Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Colombiana de Entomología
versión impresa ISSN 0120-0488
Rev. Colomb. Entomol. vol.38 no.2 Bogotá jul./dic. 2012
Feeding of Spodoptera eridania (Lepidoptera: Noctuidae)on soybean genotypes
Alimentación de Spodoptera eridania (Lepidoptera: Noctuidae) en genotipos de soya
Bruno Henrique Sardinha de Souza1,2; Arlindo Leal Boiça Júnior1,3; Júlio Cesar Janini1,4;Anderson Gonçalves da Silva1,5 and Nara Elisa Lobato Rodrigues1,6
1 Faculdade de Ciências Agrárias e Veterinárias - FCAV/UNESP, Campus de Jaboticabal, Departamento de Fitossanidade, Laboratório de Resistência de Plantas a Insetos, Via de Acesso Prof. Paulo Donato Castellane, s/nº, CEP 14884-900, Jaboticabal, SP, Brasil.
2 M. Sc. souzabhs@gmail.com, corresponding author.
3 Ph. D. and professor, aboicajr@fcav.unesp.br
4 Ph. D. juliojanini@yahoo.com.br
5 Ph. D. agroaderson.silva@yahoo.com.br
6 M. Sc. naraelr@hotmail.com
Received: 28-Jun-2011 - Accepted: 28-Oct-2012
Abstract: This work aimed to find soybean genotypes with low feeding preference by Spodoptera eridania larvae on 23 genotypes evaluated in three experiments, of which IAC 100 was established as the resistant genotype and BR 16 as the susceptible genotype. From the results obtained in these tests, a final experiment was carried out with the 10 most outstanding genotypes: IAC 100, PI 227682, PI 227687, DM 339, P 98Y51 RR, BRSGO 8360, IGRA RA 518 RR, IGRA RA 516 RR, IGRA RA 626 RR and BR 16. In all experiments, free choice and no choice tests were performed. In the former, leaf discs corresponding to the genotypes were placed in Petri dishes and then one third-instar larva per genotype was released. In the latter we used one leaf disc of each genotype per plate where one third-instar larva was released. The attractiveness of the third-instar larvae was evaluated at 1, 3, 5, 10, 15, 30, 60, 120, 360, 720 and 1080 minutes after their release, as well as the leaf area consumed. In both tests the genotype IAC 100 was the least attractive and consumed by the larvae, whereas BRSGO 8360 and P 98Y51 RR were the most consumed in free choice and no choice tests, respectively. Genotype IAC 100 was highly resistant to S. eridania in feeding preference experiments and thus can be cultivated or incorporated into breeding programs in order to obtain soybean plants with resistance characteristics to this pest.
Key words: Host plant resistance. Attractiveness. Southern armyworm. Glycine max.
Resumen: Este trabajo tuvo como objetivo encontrar genotipos de soya con baja preferencia para alimentación por larvas de Spodoptera eridania en 23 genotipos evaluados en tres experimentos, de los cuales IAC 100 se estableció como el genotipo resistente y BR 16 como el susceptible. De los resultados obtenidos en estas pruebas, se realizó un experimento final con los 10 genotipos más destacados: IAC 100, PI 227682, PI 227687, DM 339, P 98Y51 RR, BRSGO 8360, IGRA RA 518 RR, IGRA RA 516 RR, IGRA RA 626 RR y BR 16. En todos los experimentos, se realizaron pruebas con y sin opción de elección. En el primer caso, se pusieron los discos foliares relacionados a los genotipos en placas de Petri y se liberó una larva de tercer estadio por genotipo. En el segundo, se utilizó un disco de cada genotipo por placa donde se liberó una larva de tercer estadio. La atracción de las larvas de tercer estadio además del área foliar consumida por las mismas se evaluaron a 1, 3, 5, 10, 15, 30, 60, 120, 360, 720 y 1080 minutos después de su liberación. En ambas pruebas el genotipo IAC 100 fue el menos atractivo y consumido por las larvas, mientras que el BRSGO 8360 y P 98Y51 RR fueron los más consumidos en las pruebas con y sin elección, respectivamente. El genotipo IAC 100 fue altamente resistente a S. eridania en experimentos de preferencia alimenticia y por lo tanto puede ser cultivado o incorporado en programas de mejoramiento con el fin de obtener plantas de soya con características de resistencia a esta plaga.
