Atrayente.
Introduction
The guava (Psidium guajava L., Myrtaceae) is a fruit widely cultivated in many tropical and subtropical countries (Menzel 1985). One of the principal limiting factors for guava fruit production is the damage caused by insect pests such as the guava weevil, Conotrachelus psidii Marshall, 1922 (Coleoptera: Curculionidae). The guava weevil females oviposit in green fruits and the larvae tunnel into developing fruit eating seeds and pulp that dries and blackens internally. Larval injury can also cause premature fruit drop (Sampaio 1975; Bailez et al. 2003). This pest can damage up to 80% of the fruits in untreated orchards (Luckmann et al. 2009; Bóscan de Martinez and Cásares 1983).
The guava weevil is mainly controlled through frequent application of insecticides, which reduces the percentage of infested guava fruits. However, this management has not been successful in controlling this pest (Gallo et al. 2002). Moreover, intensive use of insecticides can contribute to environmental disequilibrium by selecting for resistant biotypes, and also increases the risk to human health (Innocenzi et al. 2001; Hoffmann et al. 2004).
Semiochemicals baited-traps have been used successfully in pest control of many crops. Sex pheromones are often used to control Lepidoptera pests (Howse et al. 1996). However, curculionids are usually controlled or monitored using traps baited with host-plant allelochemicals (Hunt and Raffa 1989), pheromones (Jansson et al. 1992) or combinations of pheromones and allelochemicals (Tinzaara et al. 2003). Conotrachelus nenuphar (Herbst, 1797), a pest of the same genus of guava weevil, was found to be attracted to both host plant volatiles and insect odors, both laboratory and field conditions (Leskey and Wright 2004).
Traps baited with semiochemicals used to monitor and control populations of C. psidii in guava orchards would be of great importance to improve pest management practices for this insect pest. However, no previous works have been conducted about chemical communication of this weevil. Finding semiochemicals involved in insect-plant interactions requires studies of behavioral responses to olfactory stimuli. The objective of this study was to determine if volatiles released from P. guajava and conspecifics are attractive to C. psidii.
Material and Methods
Insect. Guava weevils were collected between October to December 2004 on the day before the bioassays from an orchard located in Sao Francisco de Itabapoana, Rio de Janeiro State, Brazil. Insects were collected in the field, using beating tray method after shaking branches of the guava plants. The weevils were sexed in the laboratory (Silva-Filho et al. 2007) and were maintained individually in plastic boxes Gerbox® (10 x 10 x 5 cm) at 26 ± 2 ºC, 75 ± 5% relative humidity and photoperiod of 12:12h (light/dark) with pieces of ripe and green guava fruits as food (Bailez et al. 2003). Before testing, the weevils were deprived of food for12 hours in a room free from other odors.
Plant material and odor sources. The plant materials used as olfactory stimuli were collected the day before each test in an orchard in Campos dos Goytacazes, Rio de Janeiro State, and transported in insulated boxes to the laboratory. All plant organs from guava trees were health, without signals of damage or diseases. To reduce deterioration, this plant material was maintained in a refrigerator at 10 ± 2 ºC and 80 ± 5% relative humidity, until 30 minutes before the tests were initiated.
