Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488
Rev. Colomb. Entomol. vol.38 no.2 Bogotá July/Dec. 2012
Responses of Anastrepha fraterculus (Diptera: Tephritidae) to pesticides used in organic fruit production
Respuestas de Anastrepha fraterculus (Diptera: Tephritidae) a plaguicidas utilizados en la producción orgánica de frutas
Ricardo Bisotto-de-Oliveira1, Luiza Rodrigues Redaelli1 and Josué Sant’ Ana1
1 Pos. Doc, Ph. D., Pos Doc. respectively. PPG-Fitotecnia, Departamento de Fitossanidade. Faculdade de Agronomia Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brasil, ricardo.bisotto@ufrgs.br, corresponding author. luredael@ufrgs.br, josue.santana@ufrgs.br
Received: 2-May-2012 - Accepted: 20-Nov-2012
Abstract: The South American fruit fly, Anastrepha fraterculus (Diptera: Tephritidae), is an economically important pest of fruit production in Southern Brazil. In organically managed orchards the species has traditionally been controlled with oils, plant extracts, and solutions such as pyroligneous extract and lime sulfur. The objectives of this study were to examine the possible deterrent effect of pesticides with the highest electroantennographic bioactivity on fruit flies and to assess their effects on the viability of pupae in treated fruits. Antennae were exposed to pyroligneous extract (BioPirol7M®, 0.4%), lime sulfur solution (SulFertilizantes, 1%), neem (Organic Neem®, 0.5%), and rotenone (Rotenat®, 0.6%), taking into account fly sex, age and reproductive status. Pupal viability was assessed for larvae reared in papaya (Carica papaya var. Calyman) and guava (Psidium guajava var. Paluma) fruits treated with the pesticides that generated the strongest electrophysiological responses. The bioactivity of A. fraterculus antennae was highest when stimulated with pyroligneous extract and lime sulfur solution, for young and mated flies. Neither substance inhibited oviposition and larval development in treated fruits, a result that has important implications for A. fraterculus management in organic systems.
Key words: Fruit flies. Electroantennography. Oviposition. Pest control. Organic production.
Resumen: La mosca sudamericana, Anastrepha fraterculus (Diptera: Tephritidae), es una plaga de importancia económica en la producción de frutas en el sur de Brasil. En huertos bajo manejo orgánico la especie se controla tradicionalmente con aceites, extractos vegetales y soluciones como el extracto piroleñoso y sulfuro de cal. Los objetivos de este estudio fueron evaluar el posible efecto disuasivo de los plaguicidas que presentan la más alta bioactividad electroantenográficas en moscas de la fruta y evaluar sus efectos sobre la viabilidad de las pupas en frutas tratadas. Las antenas fueron expuestas al extracto piroleñoso (BioPirol 7M®, 0,4%), solución de sulfuro de cal (SulFertilizantes, 1%), Nim (Organic Neem®; 0,5%) y rotenona (Rotenat®, 0,6%), teniendo en cuenta el sexo de la mosca, la edad y el estado reproductivo. La viabilidad de las pupas fue evaluada en larvas criadas en frutas de papaya (Carica papaya var. Calyman) y guayaba (Psidium guajava var. Paluma) tratadas con los plaguicidas que generaron las respuestas electrofisiológicas más fuertes. La bioactividad de las antenas de A. fraterculus fue mayor cuando fueron estimuladas con extracto piroleñoso y la solución de sulfuro de cal, para moscas jóvenes y apareadas. Ninguna de estas sustancias inhibió la oviposición y el desarrollo de las larvas en los frutos tratados, un resultado que tiene implicaciones importantes en el manejo de A. fraterculus en sistemas orgánicos.
Palabras clave: Mosca de la fruta. Electroantenografia. Oviposición. Control de plaga. Producción orgánica.