Palabras clave: Resistencia de plantas a los insectos. Atractivo. Gusano ejército. Glycine max.
Introduction
Brazil stands out as the second worldwide largest soybean producer, Glycine max (L.) Merril, as also is the second largest consumer and exporter of this legume which production was 68.7 million tons in the agricultural year of 2009/2010 in a cultivated area of approximately 23.5 million hectares (Conab 2011).
Among the factors that can adversely influence the yield and quality of soybean production stand the insect pests that attack the plants from the time they sprout until physiological maturation, where larvae of some species of Lepidoptera are one of the most important pests.
Overall, the velvetbean caterpillar Anticarsia gemmatalis Hübner, 1818 (Lepidoptera: Noctuidae) is still the major defoliating pest of soybean crops (Salamina 1997; Di Oliveira et al. 2010), however, another moth species, previously considered secondary pests, have gained importance in the last seasons because of their constant injuries and regular occurrence over the crops. For instance, Spodoptera eridania (Cramer, 1782) larvae cause economical losses mainly to soybean producers from the Cerrado regions (Gazzoni and Yorinori 1995). In these areas, S. eridania was regarded as a not important pest to soybean crops. However, due to frequent outbreaks, high population densities and defoliating levels, usually higher than the control levels, this insect has become an important pest in soybean and cotton cultivated areas of Brazil (Fragoso and Silva 2007; Sosa-Gómez et al. 1993; Santos et al. 2005; Quintela et al. 2007; Santos 2007).
In general, the use of chemical insecticides is the most used tactic to control arthropod pests as it provides quick curative action when the pest population density is close to the economic injury level (Papa 2003). Nowadays, there is a lack of information about chemical control of S. eridania, but the application of phosphate, carbamate, pyrethroid and growth regulator insecticides is recommended as well as some bioinsecticides (e.g., Bacillus thurigiensis Berliner, 1911) (Gallo et al. 2002).
However, successive application of chemical insecticides increases the production costs, contaminates the environment, leaves residues on food, selects population of pests resistant to the active ingredients of the products and causes imbalances in the agroecosystem due to the mortality of natural enemies.
As an alternative to insecticides, the use of resistant plants is considered the ideal method to control agricultural pests as it can reduce their population under the economic injury level, promote balance in the agroecosystem, not encumber the producer, and also it is compatible to other control tactics (Lara 1991).
Studies have reported soybean genotypes with no preference for feeding-type resistance to various insect species, as Van Duyn et al. (1971; 1972), whom observed that soybean lines PI 229358, PI 227687 and PI 171451 showed this type of resistance to Mexican beetle Epilachna varivestis Mulsant, 1850 (Coleoptera: Coccinellidae) in experiments conducted in the field, and after in no choice tests performed in laboratory. Smith and Gilman (1981) found that Pseudoplusia includens (Walker, 1857) (Lepidoptera: Noctuidae) reduced its feeding preference on two populations of soybean derived from the line PI 229358 in free choice tests. Fugi (2003) while evaluating the resistance of four soybean genotypes to A. gemmatalis indicated that PI 229358, IAC 17 and IAC 24 showed lower values of preference indexes than the susceptible pattern IAC PL-1, indicating the presence of no preference for feeding-type resistance to the velvetbean caterpillar.
Thus, the aim of this work was to evaluate the attractiveness and no preference for feeding of third-instar larvae of S. eridania on different soybean genotypes in order to screen materials which show resistance characteristics to be cultivated or incorporated into soybean breeding programs.
