Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488
Rev. Colomb. Entomol. vol.38 no.2 Bogotá July/Dec. 2012
Entomopathogenic fungi as potential control agents against the Brazilian ground pearl Eurhizococcus brasiliensis (Hemiptera: Margarodidae)
Hongos entomopatógenos como agentes potenciales de control contra la perla de tierra, Eurhizococcus brasiliensis (Hemiptera: Margarodidae)
ROGÉRIO B. LOPES1, SILAS DUTRA SILVA1,2, MYRIAN S. TIGANO1 and MARCOS BOTTON1,3
1 Ph. D. Entomology. Embrapa Genetic Resources and Biotechnology, Parque Estação Biológica, W5 Norte, 70770-917, Brasilia, D.F., Brazil. Ph. D. - Microbiology. Embrapa Genetic Resources and Biotechnology, Parque Estação Biológica, W5 Norte, 70770-917, Brasilia, D.F., Brazil, rblopes@cenargen.embrapa.br, corresponding author.
2 Undergraduate student Agronomy. University of Brasília, Campus Darcy Ribeiro, 70910-900, Brasília, D.F., Brazil, sd_silva@hotmail.com
3 Ph. D. Entomolgy. Embrapa Grape & Wine, Livramento Street, 515, 95.700-000, Bento Gonçalves, RS, Brazil, marcos@cnpuv.embrapa.br
Received: 24-Apr-2012 - Accepted: 8-Oct-2012
Abstract: The Brazilian ground pearl Eurhizococcus brasiliensis is the most prevalent insect pest of grapes in Brazil. The natural occurrence and biological activity of entomopathogenic fungi (EF) against this pest are poorly known. In this study, we evaluate the presence of E. brasiliensis-associated EF in soil and the virulence of a ground pearl-derived strain of Isaria fumosorosea against cysts under laboratory conditions. EF were not identified on cysts in an initial survey performed in a grape-producing area in southern Brazil. However, 6% of mobile females that had emerged from cysts were infected by Metarhizium brunneum, which was the first report of this insect pathogen on ground pearls in Brazil. Cysts without the protective wax layer were inoculated with I. fumosorosea conidia suspension by immersion. The symptoms and the signs of the disease were described. Infected cysts had a yellow-ochre color and “hard-boiled egg” consistency when broken, in contrast to the intense bright yellow color and “raw egg” consistency of living cysts. Vegetative fungal cells were present inside symptomatic cysts, and later, outside conidiation was visible. The LC25 for the cysts protected with the wax layer and also inoculated by immersion was 1.31 x 107 conidia·mL-1. However, the presence of fungal structures was not observed on symptomatic individuals. Considering the motionlessness of cysts and the absence of disease signs for mortality assessment, the symptoms described may be helpful for further studies on E. brasiliensis control using I. fumosorosea.
Key words: Biological control. Isaria fumosorosea. Metarhizium brunneum.
Resumen: La perla de tierra Eurhizococcus brasiliensis es el insecto plaga más importante en las uvas de Brasil. La presencia natural y actividad biológica de hongos entomopatógenos (HE) contra esta plaga son poco conocidas. En este estudio se evaluó la presencia de E. brasiliensis asociada a HE en suelos y la virulencia de una cepa de Isaria fumosorosea proveniente de la perla de la tierra contra quistes bajo condiciones de laboratorio. No se logró identificar ningún HE en los quistes durante una evaluación inicial conducida en un área de producción de uva al sur de Brasil. Sin embargo, el 6% de las hembras móviles que emergieron de los quistes estaban infectadas con Metarhizium brunneum, el cual es el primer reporte del aislamiento de este patógeno sobre perlas de la tierra en Brasil. Los quistes sin su capa de cera protectora fueron inoculados por inmersión a una suspensión de conidios de I. fumosorosea. Se describieron los síntomas y signos de la infección. Los quistes infectados tenían un color amarillo oscuro y una consistencia de “huevo cocido” cuando rotos, en contraste a un color amarillo claro y una consistencia de “huevo crudo” de los quistes vivos. Las células fúngicas vegetativas se encontraron dentro de los quistes sintomáticos, y más tarde se hizo visible la conidiación en la parte externa. La CL25 para los quistes protegidos por su capa de cera e inoculados por inmersión fue de 1,31 x 107 conidios.mL-1. Sin embargo, la presencia de estructuras del hongo no se observó en los individuos sintomáticos. Teniendo en cuenta la inmovilidad de los quistes y la ausencia de signos patológicos para la evaluación de mortalidad, los síntomas descritos pueden ayudar en estudios futuros sobre el control de E. brasiliensis utilizando I. fumosorosea.