Introduction
Fruit flies rank among the most important pests in commercial orchards, because of the direct economic impact they have on fruit production and quarantine restrictions for fruit exports imposed by commercial patterns (Aluja 1994; Clark et al. 2005). Ovipositing flies puncture fruit that their larvae subsequently feed on, reducing their value or spoiling them altogether (Malavasi et al. 1994). Anastrepha fraterculus (Wiedemann, 1830) is common in citrus and rosaceous orchards in southern Brazil, where it outnumbers other flies in the same genus and the Mediterranean fruit fly, Ceratitis capitata (Wied. 1824) (Salles 1995).
In Brazil, fruit flies are mostly controlled with organophosphate insecticides. These are very toxic and not selective with regards to natural enemies (Kovaleski and Ribeiro 2003; Scoz et al. 2004; AGROFIT 2011). Full cover spraying is used in guava, stone fruit, and sweet passion fruit plantations, among others, while toxic baits are more commonly used in citrus orchards (Raga and Sato 2006).
In organic fruit plantations, pest control agents include plant oils and extracts such as neem (Azadirachta indica A. Juss, 1797) (Mordue and Nisbet 2000) and rotenone [Lonchocarpus utilis (Smith), Lonchocarpus urucu (Killip and Smith), Derris elliptica (Wallich) Benth and Derris malaccensis (Benth.) Prain] (Kathrina 2004; Wiesbrook 2004). Lime sulfur solution is also widely used in pest control (Bergamin Filho et al. 1995), as is pyroligneous extract (Azevedo et al. 2005; Morandi Filho et al. 2006; Kim et al. 2008). However, few studies have assessed the impacts of these substances on fruit flies (Gonçalves et al. 2005; Rupp 2005).
An electroantennography (EAG) bioassay was used to compare antennal receptivity to stimuli with neem, rotenone, lime sulfur and pyrolignous extract and to evaluate their potential as candidate substances for repelling A. fraterculus or deterring its oviposition on fruits. The results of EAG assays allow one to choose compounds perceived by the olfactory system of fruit flies and to discard those that are poorly perceived or not perceived at all.
The objectives of this study were to assess the electrophysiological activity of neem, rotenone, lime sulfur solution, and pyroligneous extract on the antennae of reared A. fraterculus of different sexes, ages, and reproductive status, and to assess the effects of the two latter substances on pupa viability.
Materials and Methods
Experiments were carried out with A. fraterculus individuals reared in the laboratory at the Universidade Federal do Rio Grande do Sul (UFRGS), southern Brazil, in 2010. Papaya (Carica papaya L. (Caricaceae) var. Calyman) fruits were used as the larval development substrate. Adults were fed on an artificial diet consisting of brown sugar, soy protein, and wheat germ at a 3:1:1 ratio. Insects were reared in an environmentally controlled chamber kept at 25 ± 2 ºC, 70 ± 10% relative humidity, and a 12-12 L-D hour photoperiod.
Adult flies up to 24 hours of age were segregated into three groups: females, males, and both sexes together in 1.5L cages containing food and water, where they remained until they reached a suitable age for the bioassays. Electrophysiological responses to neem, rotenone, lime sulfur, and pyroligneous extract were observed in the antennae of 15 male and female A. fraterculus. It was accessed using flies from different ages [young (5 to 10 days old) and old individuals (25 to 30 days old)] and reproductive status (mated and unmated), totalizing 32 treatments. Couples kept together were considered mated. The tested substances were neem (Organic Neem®; 0.5%), rotenone (Rotenat®; 0.6%), lime sulfur solution (SulFertilizantes; 1%), and pyroligneous extract (BioPirol7M®; 0.4%), which were acquired from the manufacturers Dalquim, Natural Rural, SulFertilizantes, and BioCarbo, respectively. All substances were diluted in distilled water and prepared on the day the bioassays were performed at manufacturer-recommended concentrations.
The electroantennographic methods used in this experiment are similar to those described by Trimble and Marshall (2007), in which each antenna was attached to a two-filament silver electrode using conducting gel (Spectra 360, Electrode Gel-Parker). The analog responses of the signal (in millivolts) were captured, amplified, and processed with a data acquisition controller (IDAC-4, Syntech®), and subsequently recorded using EAG 2000 software (Syntech®). Antennae were stimulated with 5µL of each substance. Twenty-four hours before the electrophysiological tests were carried out, individual flies were placed into 500mL plastic cages with only distilled water.