Materials and Methodo The experiments were carried out in Faculdade de Ciências Agrárias e Veterinárias - FCAV/UNESP, Campus de Jaboticabal, SP, Departamento de Fitossanidade, Laboratório de Resistência de Plantas a Insetos, under conditions of temperature: 25 ± 1 oC, relative humidity: 70 ± 10% and photophase: 12 hours. Initially, 23 soybean genotypes were assessed: IAC 100, Dowling, PI 227687, PI 274454, BR 16, CD 219 RR, IGRA RA 626 RR, IGRA CM 136, PI 227682, 98Y30 RR, IGRA RA 628 RR, BRSGO 8360, IGRA RA 518 RR, M-SOY 7908 RR, NK 7074 RR, BRSMG 750 SRR, IGRA RA 516 RR, BRS Valiosa RR, BR 82-12547, BRS 8160 RR, P 98Y11 RR, DM 339 and P 98Y51 RR. Seeds of these genotypes were sown in 5 L volume pots containing soil, manure and sand in the proportion of 2:1:1, and after they were put inside a greenhouse. The third-instar larvae of S. eridania used in the experiments were obtained from a laboratory rearing stock fed on an artificial diet according to Greene et al. (1976), based on beans, wheat germ, soybean bran and casein. Because of the lack of information about the resistance of soybean plants to S. eridania, the genotypes used in the tests were divided in three experiments for screening, where the genotype IAC 100 was set as the pattern of resistance once it behaved as resistant to A. gemmatalis (Oliveira et al. 1993; Salvador 2008), a species that belongs to the same taxonomic family of S. eridania, and the genotype BR 16 was used as the susceptible pattern because of its susceptibility to the velvetbean caterpillar observed in other tests (Piubelli et al. 2003, 2005). The three experiments were as follows: a) Experiment 1: IAC 100, BR 16, PI 227687, PI 274454, Dowling, CD 219 RR, IGRA RA 626 RR, IGRA CM 136 and PI 227682; b) Experiment 2: IAC 100, BR 16, 98Y30 RR, IGRA RA 628 RR, BRSGO 8360, IGRA RA 518 RR, M-SOY 7908 RR, NK 7074 RR and BRSMG 750 SRR; c) Experiment 3: IAC 100, BR 16, IGRA RA 516 RR, BRS Valiosa RR, BR 82-12547, BRS 8160 RR, P 98Y11 RR, DM 339 and P 98Y51 RR. From the results obtained in the three experiments, 10 highlighted genotypes were selected, which formed a final experiment. In all feeding preference experiments, free choice and no choice tests were performed. For both tests, leaves from the mid part of 45 days old soybean plant genotypes were collected in the greenhouse, washed in solution of distilled water and sodium hypochlorite at 0.5% and by a punch leaf discs of 2.5 cm in diameter were prepared. In free choice tests, leaf discs were arranged equidistantly from each others in Petri dishes of 14.0 cm in diameter with softly watered filter paper at the bottom, where each leaf disc represented one genotype. Then, one third-instar larva of S. eridania per genotype was released in the center of the plate. For this test, the randomized blocks design with 10 replications was used. For no choice test, only one leaf disc (genotype) per Petri dish of 8.0 cm in diameter was used, where one third-instar larva per plate was released. The complete randomized blocks design with 10 replications was used for this test. In both free choice and no choice tests, the larvae attractiveness in relation to the different soybean genotypes was evaluated at 1, 3, 5, 10, 15, 30, 60, 120, 360, 720 and 1080 minutes after their release. The leaf area consumed (L.A.C.) by the larvae was also evaluated through an electronic leaf area measurer device, model LI-COR 3100®. For this, after the closure of the tests the leftover of the discs were taken to the measurer device, and by the difference between the total area (4.91 cm2) of the disc and the leftover, the leaf area consumed was obtained. From the results of the leaf area consumed by S. eridania, preference indexes were calculated in the final experiment according to Kogan and Goeden (1970), through the formula: C = 2A / (M + A), where C = preference index; A = consumption of the tested genotype; M = consumption of the genotype used as susceptible pattern (BR 16). The interpretation of the data was according to the value of C, i.e.: C > 1, the tested genotype was preferred to larvae feeding in comparison to the pattern genotype (stimulant); C = 1, the tested genotype is similar to the pattern genotype (neutral); C < 1, the tested genotype is less preferred to larvae feeding in comparison to the pattern genotype (deterrent). An analysis of variance (ANOVA) was conducted with the obtained data through an F test, with means compared under a Tukey test at 5% probability. For analysis, data of number of larvae attracted to the genotypes in different minutes and leaf area consumed were transformed in (x + 0.5)1/2.