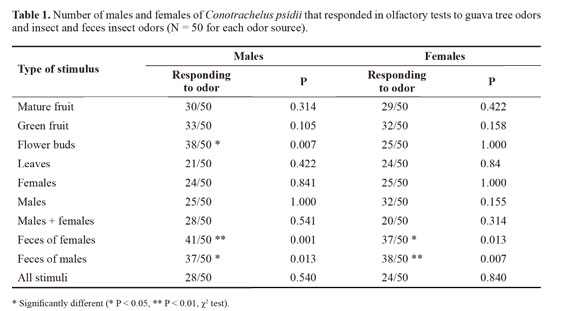
The sources and amount of the stimuli used in the experiment were: (1) ripe whole fruit (400 g of fresh weight at each test), (2) green whole fruit (400 g), (3) leaves (80 g), (4) flower buds (80 g) of guava trees, (5) males (20 individuals), (6) females (20 individuals), (7) both sexes together (10 individual of each), (7) feces males (from 20 individuals for 48 hours), (8) feces of females (as given for males),and (9) all stimuli (plants and insects odors together). The weevils used as odor sources were collected in the field on the day before the bioassays and were maintained individually in plastic boxes without feeding under the conditions described above (Cossé and Bartelt 2000). The feces of the weevils were collected during 48 hours on filter paper (10 x 10 cm) placed on the bottom of the Gerbox®.Olfactometric bioassays. The behavioral response of the individual insects to the different odor sources was tested with an olfactometer similar to a model used in another study made with weevils (Ruiz-Montiel et al. 2003). The olfactometer consisted of a Y-tube. Each arm of the Y-tube was 15 cm long with 1.5 cm internal diameter. Odors in the olfactometer were driven by a compressor, with an airflow rate of 0.35 m/s. The air was purified with an activated charcoal filter and a one-liter flask that contained 500 ml of distilled water. The air was bubbled through the distilled water to clean and humidify the airstream. The extremities of the arms of the olfactometer were connected to two-liter glass containers that held the olfactory stimulant or clean air (control).
The tests were performed from 8:00 to 13:00 h in a room maintained at 26 ± 2 ºC, 75 ± 5% relative humidity and 250 - 300 lux. Each day, 10 weevils of each sex per treatment were tested. The tests were continued in successive days until 50 individuals per treatment were tested. Weevils were tested individually and used only once. Virgin females were not used in the tests, since the great majority of field-collected females were ovipositing females. Males were used only two weeks after being collected to avoid tests with recently emerged insects. After the odor sources were placed in the glass chambers, the air compressor was turned on and the first insect placed in the main tube of the olfactometer after the air flow had stabilized at 0.35 m/s. The choice made by each weevil and the time to reach the extremity of one of the branch tubes were recorded. Based on the results of a similar experiment made with another weevil species, the maximum duration of each test was established as five minutes (Ruiz-Montiel et al. 2003). New fruits and leaves were collected each day for testing the following day. Tests occurred during the same phenological stage of guava plants (flower bud to initial flowering stages). All of the stimuli were tested daily with weevils of both sexes, in random order. The order in which the insects of each sex were tested each day was randomized. A preliminary test was made of the apparatus with 50 weevils (without odor stimuli) to determine if the olfactometer or the room had some effect, no choice tendency was found (data not shown). After each test the position of odor sources in the olfactometer was switched and the Y- tube was replaced by a clean one.Data analyses. The response to each stimulus and to the control was tested with a χ2test, assuming a null hypothesis of no preference. Insects that did not respond to odor sources (stimulus or control) were not included in the statistical analysis. We used analysis of variance (ANOVA) to compare the time spent by the weevils to respond to odors and to determine differences of velocity of response. We also compared with ANOVA the time spent by females to respond to different stimuli.
Results and Discussion
Ripe and green whole fruits, leaves and all stimuli together were not attractive for the curculionids. Between plant stimuli, flower buds significantly attracted male weevils (P = 0.007) (Table 1). Insect pests are generally attracted to their hosts by specific parts of the plant. With respect to this result, there are reports of curculionids that are attracted to specific organs of their host plants. For example, Cosmopolites sordidus (Germar, 1824)is attracted by banana rhizomes (Tinzaara et al. 2003), Metamasius hemipterus (Linnaeus, 1758) by sugar cane stems (Cerda et al. 1999) and C. nenuphar by fruits and fruit buds (Prokopy et al. 1995). Migration to the host plant is signaled to C. nenuphar by the beginning flowering phenological phase (Lafleur and Hill 1987). This weevil is attracted to apple flower bud extracts of various polarities, demonstrating that this weevil responds to various flower bud odor compounds (Leskey and Prokopy 2000). Guava weevil males are probably attracted to the flower bud odors because this part of the plant is usually consumed. In field, when we collected weevils for the experiments we frequently saw males feeding on flower buds. It is also probable that volatiles that become more conspicuous in this part of the plant in the flowering beginning are also used by guava weevil males as a kairomonal cue to find the plant. On the other hand, females were not attracted by flower buds (P = 1.000), that is probably because, rather than flower buds, females consumes fruit pulp. This consumption allows the females to produce high amount of feces that are used to seal the oviposition chambers (unpublished observations).