The data related to the variables involved in the size of the EAG responses in millivolts (mV) was analyzed via a multiple-comparison General Linear Model followed by the Least-Significant Difference (LSD) test and expressed as the eta-squared (η2) index, using SPSS 17 software. Response sizes (mV) were compared with Kruskal-Wallis (α = 0.05) and Mann-Whitney tests using BioEstat 5.0 software.
Viability of A. fraterculus pupae was assessed in papaya (var. Calyman) and guava (var. Paluma) fruits that had been submerged for five seconds in lime sulfur solution (SulFertilizantes) (1%), pyroligneous extract (Biopirol 7M®) (0.4%), or distilled water (control). These substances were selected because they generated the strongest electroantennographic responses in the previous experiment.
A set of three fruits of the same species, each subjected to one of the treatments, was placed simultaneously on regularly spaced Petri dishes inside 350 cm3 plastic cages covered with voile. In each cage were placed dishes of water, food, and 15 mated female A. fraterculus that were 20 to 25 days old. The position of fruits within the cages was randomized for each of the 18 replicates per species. The bioassay was carried out in an environmentally controlled chamber under the same conditions as rearing.
The flies remained with the fruits for 48 hours. At the end of this period fruits were removed and stored in 500 mL containers that were 1/3 full of sterilized sand and covered with voile. After 20 days the fruits were removed, the sand sifted, and the pupae counted. Pupae were transferred to 500 mL containers with 2 cm of sterilized sand. The containers remained covered with voile for up to 30 days, during which time the number and sex of emerging insects were recorded.
Three guava and three papaya were stored in containers with sterilized sand, covered with voile, for 30 days, in order to determine the potential for prior infestation by fruit flies. Three other fruits (guava and papaya) were placed inside a rearing cage with approximately 150 pairs of A. fraterculus for 48 hours to ensure that the fruits were appropriate for insect development. These fruits were then transferred to 500 mL containers with sterilized sand and kept there for 30 days, at which time the sand was sifted and the pupae counted.
The numbers of pupae and emerged insects were square root-transformed and compared among treatments using the Kruskal-Wallis test (α = 0.05).
Results
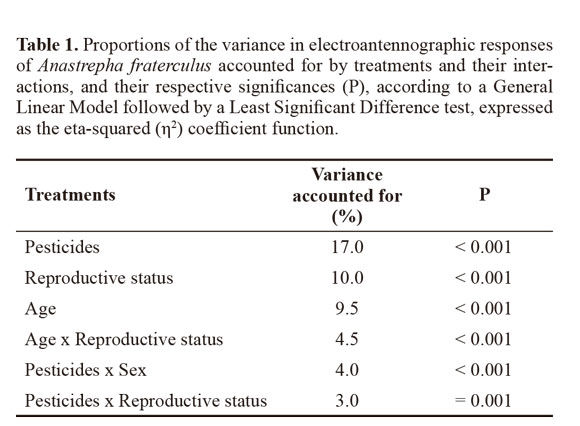
Electroantennography. Tested substances, reproductive status, and age accounted for 17.0, 10.0, and 9.5% of variance in the electroantennographic responses of A. fraterculus, respectively, according to the multiple comparisons method using the GLM and the LSD test (Table 1).