Results and Discussion
When we analyze the results in experiment 1, significant differences in the attractiveness of third-instar larvae of S. eridania were observed only at 1080 minutes after their release, in free choice feeding preference test (Table 1). In this period, a higher number of larvae were attracted to genotype IGRA RA 626 RR, whereas fewer larvae were observed on PI 227687, IGRA CM 136, Dowling and PI 227682.
Moreover, there was also a significant difference in the means of the larvae attracted to the soybean genotypes, where IGRA RA 626 RR and IGRA CM 136 were the most and the least attractive to S. eridania, respectively (Table 1).
The leaf area consumed differed significantly among the genotypes in free choice test (Table 1). Among them, IGRA RA 626 RR was the most consumed, with 0.68 cm2, whereas IAC 100, PI 227682 and PI 227687 were the least preferred by the larvae, with means of 0.04, 0.04 and 0.09 cm2, respectively. Luedders and Dickerson (1977) observed that PI 227687 showed lower defoliating index to Trichoplusia ni (Hübner, 1802) (Lepidoptera: Noctuidae) in comparison to other susceptible soybean genotypes, in experiments carried out in field conditions. Hoffmann-Campo et al. (1994) verified that PI 227687 also behaved as one of the least preferred genotypes to A. gemmatalis, in no preference for feeding tests.
The lower leaf consumption of S. eridania third-instar larvae on these genotypes is probably due to the presence of morphological and/or chemical factors intrinsic to them which provide degrees of resistance to insects. Hoffmann-Campo (1995) reported that rutin, phenolic compound which performs antibiotic and/or anti-feedant effect in various insects, was one of the flavonoids found in genotype PI 227687.
In no choice test we did not observe significant differences in larvae attractiveness in the time periods evaluated (Table 1). However, the mean of the attracted larvae differed significantly among the soybean genotypes, where a higher number of insects were observed on CD 219 RR and IGRA CM 136, and a lower value was verified on PI 227687 (Table 1). Regarding the leaf area consumed, all genotypes were equally preferred to S. eridania feeding (Table 1).
In experiment 2, there were significant differences in the attractiveness of S. eridania third-instar larvae among the genotypes at 120 and 720 minutes after their release in free choice test (Table 2). In these two time periods, overall, IGRA RA 628 RR and NK 7074 RR were the most and the least attractive genotypes, respectively.
There were significant differences in the means of the larvae attracted to the genotypes (Table 2). The genotypes IGRA RA 628 RR, IAC 100 and BRSGO 8360 showed the highest numbers of larvae, whereas NK 7074 RR, M-SOY 7908 RR and BRSMG 750 SRR showed the lowest means.
Genotype IGRA RA 628 RR was the most consumed by S. eridania, with 2.20 cm2, whereas the others were equally consumed by the larvae (Table 2). It is important to note that the tests belonging to experiment 2 were finished with the last larvae attractiveness evaluation at 720 minutes after their release, once the leaf discs of at least one of the treatments had already been consumed in 70% of the total area.
In no choice test, we observed that soybean genotypes differed significantly regarding the larvae attractiveness only at 120 minutes after their release (Table 2). In this time, genotype BRSGO 8360 was the most attractive to S. eridania, and BR 16 showed the lowest number of larvae.
The mean of attracted larvae, considering all the minutes assessed, also differed among the genotypes (Table 2). The genotypes IGRA RA 518 RR, BRSGO 8360, IGRA RA 628 RR and NK 7074 RR stood out with the highest means, whereas BR 16 and IAC 100 were the least attractive to the larvae. Regarding the leaf area consumed, all soybean genotypes were equally preferred by S. eridania, and did not differ significantly from each other (Table 2).
From the results obtained in experiment 3, we verified that the number of attracted larvae differed significantly among the genotypes at 360, 720 and 1080 minutes, in free choice test (Table 3). At 360 minutes, the highest number of larvae was observed on genotype BRS 8160 RR, and the lowest mean occurred on IAC 100. At 720 minutes, a higher number of attracted larvae were found on BR 82-12547, whereas BRS 8160 RR, IAC 100 and BRS Valiosa RR were the least attractive genotypes, and the same values occurred at 1080 minutes.