Palabras clave: Control biológico. Isaria fumosorosea. Metarhizium brunneum.
Introduction
The Brazilian ground pearl Eurhizococcus brasiliensis (Hempel, 1922) (Hemiptera: Margarodidae) is an underground scale that attacks the roots of over 70 wild and cultivated plant species, especially grapevines (Foldi 2005). Ground pearls present a serious problem in vineyards in southern Brazil but have also been found in the state of São Paulo in southeastern Brazil (Figueredo Jr. 1970; Lourenção et al. 1989) and recently in the São Francisco Valley, Petrolina, in the state of Pernambuco (PE) in northeastern Brazil (Haji et al. 2004). The insect remains in the root area and is harmful only in the juvenile stage (cyst) because adults are devoid of mouthparts.
Ground pearl control has been achieved with the use of neonicotinoid insecticides that act primarily via ingestion (Teixeira et al. 2002; Botton et al. 2010) with a reduced contact effect in the cyst stage (Hickel et al. 2001). In general, chemical control of the insect has been unsatisfactory, particularly when the attack occurs in adult grapevine plants (Botton et al. 2010). In this sense, an alternative to chemical control, mostly through the use of biological control agents, is a growing demand for sustainable production. The main natural enemies of the Brazilian ground pearl are the dipteran predator Prolepsis lucifer (Wiedemann, 1928) (Soria et al. 2004) and entomopathogenic fungi, especially Isaria fumosorosea (Carneiro et al. 1994).
The study of biological control agents for ground pearls is essential for understanding the potential use of biological agents for controlling the pest. The I. fumosorosea strain CG259, deposited in the Embrapa Genetic Resources and Biotechnology Invertebrate Fungi Collection, was originally obtained from infected cysts collected in the region of Vila Conceição, Caxias do Sul, in the state of Rio Grande do Sul, Brazil, in the early 1990s (Carneiro et al. 1994). Preliminary studies of pathogenicity to cysts in the laboratory indicated its potential as a control agent against the pest. However, little information concerning the symptoms and signs of the disease is available. In the specific case of ground pearls, the cyst stage is long (6-8 months), immobile, and devoid of appendices, which hampers differentiation between living and dead individuals in studies with entomopathogenic fungi. Another key factor is that the pest lives in the soil and produces an intense secretion of protective wax during the cyst stage. This characteristic, combined with the presence of other soil microorganisms on the host plant, may interfere with pathogen action and the evolution of disease signals (Pereira et al. 1993; Inglis et al. 1998). The determination of clear symptomatological parameters is essential for the confirmation of cyst mortality and a better analysis of the results. It is also important to investigate other microbial agents for their capacity to control ground pearls. The objective of this study was to evaluate the natural occurrence of fungi on E. brasiliensis cysts and females, the virulence of I. fumosorosea strain CG259 to cysts under laboratory conditions, and the disease symptoms during the cystic stage of insect development.
Materials and Methods
Sampling of Eurhizococcus brasiliensis cysts. Cysts of E. brasiliensis were sampled at a commercial vineyard (Yves cultivar) in Flores da Cunha, Rio Grande do Sul in November 2009. Cysts were sampled by digging trenches 0.5 m in depth near the bases of the plants and sieving soil samples. The insects were placed in plastic boxes (25 x 15 x 10 cm) with moistened soil (95% RH) from the sampling site for 25 days (25 ± 1 °C and 24 h scotophase). After this period, insects were counted, and dead cysts and mobile females were transferred to a moisture chamber for five days (25 ± 1 °C and 24 h scotophase) for better growth and conidiogenesis of fungi associated with the insects.