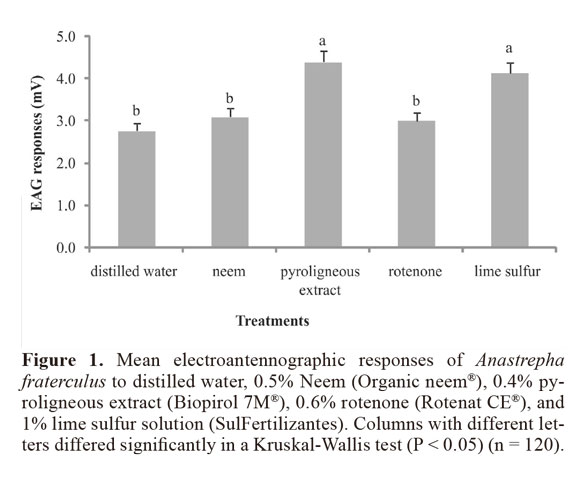
Regardless of age, sex, and reproductive status, electroantennographic responses of adult A. fraterculus were significantly stronger when insects were stimulated with lime sulfur solution and the pyroligneous extract than with rotenone or neem (H = 77.183; df = 4; P < 0.0001) (Fig. 1). Males only showed significantly stronger responses than females for neem (H = 97.130; df = 9; P = 0.023), reflecting the low explanatory power (4%) of the association between pesticide and sex (Table 1).
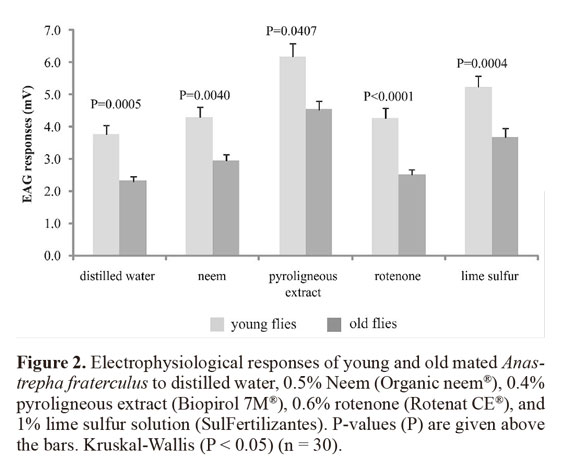
Mated flies showed stronger responses than unmated flies (Z = 6.454; df = 2; P < 0.0001) and young flies showed stronger responses than old flies (Z = 6.282; df = 2; P < 0.0001). The eta-squared coefficient indicated that 4.5% of the variance in electroantennographic responses of A. fraterculus was accounted by the association between age and reproductive status, while 3% by reproductive status and pesticide (Table 1). Young mated flies showed significantly stronger responses than old mated flies, for all treatments (H = 104.76; df = 9; P < 0.0001) (Fig. 2). Young unmated flies only showed stronger responses than old unmated ones for pyroligneous extract (H = 45.167; df = 9; P = 0.025).
Viability of pupae. The number of pupae did not differ between the guava fruits treated with distilled water (control), lime sulfur solution, and pyroligneous extract (H = 3.311; df = 2; P = 0.191). The same was true for papayas (H = 2.345; df = 2; P = 0.309). Likewise, there was no significant difference in the number individuals that emerged from guava (H = 0.890; df = 2; P = 0.640) or papaya fruits (H = 1.959; df = 2; P = 0.375). Pupa viability was 99, 70, and 71% for individuals that developed in guava fruits treated with distilled water, pyroligneous extract, and lime sulfur solution, respectively, and 84, 85, and 90% in papaya fruits.No pupae were observed in the fruits stored in containers with sterilized sand. By contrast, pupae were recorded in the fruits stored in a rearing cage. These results were not statistically analyzed.
Discussion
Although female A. fraterculus responded selectively to pyroligneous extract and lime sulfur solution in the electroantennographic bioassays, the results of the oviposition test showed that these substances did not prevent egg laying and the subsequent development and emergence of A. fraterculus. Lime sulfur solution is a leaf fertilizer and fungicide traditionally used to repel certain species of insects. The elemental sulfur naturally present in the waxy cuticle of gymnosperms and angiosperms may play a role in plant defense mechanisms (Burow and Wittstock 2008), and can also induce the production of antifungal substances (Cooper and Williams 2004). Under field conditions, the toxic effect of lime sulfur solution on insects and mites is produced by the release of hydrogen sulfide (H2S) and sulfur colloids (Abbot 1945).