The mean of attracted larvae also differed significantly among the genotypes, being BR 82-12547, BRS 8160 RR, IGRA RA 516 RR and P 98Y51 RR the most preferred to S. eridania, and BRS Valiosa RR showed the lowest number of larvae (Table 3). Soybean genotypes differed from each other regarding the leaf area consumed, where BR 82-12547 and IAC 100 were respectively the most and the least consumed genotypes, with 1.21 and 0.20 cm2 (Table 3). Lustosa et al. (1989) studying the feeding preference of A. gemmatalis on 10 genotypes in free choice test verified the occurrence of resistance in genotype BR 82-12547.
In no choice test, there were significant differences among the genotypes at 360 and 1080 minutes after the larvae release (Table 3). In both time periods, a higher number of larvae were attracted to genotype BR 82-12547, whereas the lowest number was observed on BRS Valiosa RR.
The number of S. eridania third-instar larvae attracted to the genotypes in the mean of the assessed minutes differed significantly (Table 3). The highest mean number of larvae occurred on BR 82-12547, whereas BRS Valiosa RR, DM 339, IAC 100, P 98Y11 RR and BR 16 were the least attractive genotypes.
The leaf area consumed also showed significant differences among the genotypes, where IGRA RA 516 RR was the most consumed by S. eridania, with 1.64 cm2, and the genotypes DM 339 and P 98Y51 RR were the least preferred, with 0.13 and 0.16 cm2, respectively.
After performing the three experiments, in general, we observed that IAC 100 was the least consumed in comparison to the other genotypes, in free choice tests. Such repeatability confirms the presence of features in this genotype which express resistance to S. eridania larvae as well. Piubelli et al. (2005) studying leaf extracts of IAC 100 to A. gemmatalis feeding, identified and quantified the flavonoid rutin and isoflavonoid genistin in this genotype, substances that play a role in the plant defense to insects (Dixon and Steele 1999), which may be classified as attractive, repellent, deterrent or toxic to them (Hoffmann-Campo et al. 2001).
From the results obtained in the three experiments, the following 10 genotypes were screened for a final experiment: IAC 100 (resistant pattern), PI 227682, PI 227687, DM 339, P 98Y51 RR (less preferred), BRSGO 8360, IGRA RA 518 RR (moderately preferred), IGRA RA 516 RR, IGRA RA 626 RR (more preferred) and BR 16 (susceptible pattern) (Table 4).
There were significant differences in the larvae attractiveness at 10, 15 and 360 minutes after their release, in free choice test (Table 4). At 10 minutes, a higher number of larvae attracted to genotype DM 339 were observed, whereas IAC 100 and IGRA RA 626 RR were less attractive than the others. In the time period of 15 minutes, DM 339 remained as the most preferred to S. eridania, and the genotypes PI 227682, IGRA RA 626 RR and BR 16 showed the lowest numbers of larvae. Finally, at 360 minutes after initiating the test, a higher number of larvae occurred on the genotypes DM 339 and BRSGO 8360, and the lowest value was found on IAC 100.
Regarding the mean of attracted larvae in all the evaluated times, there were significant differences among the genotypes, where DM 339 showed the highest mean, whereas PI 227682 and IAC 100 were the least attractive to S. eridania larvae (Table 4).
Leaf area consumed differed significantly among the soybean genotypes (Table 4). Genotype BRSGO 8360 was the most preferred for larvae consumption, with 1.51 cm2, and IAC 100 was the least consumed, with 0.29 cm2.
Significant differences were observed in larvae attractiveness among the genotypes at 10, 15 and 120 minutes after their release, in no choice test (Table 4). At 10 minutes, a higher and lower number of insects were attracted to the genotypes PI 227682 and IAC 100, respectively. At 15 minutes, PI 227682, BRSGO 8360 and IGRA RA 518 RR were the most attractive and IAC 100 showed the lowest number of larvae. Finally, at 120 minutes, genotype BRSGO 8360 was the least attractive, whereas PI 227682 had the lowest number of larvae.