Morphological identification and phylogenetic analyses of a Metarhizium strain isolated from Eurhizococcus brasiliensis females. Fungal propagules on cysts or mobile female cadavers were first prepared on slides using cotton blue stain and were observed under a microscope (1000x). Insect-associated fungi were purified and plated on culture media (potato dextrose agar (PDA) - Difco Laboratories, Detroit, MI, USA) and diagnosed based on the morphological characteristics of reproductive cells (conidia and conidiogenous cell) (Bischoff et al. 2009). Conidia and conidiophores were measured using an Olympus OSM-4 10x/13 ocular micrometer lens coupled to the microscope.
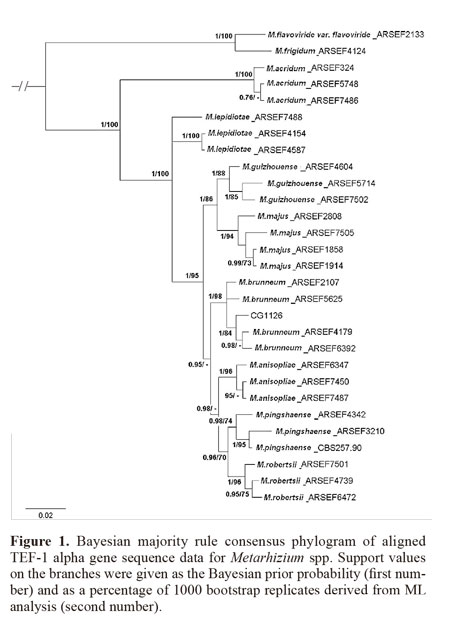
For phylogenetic identification of the Metarhizium strain isolated from dead E. brasiliensis females, monosporic colonies were prepared in PDA medium using the material obtained from purified colonies. Ten-day culture discs were inoculated into Erlenmeyer flasks with 250 mL of sabouraud dextrose agar with yeast extract (SDAY) liquid media (1% glucose; 0.3% malt extract; 0.5% peptone; 0.3% yeast extract) and incubated at 25 ± 0.5 °C at 250 rpm for 3 days. Mycelium samples were collected on filter paper using vacuum filtration and were frozen at –80 °C. Approximately 25 mg of mycelia were frozen in liquid nitrogen and crushed in a mortar, and the total genomic DNA was extracted using a DNA extraction protocol described by Raeder and Broda (1985). The phylogenetic identification was performed by amplifying the translation elongation factor1-α (TEF1-α) gene fragment based on methodology described by Bischoff et al. (2009). Sequencing was performed by Macrogen Inc. (Seoul, Korea), and the sequences were edited using the DNA baser V.3 program (Heracle BioSoft S.R.L.). Consensus sequences were analyzed using the MEGA 5.03 program, and the final alignment was adjusted manually. Selected TEF sequences from the GenBank database (strains from the ARS Collection of Entomopathogenic Fungal Cultures) were included in the analysis. Phylogenetic analysis was performed with phylogenetic analysis using parsimony (PAUP, version 4.0) under the Maximum Likelihood (ML) method, and 1000 bootstrap (BS) repetitions were performed. Heuristic searches identified the most likely tree, and bootstrap support values were provided. Clades with 70% ML BS or greater were considered significantly supported by the data. Additionally, a second analysis was conducted using a Bayesian phylogenetic inference performed by MrBayes v.3.1.2. The jModelTest program was previously used to identify the distribution model. Analysis was performed over five million generations, with tree sampling every 100 generations; the first 25% of trees were discarded prior to consensus tree calculation (the “burn-in”). The MP BS values were finally included in the Bayesian tree.