Sulfurous volatiles emitted by plants play a role in chemical defense (Rouseff et al. 2008). According to Rouseff et al. (2008), the dimethyl disulfide and trimethyl disulfide emitted by guava leaves may be primarily responsible for protecting against attacks by the psyllid Diaphorina citri Kuwayama, 1908 (Hemiptera, Psyllidae). By contrast, sulfurous compounds emitted by onion, Allium cepa L. (Liliaceae), attract Delia antiqua (Meigen) (Diptera, Anthomyiidae) (Matsumoto, 2008). In electroantennographic bioassays, Gouinguené et al. (2005) demonstrated that females of that species oviposited significantly more in the presence of n-propyl disulphide (Pr2S2).
In field conditions, Afonso et al. (2007) reported a decrease of 79.1% in infestations of the European peach scale, Parthenolecanium persicae (Fabricius, 1776)(Hemiptera, Coccidae), in vineyards treated with lime sulfur solution (0.5%). Likewise, Bellon et al. (2009) documented a 28.9% decrease in oviposition by Vatiga manihotae (Drake, 1922) (Hemiptera: Tingidae) in leaves of Manihot esculenta Crantz.
According to Afonso et al. (2007) and Bellon et al. (2009), these species deposit their eggs on the host’s cuticle, where the presence of sulfurous compounds may have a deterrent effect and inhibit oviposition. Such an effect, however, was not evident in A. fraterculus.The stronger electroantennographic responses of A. fraterculus to Biopirol may be attributed to the presence of acetic acid in its composition. That compound is typically present in fruits, where it is a product of the fermentation process (IAEA 2003; Zhu et al. 2003). The same acid, in the form of vinegar, has been used in traps to monitor A. fraterculus populations (Salles 1999; Lemos et al. 2002; Monteiro et al. 2007) and has been characterized as an attractant for A. suspensa (Robacker et al. 1997; Robacker and Heath 1997; Robacker et al. 1998; Robacker et al. 2011) and C. capitata (Joachim-Bravo et al. 2001). Santos and Wansen (2006), however, noted that pyroligneous extract was ineffective at controlling A. fraterculus in organically managed apple orchards in Caçador, Santa Catarina, Brazil. Similarly, Morandi Filho et al. (2006) reported that the substance did not affect survival of Trichogramma pretiosum Riley, 1879 (Hymenoptera, Trichogrammatidae) in laboratory conditions.
In our study, while A. fraterculus showed electroantennographic responses to volatiles of lime sulfur solution and pyroligneous extract, we observed no deterrent effect on oviposition in fruits exposed to these substances. The lack of significant differences in pupae viability between fruits treated with water, solution, and extract may be explained by the fact that eggs are deposited in the fruit interior, away from the substances’ potential insecticidal effects. Our results support those of Efrom et al. (2011), who also demonstrated that treating artificial fruits made of agar with both substances had an ineffective deterrent effect on A. fraterculus oviposition. Those authors also demonstrated that even the topical application of these substances on flies had no insecticidal action.
The fact that mated flies showed stronger electroantennographic responses than unmated flies might be related to physiological changes following mating. In Anastrepha ludens (Loew, 1873) (Diptera, Tephritidae) it has been observed that the olfactory perception of antennae changed after mating, in such a way that certain odor became more or less perceptible than others, including those involved in signaling attractiveness or repellence (Robacker et al. 1990). According to Metcalf and Metcalf (1992), mated females’ greater olfactory sensitivity to host plant volatiles reflects the need for quick and selective orientation in finding the best oviposition sites, thereby favoring the survival of offspring.
Electroantennographic responses of young A. fraterculus were stronger than those of old individuals. A similar result was found by Kendra et al. (2005) for A. ludens when exposed to a bait of ammonium bicarbonate.
Our results suggest that, despite the traditional use of lime sulfur solution and pyroligneous extract in organic fruit orchards, these substances are not effective in reducing populations of A. fraterculus or in deterring oviposition and avoid damage in fruit.