Regarding the number of larvae attracted to the soybean genotypes in the mean of the evaluated times, BRSGO 8360 and P 98Y51 were the most preferred, and IAC 100 behaved as the least attractive (Table 4). Leaf consumption also showed differences among the genotypes, being P 98Y51 RR and IAC 100 the most and the least consumed, with means of 1.47 and 0.57 cm2, respectively (Table 4).
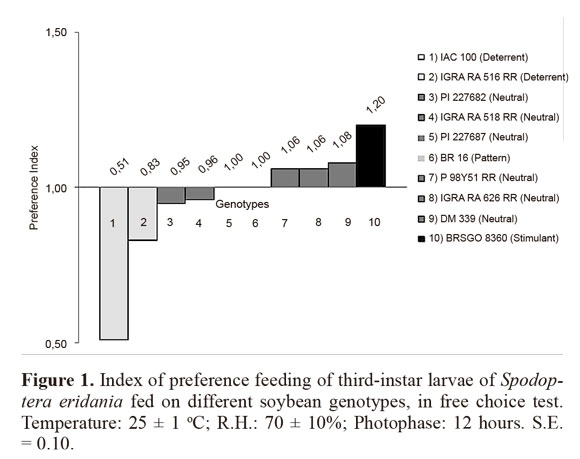
Differences of the feeding preference index among the genotypes in free choice test were found (Fig. 1). When the tested genotypes were compared to the susceptible pattern BR 16, we observed that IAC 100 and IGRA RA 516 RR showed the lowest preference indexes, 0.51 and 0.83, respectively, and both behaved as deterrent to S. eridania feeding. On the other hand, BRSGO 8360 showed the highest preference index, 1.20, and it was classified as stimulant. The other genotypes were as consumed as the susceptible pattern and were neutral to S. eridania larvae feeding.
In no choice test, the genotypes IAC 100, IGRA RA 516 RR and BRSGO 8360 showed the lowest preference indexes, and they were classified as deterrent to S. eridania larvae feeding, whereas the genotypes P 98Y51 RR and IGRA RA 518 RR had the highest indexes, and behaved as stimulant (Fig. 2). The other genotypes had similar preference indexes to the pattern BR 16, and they were neutral to S. eridania feeding.
Studies about resistance of soybean genotypes to S. eridania are scarce mainly due to the recent importance of this pest in the economic scenario of Brazilian soybean production. However, results obtained for the genotype IAC 100 in the present work corroborate studies in the literature when the authors evaluated the resistance of soybean genotypes to A. gemmatalis.
For example, Hoffmann-Campo et al. (1994) evaluated the feeding preference of A. gemmatalis to soybean genotypes and indicated that IAC 100 behaved as resistant in comparison to the genotypes BR 79-15149, Davis and BR 80-25896, all early cycle genotypes.
Lourenção et al. (2000), in experiments performed with early and semi early cycle soybean genotypes in the field observed that IAC 100 showed the lowest mean of cut leaf area percentage, and it was classified as resistant to A. gemmatalis.
Genotype IAC 100 holds resistance factors, chemical and/or morphological, which provides it with no preference for feeding-type resistance to S. eridania, reflected in lower larvae attractiveness and leaf consumption.
Conclusions
Genotype IAC 100 showed a high degree of no preference for feeding-type resistance to S. eridania, in free choice and no choice tests; Genotype IAC 100 can be cultivated or incorporated into breeding programs in order to obtain soybean plants with resistance features to this pest.
Acknowledgements
To Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq, by the concession of Master of Science scholarship to the first author, research productivity to the second author and Doctorate scholarship to the fifth author. To Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES, by the concession of Doctorate scholarships to the third and fourth authors. To Ph.D. Clara Beatriz Hoffmann-Campo, researcher of EMBRAPA - Centro Nacional de Pesquisa de Soja and to Prof. Dr. Flávio Gonçalves de Jesus, professor of Instituto Federal Goiano, by the seeds of soybean genotypes used in the experiments.