Production of Isaria fumosorosea inocula.Conidia of the strain CG259, stored in the Embrapa Genetic Resources and Biotechnology Invertebrate Fungi Collection (Brasília, Federal District), were transferred to Petri dishes with PDA culture medium and incubated at 25 ± 1 °C and with a 12 h photophase. The conidia produced after a 15-day period were scraped and immediately used in bioassays. Before each bioassay, the viability of conidia was assessed by plating a 5 x 105 conidia.mL-1 suspension in PDA medium and counting, a random sample of 300 germinated and non-germinated conidia under a microscope (400x) after 20 h of incubation (25 ± 1 °C and 24 h scotophase). The initial viability of conidia at the time of the implementation of the bioassays was greater than 95%.
Description of symptoms and signs of the disease caused by Isaria fumosorosea in Eurhizococcus brasiliensis cysts. Healthy cysts obtained during sampling and previously washed in distilled water for 30 s were selected according to size (4 - 5 mm) and aspect (light color and no signs of damage). The chitinous protection of the cysts was removed using a needle. To ensure the complete removal of soil and chitin residues, the cysts were subsequently washed in a sodium hypochlorite solution (0.25% v/v) for 2 min and rinsed twice in sterile water. The cysts were transferred to Petri dishes lined with moistened filter paper for 24 h at 25 ± 1 °C in the dark. Subsequently, cysts without signs of injury (i.e., those with bright yellow color and early wax production) were selected and divided into two groups of 20 cysts each.
Conidia were suspended in sterile water with Tween 80® solution (0.01% v/v), and the concentration was adjusted to 1x109 conidia.mL-1 using a Neubauer chamber to ensure infection and disease symptoms. Cysts of one group were immersed in fungal suspension for 90 s while the control group was immersed only in sterile water and Tween 80® solution. The treated and untreated insects were transferred to Petri dishes lined with moistened filter paper for 20 d at 25 ± 1 oC in the dark (adapted from Carneiro et al. 1994).
Petri dishes were inspected every five days, and all cysts with symptoms (change in color or shape and dehydration) or signs (presence of fungal structures) were reserved for further analysis. Symptomatic cysts were washed once more in sodium hypochlorite solution (0.25% v/v) for 2 min and were rinsed twice in sterile water. Internal liquid from the cysts (5 µL) was removed with a glass microsyringe and was transferred to Petri dishes with PDA culture medium. The Petri dishes were kept in an incubator for 5 d (25 ± 1 °C; 12 h photophase). Slides with the liquid and parts of the internal tissue of the cysts were prepared with lactophenol cotton blue dye for microscopic examination (1000x). When present, slides of fungal structures on cysts were also prepared, and morphological characteristics of the fungi (conidia and conidiogenous cell) were observed under an optical microscope (400x). In this case, the mycelium cover was completely removed before the process of external disinfection and the internal evaluation of the cysts. At the end of the 20-day period, all asymptomatic cysts remaining, both treated and untreated, were subjected to external disinfection, microscopic observation and plating of the internal liquid in culture medium.
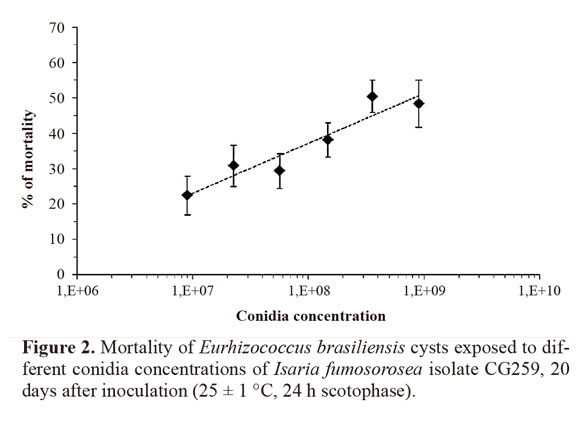
Determination of the concentration-response curve of Isaria fumosorosea strain CG259 to Eurhizococcus brasiliensis cysts. The procedures for the preparation of suspensions and the inoculation of cysts were the same as described in the previous section. However, to simulate field conditions, the protective layer was not removed, and the external disinfection of the cysts in sodium hypochlorite was not performed. The following concentrations were used in the concentration-response bioassays: 9x106, 2.26 x 107, 5.67 x 107, 1.48 x 108, 3.58 x 108 and 9 x 108 conidia.mL-1. Concentrations were determined based on preliminary tests (data not shown), ensuring at least 25% of cysts mortality. After inoculation, each treatment with 60 cysts (6 - 7 mm) divided into four replicates of 15 cysts each was transferred to plastic cups (6 cm in height and 5 cm in diameter) with sterile vermiculite in the bottom. The cysts were then covered with another layer of vermiculite, totaling 4.5 g of substrate per cup. The top of the cup was covered with cotton, which was moistened on a daily basis to keep the relative humidity inside the cup above 90%. The cups were then transferred to a climatic chamber at 25 ± 1 °C in the dark, and cyst removal was performed on the twelfth day. The insects were kept in a moisture chamber for 10 more days prior to analysis based on the criteria established in the study that described the symptoms of the disease caused by I. fumosorosea. Bioassay for determining the concentration-response was repeated three times on different dates.