Acknowledgements
The authors are grateful for the financial support provided by Brazil’s National Council for Scientific and Technological Development (CNPq).
Literature cited
ABBOT, C. E. 1945. The toxic gases of lime-sulfur. Journal of Economic Entomology 38: 618-620. [ Links ]
AGROFIT. 2011. Desenvolvido pelo Ministério da Agricultura Pecuária e Abastecimento. Apresenta informações sobre produtos fitossanitários. Available in: www.agricultura.gov.br. [Review date: 15 July 2011] [ Links ].
AFONSO, A. P. S.; FARIA, J. L. C.; BOTTON, M.; ZANARDI, O. Z. 2007. Avaliação da calda sulfocálcica e do óleo mineral no controle da cochonilha-parda Parthenolecanium persicae (Hemiptera, Coccidae) na cultura da videira. Arquivos do Instituto Biológico 74 (2): 167-169. [ Links ]
ALUJA, M. 1994. Bionomics and management of Anastrepha. Annual Review of Entomology 39: 155-178. [ Links ]
AZEVEDO, F.; GUIMARÃES, J.; SOBRINHO, R.; LIMA, M. 2005. Eficiência de produtos naturais para o controle de Bemisia tabaci biótipo B (Hemiptera: Aleyrodidae) em meloeiro. Arquivos do Instituto Biológico 72: 73-79. [ Links ]
BELLON, P. P.; RHEINHEIMER, A. R.; GAZOLA, D.; MIRANDA, A. M.; SANTOS, A. A.; MONDARDO, D.; PIETROWSKI, V; ALVES, L. F. A. 2009. Ação de produtos utilizados no sistema agroecológico na oviposiçao do percevejo da renda (Vatiga manihotae) (Hemiptera: Tingidae) em mandioca. Revista Raízes e Amidos Tropicais 5: 207-211. [ Links ]
BERGAMIN FILHO, A.; KIMATI, H.; AMORIM, L. 1995. Manual de Fitopatologia: Princípios e conceitos. v. 1. São Paulo: Ceres. 919 p. [ Links ]
BUROW, M.; WITTSTOCK, U. 2008. Sulfur-containing secondary metabolites and their role in plant defense. pp. 201-222. In: Hell, R.; Dahl,C.; Knaff, D.; Leustek, T. (Eds.). Sulfur metabolism in phototrophic organisms. Springer: Netherlands. 516 p. [ Links ]
CLARK, A. R.; ARMSTRONG, K. F.; CARMICHAEL, A. E.; MILNE, J. R. ; RAGHU, S. 2005. Invasive phytophagous pests arising through a recent tropical evolutionary radiation: the Bactrocera dorsalis complex of fruit flies. Annual Review Entomology 50: 293-319. [ Links ]
COOPER, R. M.; WILLIAMS, J. S. 2004. Elemental sulphur as an induced antifungal substance in plant defence. Journal of Experimental Botany 55 (404): 1947-1943. [ Links ]
EFROM, C. F. S.; REDAELLI, L. R.; MEIRELLES, R. N.; OURIQUE, C. B. 2011. Laboratory evaluation of phytosanitary products used for control of the South American fruit fly, Anastrepha fraterculus, in organic farming. Crop Protection 30: 1162-1167. [ Links ]
GONÇALVES, P.; DEBARBA, J.; KESKE, C. 2005. Incidência da mosca-das-frutas, Anastrepha fraterculus (Diptera: Tephritidae), em cultivares de ameixa conduzidas sob sistema orgânico. Revista de Ciencias Agroveterinarias 4: 101-108. [ Links ]
GOUINGUENÉ, S. P.; BUSER, HANS-RUEDI; STÄDLER, E. 2005. Host-plant leaf surface compounds influencing oviposition in Delia antiqua. Chemoecology 15: 243-249. [ Links ]
IAEA - TRAPPING GUIDELINES FOR AREA-WIDE FRUIT FLY PROGRAMMES. 2003. Insect Pest Control Section. Printed by the IAEA in Austria. 48 p. [ Links ]