Literature cited
CONAB. 2011. Acompanhamento da safra brasileira. 2011.Available in: http://www.conab.gov.br/OlalaCMS//uploads/arquivos/11_03_10_09_03_02_boletim_marco-11[1.pdf [Review date: 31 March 2011. [ Links ]
DI OLIVEIRA, J. R. G.; FERREIRA, M. C.; ROMÁN, R. A. A. 2010. Diferentes diâmetros de gotas e equipamentos para aplicação de inseticida no controle de Pseudoplusia includens. Engenharia Agrícola 30 (1): 92-99. [ Links ]
DIXON, R. A.; STEELE, C. L. 1999. Flavonoids and isoflavonoids - a gold mine for metabolic engineering. Trends in Plant Science 4: 394-400. [ Links ]
FRAGOSO, D. B.; SILVA, R. Z. 2007. Na soja!Revista Cultivar Grandes Culturas 94: 20-22. [ Links ]
FUGI, C. G. Q. 2003. Aspectos biológicos de Anticarsia gemmatalis Hübner, 1818 em genótipos de soja com diferentes graus de resistência a insetos. Dissertação, Instituto Agronômico. 59 p. [ Links ]
GALLO, D.; NAKANO, O.; SILVEIRA NETO, S.; CARVALHO, R. P. L.; BAPTISTA, G. C.; BERTI FILHO, E.; PARRA, J. R. P.; ZUCCHI, R. A.; ALVES, S. B.; VENDRAMIM, J. D.; MARCHINI, L. C.; LOPES, J. R. S.; OMOTO, C. 2002. Entomologia Agrícola. Piracicaba: FEALQ. 920 p. [ Links ]
GAZZONI, D. L.; YORINORI, J. T. 1995. Manual de identificação de pragas e doenças da soja. Brasília: EMBRAPA - SPI. (Manuais de identificação de pragas e doenças, 1). 128 p. [ Links ]
GREENE, G. L.; LEPPLA, N. C.; DICKERSON, W. A. 1976. Velvet bean caterpillar: a rearing procedure and artificial medium. Journal of Economic Entomology 69 (4): 487-488. [ Links ]
HOFFMANN-CAMPO, C. B.; MAZZARIN, R. M.; LUSTOSA, P. R. 1994. Mecanismos de resistência de genótipos de soja: teste de não-preferência para Anticarsia gemmatalis Hübner, 1818 (Lep.: Noctuidae). Pesquisa Agropecuária Brasileira 29 (4): 513-519. [ Links ]
HOFFMANN-CAMPO, C. B. 1995. Role of the flavonoids in the natural resistance of soybean to Heliothis virescens (F.) and Trichoplusia ni (Hübner). Ph. D. thesis, University of Reading, UK. 165 p. [ Links ]
HOFFMANN-CAMPO, C. B.; HARBONE, J. B.; MCAFERRY, A. R. 2001. Pre-ingestive and post-ingestive effects of soya bean extracts and rutin on Trichoplusia ni growth. Entomologia Experimentalis et Applicata 98: 181-194. [ Links ]
KOGAN, M.; GOEDEN, R. D. 1970. The host-plant ranger of Lema trilineata daturaphila (Coleoptera: Chrysomelidae). Annals of the Entomological Society of America 63 (4): 1175-1180. [ Links ]
LARA, F. M. 1991. Princípios de resistência de plantas a insetos. São Paulo: Ícone. 336 p. [ Links ]
LOURENÇÃO, A. L.; PEREIRA, J. C. V. N. A.; MIRANDA, M. A. C.; AMBROSANO, G. M. B. 2000. Avaliação de danos causados por percevejos e por lagartas em genótipos de soja de ciclos precoce e semiprecoce. Pesquisa Agropecuária Brasileira 35 (5): 879-886. [ Links ]
LUEDDERS, V. D.; DICKERSON, W. A. 1977. Resistance of selected soybean genotypes and segregating population to cabbage looper feeding. Crop Science 17: 395-396. [ Links ]
LUSTOSA, P. R.; HOFFMANN-CAMPO, C. B.; MAZZARIN, R. M. 1989. Teste de preferência alimentar e ganho de peso em Anticarsia gemmatalis Hübner, 1818 (Lep., Noctuidae) em genótipos de soja com características de resistência a insetos. pp. 382. In: Congresso Brasileiro de Entomologia, 12, Belo Horizonte. Abstracts. Porto Alegre, Sociedade Entomológica do Brasil. [ Links ]