Statistical analyses. Data on confirmed mortality were analyzed using a generalized linear mixed model (GLMM) adjusted for restricted maximum likelihood, with the response variable (deaths) assigned a binomial distribution with logit link function. The data from the three experiments were analyzed together using R statistical software version 2.8 (R Development Core Team 2006).
Results
Occurrence of entomopathogenic fungi on Eurhizococcus brasiliensis.A total of 2480 cysts were sampled, and the occurrence of entomopathogenic fungi was not observed in this stage after the quarantine period. Opportunistic fungi in the genera Trichoderma, Aspergillus, Fusarium, and Penicillium were identified on the surfaces of cysts, which are often associated with dead individuals during sampling and transport or soil adhered to the chitinous protection of living individuals.
Only 166 cysts (6.7% of the total sampled) became mobile females during the quarantine period. Only 6% of all females that emerged from the cysts were infected by Metarhizium spp. The cadavers were mummified, yellow-orange in color and covered with an olive-green mass of fungal conidia. Microscopic examination of conidia and conidiophores showed the typical morphology of species in the M. anisopliae complex (conidia that were 5.1 - 6.8 x 2.1 - 3.2 µm in size and phialides that were 7.4 - 13.1 x 2.0 - 3.5 µm in size) (Bischoff et al. 2009).The size of the amplified DNA product was 681 bp after the overlap of the two sequences; from a set of 29 isolates, the aligned length of this locus was 553 bp. The phylogenetic tree shows that the new strain isolated from E. brasiliensis females is closely related to all representative strains of Metarhizium brunneum, supported by 100% probability and 98 ML BS (Fig. 1). The new strain was preserved in the Invertebrate Fungal Collection of Embrapa Genetic Resources and Biotechnology under the access code CG1126.
Description of symptoms and signs of the disease caused by Isaria fumosorosea in Eurhizococcus brasiliensis cysts. The outward symptoms of disease caused by I. fumosorosea in cysts were initially characterized by a loss in brightness and slight color change from bright yellow to yellow-ocher. Initially, a white mycelial growth appeared that evolved into a rosy color, which is typical of this species of fungus and was later confirmed in the microscopic examination of reproductive structures. The main characteristic used in the virulence bioassays to differentiate living and dead cysts was the difference in appearance and consistency of the cysts when cut or pierced. Healthy cysts had a thin membrane and a “raw egg” appearance when pierced, releasing an intense yellow liquid. Infected cysts had a “hard-boiled egg” appearance when pierced, retaining their original shape. Cross-section cuts of infected cysts revealed a thick wall and small amount of transparent liquid in a hollow central portion.