JOACHIM-BRAVO, I. S.; GUIMARÃES, A. N.; MAGALHÃES, T. C. 2001. Influência de substâncias atrativas no comportamento alimentar e na preferência de oviposição de Ceratitis capitata (Diptera, Tephritidae). Sitientibus série Ciências Biológicas 1 (1): 60-65. [ Links ]
KATHRINA, G. A. 2004. Control biológico de insectos mediante extractos botánicos. pp. 137-160. En: CARBALL, M.; GUAHARAY, F. (Ed.). Control biológico de plagas agrícolas. Managua: CATIE. 232 p. [ Links ]
KENDRA, P. E.; MONTGOMERY, W. S.; MATEO, D. M.; PUCHE, H.; EPSKY, N. D.; HEATH, R. R. 2005. Effect of age on EAG response and attraction of female Anastrepha suspensa (Diptera: Tephritidae) to ammonia and carbon dioxide. Environmental Entomolology 3 (34): 584-590. [ Links ]
KIM, D. H.; E. S. HAN; LEE, S. C.; LEE, K. Y. 2008. Effects of wood vinegar mixted with insecticides on the mortalities of Nilaparvata lugens and Laodelphax striatellus (Homoptera: Delphacidae). Animal Cells and Systems 12: 47-52. [ Links ]
KOVALESKI, A.; RIBEIRO, L. G. 2003. Manejo de pragas na produção integrada de maçãs. pp. 61-76. In: Protas, J. F. S.; Sanhueza, R. M. V. (Eds.). Produção integrada de frutas: O caso da maçã no Brasil. Embrapa Uva e Vinho, Bento Gonçalves, Brasil. 192 p. [ Links ]
LEMOS, R. N. S.; SILVA, C. M. C.; ARAUJO, J. R. G.; COSTA, L. J. M. P.; SALLES, J. R. J. 2002. Eficiência de substâncias atrativas na captura de moscas-das-frutas (Diptera: Tephritidae) em goiabeiras no município de Itapecuru-Mirim (MA). Revista Brasileira de Fruticultura 24 (3): 687-689. [ Links ]
MALAVASI, A.; NASCIMENTO, A. S.; CARVALHO, R. S. 1994. Moscas-das-frutas no MIP-Citros. pp. 211-231. In: Donadio, L.C.; Gravena, S. (Eds.). Manejo integrado de pragas dos citros. Fundação Cargill, Campinas-São Paulo, Brasil. 228 p. [ Links ]
MATSUMOTO, Y. 2008. Attraction of insects to organic sulfur compounds in plants. pp. 424-426. En: Capinera, J. L. (Ed.). Encyclopledia of Entomology. 2ed. Springer, Netherlands. 4346 p. [ Links ]
METCALF, R. L.; METCALF, E. R. 1992. Plant kairomones in insect ecology and control. Chapman and Hall. New York .168 p. [ Links ]
MONTEIRO, L. B.; MAY-DE-MIO, L. L.; MOTTA, A. C. V; SERRAT, B. M.; CUQUEL, F. L. 2007. Avaliação de atrativos alimentares utilizados no monitoramento de mosca-das-frutas em pessegueiro na Lapa - PR. Revista Brasileira de Fruticultura 29 (1): 72-74. [ Links ]
MORANDI-FILHO, W.; BOTTON, M.; GRÜTZMACHER, A.; GIOLO, F.; MANZONI, C. 2006. Ação de produtos naturais sobre a sobrevivência de Argyrotaenia sphaleropa (Meyrick) (Lepidoptera: Tortricidae) e seletividade de inseticidas utilizados na produção orgânica de videira sobre Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae). Ciência Rural 36 (4): 1072-1078. [ Links ]
MORDUE (LUNTZ), A. J.; NISBET, A. J. 2000. Azadirachtin from the neem tree Azadirachta indica: its actions against insects. Anais da Sociedade Entomologica do Brasil 29 (4): 615-632. [ Links ]