OLIVEIRA, L. J.; HOFFMANN-CAMPO, C. B.; MAZZARIN, R. M. 1993. Aspectos biológicos e nutricionais de Anticarsia gemmatalis Hüb. (Lepidoptera: Noctuidae) em diversos genótipos de soja. Anais da Sociedade Entomológica do Brasil 22 (33): 547-552. [ Links ]
PAPA, G. 2003 . Manejo integrado de pragas. pp. 203-231. In: Zambolim, L.; Conceição, M. Z.; Santiago, T. (Ed.). O que engenheiros agrônomos devem saber para orientar o uso de produtos fitossanitários. Viçosa: UFV. 376 p. [ Links ]
PIUBELLI, G. C.; HOFFMANN-CAMPO, C. B.; ARRUDA, I. C.; LARA, F. M. 2003. Nymphal development, lipid content, growth and weight gain of Nezara viridula (L.) (Heteroptera: Pentatomidae) fed on soybean genotypes. Neotropical Entomology 32 (1): 127-132. [ Links ]
PIUBELLI, G. C.; HOFFMANN-CAMPO, C. B.; MOSCARDI, F.; MIYAKUBO, S. H.; OLIVEIRA, M. C. N. 2005. Are chemical compounds important for soybean resistance to Anticarsia gemmatalis? Journal of Chemical Ecology 31: 1509-1525. [ Links ]
QUINTELA, E. D.; TEIXEIRA, S. M.; FERREIRA, S. B.; GUIMARÃES, W. F. F.; OLIVEIRA, L. F. C.; CZEPAK, C. 2007. Desafios do manejo integrado de pragas da soja em grandes propriedades no Brasil Central. Santo Antônio de Goiás: Embrapa Arroz e Feijão. 65 p. (Comunicado Técnico, 149). [ Links ]
SALAMINA, B. A. Z. 1997. Bioecologia de Trichogramma pretiosum Riley, 1879, para o controle de Anticarsia gemmatalis Hubner, 1818, na cultura da soja. Tese, Escola Superior de Agricultura "Luiz de Queiroz", Universidade de São Paulo. 106 p. [ Links ]
SALVADOR, M. C. 2008. Efeito de genótipos de soja e de flavonoides na biologia e no intestino médio de Anticarsia gemmatalis. Dissertação, Faculdade de Ciências Agrárias e Veterinárias, Universidade Estadual Paulista. 116 p. [ Links ]
SANTOS, K. B.; NEVES, P. J.; MENEGUIM, A. M. 2005. Biologia de Spodoptera eridania (Cramer) (Lepidoptera: Noctuidae) em diferentes hospedeiros. Neotropical Entomology 34 (6): 903-910. [ Links ]
SANTOS, W. J. 2007. Manejo das pragas do algodão com destaque para o cerrado brasileiro. pp. 403-478. In: Freire, E. C. (Ed.). Algodão no cerrado do Brasil. Brasília: Associação Brasileira dos Produtores de Algodão. 918 p. [ Links ]
SMITH, C. M.; GILMAN, D. F. 1981. Comparative resistance of multiple insect-resistance soybean genotypes to the soybean looper. Journal of Economic Entomology 74: 400-403. [ Links ]
SOSA-GÓMEZ, D. R.; GAZZONI, D. L.; CORRÊA-FERREIRA, B.; MOSCARDI, F. 1993. Pragas da soja e seu controle. pp. 299-331. In: Arantes, N. E.; Souza, P. I. M. (Ed.). Cultura da soja nos cerrados. Piracicaba: Potafos. 535 p. [ Links ]
VAN DUYN, J. W.; TURNIPSEED, S. G.; MAXWELL, J. D. 1971. Resistance in soybean to the Mexican beetle to resistant plants. I. Sources of resistance. Crop Science 11 (4): 572-573. [ Links ]
VAN DUYN, J. W.; TURNIPSEED, S. G.; MAXWELL, J. D. 1972. Resistance in soybean to the Mexican beetle to resistant plants. II. Reaction to the beetle to resistant plants. Crop Science 12 (5): 561-562. [ Links ]