Cysts with disease signs and symptoms were observed from the fifth day onward after inoculation (15% of individuals). The percentage of symptomatic cysts increased with incubation time, reaching 40% and 55% 10 and 15 days after incubation, respectively. At the end of the evaluation period (20 d), this percentage reached 65%. Pathogen cells (blastospores or mycelium) were rare or absent in the transparent liquid from the central portion of symptomatic cysts when observed under the microscope. However, vegetative cells were found in large quantities in the inner solid portion of the cyst wall. Abundant fungal growth in culture medium was observed in all symptomatic cysts after the internal contents of the host were plated. Conversely, no fungi were detected in asymptomatic cysts under the microscope and after plating in culture medium for both inoculated and non-inoculated groups at the end of the incubation period.Concentration-response curve (LC25) for cysts inoculated with Isaria fumosorosea. The estimated LC25 for the I. fumosorosea isolate CG259 was 1.31 x 107 conidia.mL-1(d =144.33; P = 6.47e-10; df = 66; IC = 8.711 x 106 - 1.96 x 107). The increase in concentration resulted in increased cyst mortality (Fig. 2). Mortality rates were 22.4 ± 5.4% at the lowest concentration (9 x 106 conidia.mL-1) and reached 50.4 ± 4.5% at 3.58 x 108 conidia.mL-1. All dead insects had a “hard-boiled egg” appearance that was typical of infection but rarely presented pathogen structures on the cadavers.
Discussion
This is the first report of the natural occurrence of M. brunneum infecting ground pearls. Recent studies have identified naturally occurring M. brunneum from other hosts, usually from coleopterans and lepidopterans (Bischoff et al. 2009; Cossentine et al. 2010), but hosts from the scale insect family Margarodidae have yet to be identified. Our study also describes a simple and precise method to identify cysts infected by I. fumosorosea in the absence of the typical fungal structures on the insect cadaver but based on several specific symptoms. Additionally, we observed that a great amount of conidia of the ground pearl-derived I. fumosorosea strain is necessary to cause limited levels of mortality.
The abundance of insect-associated fungi in soil differs greatly for each agroecosystem or environmental condition, and species in the M. anisopliae complex seem to be more abundant in agricultural habitats (Bidochka et al. 1998; Sun et al. 2008), including M. brunneum, which was already isolated from grape rhizospheres (Fisher et al. 2011). Although ground pearls are subterranean plant-sucking parasites found on the roots of a wide variety of cultivated plants around the world and entomopathogenic fungi species are frequently present in soil, little information regarding fungal infection for this insect group is published. The life cycle of the Brazilian ground pearl is peculiar. Nymphs feed on the roots, and an excreted waste fluid serves to construct a shell, which ensures strong protection against adverse environmental conditions (Foldi 2005). At this stage, fungal infection throughout the cyst tegument is likely blocked by this protective layer, either physically or by the presence of chemical compounds. The structure of the cyst wax of Eurhizococcus colombianus (Jakubski, 1965) indicates the presence of a triglyceride and the subsequent formation of unsaturated diglycerides and aldehyde during the cyst development (Quiñones et al. 2008). An aldehyde was already detected in cuticle extracts of the stink bug Nezara viridula (Linnaeus, 1758) and was found to be fungistatic to certain entomopathogenic fungi, including M. anisopliae (Sosa-Gómez et al. 1997). This cyst shell might protect the insect against natural infections in soil, a hypothesis supported by the low mortality of cysts exposed to high concentrations of I. fumosorosea conidia.
However, at this stage, we observed a low proportion of mobile females in the collected population and out of the protective shell. Reproductive ground pearl females are devoid of functional mouthparts and do not feed but may live for several weeks in some species (Foldi 2005); during this period, they are exposed to inhabiting soil entomopathogenic fungi. Although the frequency of diseased females was low, the presence of propagules of M. brunneum in the soil allowed some insects to be infected. As already discussed, it is not reasonable that infection occurs during the cyst stage and the disease later manifests in females.
Behavioral parameters, such as response to a stimulus, spontaneous movement, or feeding activity, are often used to evaluate the mortality of the target insect. Observations that provide an accurate and immediate response concerning the state of the target insect are the key for the rapid acquisition and interpretation of results during bioassays. The absence of appendices and any activity in E. brasiliensis cysts combined with the long duration of this stage (4-6 months) hampers the assessment of control method efficiency. In the specific case of entomopathogenic fungi studies, the presence of specific signs of infection (i.e., fungal growth and reproduction on the host) is often used as a parameter for confirmation of the cause of death. However, the abundant presence of microorganisms in the soil adhering to cysts might have interfered with the clear manifestation of signs of infection. In fact, this effect was observed in this study, where infected cysts did not manifest signs of infection.