In the tests made with guava weevil odors, both sexes were significantly attracted to feces odors, but not toward volatiles from conspecific (Table 1). Since weevils used to obtain feces were in constant contact with the filter paper used to collect feces it may be that volatiles released from the weevils themselves and not from the feces were attractive. However, when tested volatiles of females and males with their conspecifics, there was no significant response. Because odors from feces attracted males and females, we suggest that an aggregation rather than a sex pheromone is present in guava weevil feces. However, attraction to both sexes by odor of both sexes was described only once by Löfstedt et al. (1994) in Trichoptera and recently by Schwarz and Gries (2010) in Heteroptera. Usually, only the males (e.g. Perez et al. 1997, Beran et al. 2011) or the females release aggregation pheromones (e.g. Renwick and Vité 1969; Arakaki et al. 2003). An alternative explanation to this attraction may be allelochemicals (undigested plant compounds presents in weevil feces). Certain insects can acquire host plant compounds and use them as attractants (Landolt and Phillips, 1997). Thus, semiochemicals in feces could give a reliable indication of the availability of host plants with suitable fruits for ovipositing females and availability of females for males searching mates. However the fact that ripe or green fruits were not attractive to curculionids in different tests situations is a strong objection to this hypothesis.
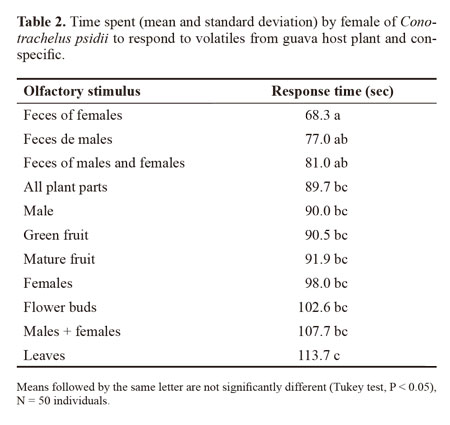
When we analyzed the time elapsed to respond to different stimuli in the olfactometer tests weevil females responded significantly faster than males (88.0 ± 2.35 seconds and 97.7 ± 2.68 seconds, respectively, F = 7.35; df =1; P < 0.035, Table 2). The velocity of the female response also varied significantly with odor source (F = 3.28; df =10; P < 0.001, Table 2) showing a shorter response time to feces odors. This difference may be explained by the hypothesis of Vanderbilt et al. (1998) who suggest a faster response to aggregation pheromone in order to increase reproductive success. So, the pheromone should both promote insect aggregation and maintain them competitive. Along this line, the approximation of C. psidii weevils promoted by feces odors would be accompanied by increasing movement and consequently increased probability of finding sexual partners.
In conclusion, we showed the first evidence of odors in flower buds of guava plants and in weevil feces that attract males and either males or females of C. psidii, respectively The attractiveness to both weevil sexes indicates that semiochemicals in the feces may be a prevalent component of chemical communication between guava weevils. The guava weevil odor of feces and floral buds could be used as bait to capture adult weevils in monitoring or control traps in the context of developing new pest management programs. Further studies should be performed to isolate and to characterize these substances and to determine if this attractants would be efficient enough to compete with natural sources of odors from the host and from the conspecific.Acknowledgements
The authors thank Arli de Fátima and Alexandre Almeida for help with the experiments. To the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro for a research scholarship.