RAGA, A.; SATO, M. E. 2006. Time-mortality for fruit flies (Diptera: Tephritidae) exposed to insecticides in laboratory. Arquivos do Instituto Biológico 73 (1): 73-77. [ Links ]
ROBACKER, D. C.; HEATH, R. R. 1997. Decreased attraction of Anastrepha ludens to combinations of two types of synthetic lures in a citrus orchard. Journal of Chemical Ecology23 (5): 1253-1262. [ Links ]
ROBACKER, D. C.; GARCÍA, J. A.; HART, W. G. 1990. Attraction of a laboratory strain of Anastrepha ludens (Diptera: Tephritidae) to the odor of fermented chapote fruit and to pheromone in laboratory experiments. Environmental Entomology 19: 403-408. [ Links ]
ROBACKER, D. C.; DEMILO, A. B.; VOADEN, D. J.1997. Mexican fruit fly attractants: effects of 1-pyrroline and other amines on attractiveness of a mixture of ammonia, methylamine, and putrescine.Journal of Chemical Ecology 23 (5): 1263-1280. [ Links ]
ROBACKER, D. C.; DELAIDO, A.; MARTÍNEZ, J.; GARCÍA, J. A; BARTELT, R. J. 1998. Volatiles attractive to the Mexican fruit fly (Diptera: Tephritidae) from eleven bacteria taxa. Florida Entomologist81 (4): 497-508. [ Links ]
ROBACKER, D. C.; MASSA, M. J.; SACCHETTI, P.; BARTELT, R. J. 2011. A novel attractant for Anastrepha ludens (Diptera: Tephritidae) from a Concord Grape product. Journal of Economic Entomology 104 (4): 1195-1203. [ Links ]
ROUSEFF, R. L.; ONAGBOLA, E. O.; SMOOT, J. M.; STELINSKI, L. L. 2008. Sulfur volatiles in guava (Psidium guajava L.) leaves: Possible defense mechanism. Journal Agricultural and Food Chemistry 56: 8905-8910. [ Links ]
RUPP, L. 2005. Percepção dos agricultores orgânicos em relação à Anastrepha fraterculus (Wied.) (Diptera: Tephritidae) e efeito de preparados homeopáticos no controle da espécie em pomares de pessegueiro. Master's Thesis. Universidade do Estado de Santa Catarina, Lages, SC. 89 p. [ Links ]
SALLES, L. A. B. 1995. Bioecologia e controle da mosca-das-frutas sul-americana. Pelotas: Embrapa - CPACT (Brasil). 58 p. [ Links ]
SALLES, L. A. 1999. Efeito do envelhecimento e da decomposição do atrativo na captura de adultos de Anastrepha fraterculus (Wied.) (Diptera: Tephritidae). Revista Brasileira de Agrociência 5 (2): 147-148. [ Links ]
SANTOS, J. P; WANSEN, A. F. 2006. Eficiência de produtos orgânicos sobre danos de moscas-das-frutas em pomar de macieira. Jornal da Fruta 14 (173): 9. [ Links ]
SCOZ, P. L.; BOTTON, M.; GARCÍA, M. S. 2004. Controle químico de Anastrepha fraterculus Wied (Diptera: Tephritidae) em laboratório. Ciência Rural 34 (6): 1689-1694. [ Links ]
TRIMBLE, R. M.; MARSHALL, D. B. 2007. Quantitative method for pheromone delivery in studies of sensory of moth antennae. Physiological Entomology 32 (4): 388-393. [ Links ]
WIESBROOK, M. L. 2004. Natural indeed: Are natural insecticides safer and better than conventional insecticides? Illinois Pesticide Review 17 (3): 1-8. [ Links ]
ZHU, J., PARK, K.C; BAKER, T. C. 2003. Identification of odors from overripe mango that attract vinegar flies, Drosophila melanogaster. Journal of Chemical Ecology 29 (4): 899-909. [ Links ]