The species I. fumosorosea is present in soil in both natural and cultivated areas (Sookar et al. 2008; Sun et al. 2008). However, reports of the use of this fungus for ground pearl control are scarce. Although the pest can attack several host plants, it is restricted to the Neotropical region (Foldi 2005). Thus, research on E. brasiliensis is limited to Brazil. The results of this study complement previous information and provide the basis for future research on the biological control of ground pearls. The pathogenic activity of I. fumosorosea isolate CG259 was confirmed in the laboratory, supporting the study by Carneiro et al. (1994). However, conidial concentrations needed to cause high mortality of the insect were higher than those observed by Carneiro et al. (1994) (CL50 = 8.1 x 102 conidia.mL-1). Despite methodological similarities, the surface disinfection of cysts in the latter study possibly removed the protective layer and the antagonistic microorganisms present in the soil, favoring invasion by the entomopathogenic fungus and the subsequent manifestation of disease signs. It is worth noting that under natural conditions, cysts are covered in a waxy protective layer and are found in soil aggregates, which may directly affect the infection process and the subsequent colonization of hosts. The antagonistic action of soil microorganisms has been reported for other insect species that in some way inhabit the soil, as in the red imported fire ant Solenopsis invicta (Buren, 1972) (Pereira et al. 1993) and the grasshopper Melanoplus sanguinipes (Fabricius, 1798) (Inglis et al. 1998).
Conclusions
This study demonstrated that, despite the susceptibility of E. brasiliensis to I. fumosorosea isolate CG259 previously observed by Carneiro et al. (1994), high concentrations of this pathogen are needed to cause death in cysts covered in a protective layer. Moreover, even though the pest is susceptible to M. brunneum fungus naturally present in soil, infection occurred only in mobile females, which comprise a small proportion of the population, significantly limiting the use of M. brunneum for ground pearl control. Detailed analysis of the effects of fungi on females and cysts at different ages and methods of pathogen release in the field are the key for determining the viability of entomopathogenic fungi for ground pearl control.
Literature cited
Bidochka, M. J.; Kasperski, J. E.; Wild, G. A. M. 1998. Occurrence of the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana in soils from temperate and near-northern habitats. Canadian Journal of Botany 78: 1198-1204. [ Links ]
Bischoff, J. F.; Rehner, S.A.; Humber, R. A. 2009. A multilocus phylogeny of the Metarhizium anisopliae lineage. Mycologia 101: 508-528. [ Links ]
BOTTON, M.; TEIXEIRA, I.; BAVARESCO, A.; PASTORI, P. L. 2010. Use of soil insecticides to control the Brazilian ground pearl in vineyards. Revista Colombiana de Entomología 36: 20-24. [ Links ]
CARNEIRO, R. M. D. G.; SORIA, S. J.; KULCZYNKI, S. M.; SILVA, J. B. 1994. Patogenicidade de Paecilomyces fumosoroseus isolado CG 259 à Eurhizococcus brasiliensis Hempell (Homoptera: Margarodidae). Anais da Sociedade Entomológica do Brasil 23 (2): 345-348. [ Links ]
COSSENTINE, J. E.; JUDD, G. J. R.; BISSETT, J. D.; LACEY, L. A. 2010. Susceptibility of apple clearwing moth larvae, Synanthedon myopaeformis (Lepidoptera: Sesiidae) to Beauveria bassiana and Metarhizium brunneum Biocontrol Science and Technology 20 (7): 703-707. [ Links ]