Literature cited
ARAKAKI, N.; WAKAMURA, S.; YASUI, H.; SADOYAMA, Y.; KISHITA, M. 2003. Sexually differential functions of female-produced pheromone of the black chafer Holotrichia loochooana loochooana (Sawada) (Coleoptera: Scarabaeidae). Chemoecology 13: 183-186. [ Links ]
BAILEZ, O. E.; VIANA-BAILEZ, A. M. M.; LIMA, J. O. G.; MOREIRA, D. O. 2003. Life History of the guava weevil, Conotrachelus psidii Marshall (Coleoptera: Curculionidae), under laboratory conditions. Neotropical Entomology 32 (2): 203-207. [ Links ]
BERAN, F.; MEWIS, I.; SRINIVASAN, R.; SVOBODA, J.; VIAL, C.; MOSIMANN, H. 2011. Male Phyllotreta striolata (F.) produce an aggregation pheromone: Identification of male-specific compounds and interaction with host plant volatiles. Journal of Chemical Ecology 37: 85-97. [ Links ]
BOSCÁN DE MARTÍNEZ, N.; CÁSARES, R. 1983. Distribución en el tiempo de las fases del gorgojo de la guayaba Conotrachelus psidii Marshall (Coleoptera: Curculionidae) en el campo. Agronomia Tropical 31 (2): 123-130. [ Links ]
CERDA, H.; FERNÁNDEZ, G.; LÓPEZ A.; VARGAS, J. 1999. Olfactory attraction of the sugar cane weevil (Coleoptera, Curculionidae) to host plant odors and its aggregation pheromone. Florida Entomologist 82 (1): 103-112. [ Links ]
Cossé, A.; Bartelt, R. J. 2000. Male-produced aggregation pheromone of Coleopterus truncatus; structure electrophysiological, and behavioral activity. Journal of Chemical Ecology 26: 1735-1748. [ Links ]
GALLO, D.; NAKANO, O.; SILVEIRA-NETO, S. 2002. Manual de entomologia agrícola. 3rd ed. Piracicaba: FEALQ, Sao Paulo. 920 p. [ Links ]
HOFFMANN, E. J.; COOMBS, A. B.; WHALON, M. E. 2004. Reproductive development of northern and southern strains of plum curculio (Coleoptera: Curculionidae). Journal of Economic Entomology 97 (1): 27-32. [ Links ]
Howse, P.; Stevens, I.; Jones, O. 1996. Insect phromones and their use in pest management. Chapman and Hall, London. 256 p. [ Links ]
HUNT, D. W. A.; RAFFA, K. F. 1989. Attraction of the pine root collar weevil, Hylobius radicis, and the pitch-eating weevil, Pachylobius picivorus (Coleoptera: Curculionidae), to ethanol and turpentine in pitfall traps. Environmental Entomology 18 (3): 351-355. [ Links ]
INNOCENZI, P. J.; HALL, D. R.; CROSS, J. V. 2001. Components of male aggregation pheromone of strawberry blossom weevil, Anthonomus rubi Herbst (Coleoptera: Curculionidae). Journal of Chemical Ecology 27 (6): 1203-1212. [ Links ]
JANSSON, R.; MASON, L.; HEATH, R.; SORENSEN, K.; HAMMOND, A.; ROBINSON, J. 1992. Pheromone trap monitoring system for sweet potato weevil in the southern United States: effects of trap type and pheromone dose. Journal of Economic Entomology 85 (2): 416-423. [ Links ]
LAFLEUR, G.; HILL, S. B.; VINCENT, C. 1987. Fall migration, hibernation sites selection and associated winter mortality of plum curculio (Coleoptera: Curculionidae) in a Quebec apple orchard. Journal of Economic Entomology 80 (6): 1152-1172. [ Links ]
LANDOLT, P. J.; PHILLIPS, T. W. 1997. Host plant influences on sex pheromone behavior of phytophagous insects. Annual Review of Entomology 42: 371-391. [ Links ]
LESKEY, T. C.; PROKOPY, R. J. 2000. Sources of apple odor attractive to adult plum curculios. Journal of Chemical Ecology 26 (3): 639-653. [ Links ]
LESKEY, T. C.; WRIGHT, S. E. 2004. Monitoring plum curculio, Conotrachelus nenuphar (Coleoptera: Curculionidae), populations in apple and peach orchards in the Mid-Atlantic. Journal of Economic Entomology 97 (1): 79-88. [ Links ]
LÖFSTEDT, C.; HANSSON, B. S.; PETERSSON, E.; VALEUR, P.; RICHARDS, A. 1994. Pheromonal secretions from glands on the 5th abdominal sternite of hydropsychid and rhyacophilid caddisflies (Trichoptera). Journal of Chemical Ecology 20: 153-170. [ Links ]
LUCKMANN, A.; MACHADO, R.; BOFF, P. 2009. Danos e dispersão do gorgulho Conotrachelus sp. em goiabeira serrana (Acca sellowiana) sob monocultivo e ecossistemas "Capões". Revista Brasileira de Agroecologia 4: 1224-1228. [ Links ]
MENZEL, C. M. 1985. Guava: an exotic fruit with potential in Queensland. Queensland Agricultural Journal 111: 93-98. [ Links ]
PEREZ, A. L.; CAMPOS, Y.; CHINCHILLA, C. M.; OEHLSCHLAGER, A. C.; GRIES, G.; GRIES, R.; GILBLIN-DAVIES, R. M.; CASTRILLO, G.; PEÑA, J. C.; DUNCAN, R. C.; GONZÁLEZ, L. M.; PIERCE, H. D.; MCDONALD, R.; ANDRADE, R. 1997. Aggregation pheromones and host kairomones of West Indian sugarcane weevil, Metamasius hemipterus sericeus. Journal of Chemical Ecology 23 (4): 869-889. [ Links ]
PROKOPY, R. J.; COOLEY, S. S.; PHELAN, P. L. 1995. Bioassay approaches to assessing behavioral responses of plum curculio adults (Coleoptera: Curculionidae) to host fruit odor. Journal of Chemical Ecology 21 (8): 1073-1084. [ Links ]
RENWICK, J. A. A.; VITÉ, J. P. 1969. Bark beetle attractants: mechanisms of colonization by Dendroctonus frontalis. Nature 224: 1222-1223. [ Links ]
RUIZ-MONTIEL, C. H.; GONZÁLES-HERNÁNDEZ, J.; LEYVA, J.; LANDERAL-CAZARES, C.; CRUZ-LÓPES, L. E.; ROJAS, J. C. 2003. Evidence for a male-produced aggregation pheromone in Scyphophorus acupunctatus Gyllenhal (Coleoptera: Curculionidae). Journal of Economic Entomology 96 (7): 1126-1131. [ Links ]
SAMPAIO, A. 1975. O gorgulho-da-goiaba tem agora um moderno controle. Correio Agricola 2:20-21. [ Links ]
SCHWARZ, J.; GRIES, G. 2010. 2-Phenylethanol: Context-Specific Aggregation or Sex-Attractant Pheromone of Boisea rubrolineata (Heteroptera: Rhopalidae). The Canadian Entomologist 142 (5): 489-500. [ Links ]
SILVA-FILHO, G.; BAILEZ, O.; VIANA-BAILEZ, A. M. 2007. Dimorfismo sexual do gorgulho-da-goiaba Conotrachelus psidii Marshall (Coleoptera: Curculionidae). Neotropical Entomology 36 (4): 420-524. [ Links ]
TINZAARA, W.; DICKE, M.; VAN HUIS, A.; VAN LOON, J. J. A.; GOLD, A. S. 2003. Different bioassays for investigating orientation responses of the banana weevil, Cosmopolites sordidus, show additive effects of host plant volatiles and a synthetic male-produced aggregation pheromone. Entomologia Experimentalis et Applicatta 106 (2): 169-175. [ Links ]
VANDERBILT, C. F.; GIBLIN-DAVIS, R. M.; WEISSLING, T. J. 1998. Mating behavior and sexual response aggregation pheromone of Rhynchophorus cruentatus (Coleoptera: Curculionidae). Florida Entomologist 81 (3): 351-360. [ Links ]