FIGUEIREDO JR., E. R. de. 1970. Nova praga da videira em São Paulo - Eurhizococcus brasiliensis (Hempel). O Biológico 36 (9): 229-234. [ Links ]
FISHER, J. J.; REHNER, S. A.; BRUCK, D. J. 2011. Diversity of rhizosphere associated entomopathogenic fungi of perennial herbs, shrubs and coniferous trees. Journal of Invertebrate Pathology 106: 289-295. [ Links ]
FOLDI, I. 2005. Ground pearls: a generic revision of the Margarodidae sensu stricto (Hemiptera: Sternorrhyncha: Coccoidea). Annales da la Société Entomologique de France 41 (1): 81-125. [ Links ]
HAJI, F. N. P.; LIMA, M .P. L.; ALENCAR, J. A.; BARBOSA, F. R.; FERREIRA, R. C. F.; MATTOS, M. A. A. 2004. Cochonilha-pérola-da-terra: praga emergente na cultura da uva, no submédio do Vale do São Francisco. Petrolina, Embrapa Semi-Árido, 5 p. (Circular Técnica 78). [ Links ]
HICKEL, E. R.; PERUZZO, E. L.; SCHUCK, E. 2001. Controle da pérola-da-terra, Eurhizococcus brasiliensis (Hempel) (Homoptera:Margarodidae), através da insetigação. Neotropical Entomology30 (1): 125-132. [ Links ]
Inglis, G. D.; Johnson, D. L.; Kawchuk, L. M.; Goettel, M. S. 1998. Effect of soil texture and soil sterilization on susceptibility of ovipositing grasshoppers to Beauveria bassiana. Journal of Invertebrate Pathology 71 (1): 73-81. [ Links ]
LOURENÇÃO, A. L.; MARTINS, F. P.; ALARCON, L. C. M. 1989. Ocorrência de Eurhizococcus brasiliensis (Hempel) (Homoptera: Margarodidae) em videira no município de Louveira, Estado de São Paulo. Bragantia 48 (2): 205-208. [ Links ]
PEREIRA, R. M.; STIMAC, J. L.; ALVES, S. B. 1993. Soil antagonism affecting the dose response of workers of the red imported fire ant, Solenopsis invicta, to Beauveria bassiana conidia. Journal of Invertebrate Pathology 61: 156-161. [ Links ]
Quiñones, W.; Vicente, B.; Torres, F.; Archbold, R.; Murillo, W.; Londoño, M.; Echeverri, F. 2008. Chemical composition of ground pearl (Eurhizococcus colombianus) cysts.Molecules13: 190-194. [ Links ]
R DEVELOPMENT CORE TEAM. 2006. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, ISBN 3-900051-07-0. [ Links ]
Raeder, U.; Broda, P. 1985. Rapid preparation of DNA from filamentous fungi. Letters Applied Microbiology 1:17-20. [ Links ]
SOOKAR, P.; BHAGWANT, S.; AWUOR OUNA, E. 2008. Isolation of entomopathogenic fungi from the soil and their pathogenicity to two fruit fly species (Diptera: Tephritidae) Journal of Applied Entomology 132: 778-788. [ Links ]
SORIA, S. J.; MELLO R. P.; OLIVEIRA, A. M. 2004. Novos registros de Prolepsis lucifer (Wiedemann, 1928) (Diptera, Asilidae) como predador de Eurhizococcus brasiliensis (Hempel in Wille, 1922) (Hemiptera, Margarodidae) em diferentes regiões viticultoras do Rio Grande do Sul, Brasil. Entomología y Vectores 11: 323-331. [ Links ]
Sosa-Gómez, D. R.; Boucias, D. G.; Nation, J. L. 1997. Attachment of Metarhizium anisopliae to the southern green stink bug Nezara viridula cuticle and fungistatic effect of cuticular lipids and aldehydes. Journal of Invertebrate Pathology 69: 31-39. [ Links ]
SUN, B.; YU, H.; CHEN, A. J.; LIU, X. 2008. Insect-associated fungi in soils of field crops and orchards. Crop Protection 27 (11): 1421-1426. [ Links ]
TEIXEIRA, I.; BOTTON, M.; LOECK, A. E. 2002. Avaliação de inseticidas visando ao controle de Eurhizococcus brasiliensis (Hempel) (Hemiptera: Margarodidae) em novos plantios de videira. Neotropical Entomology 31 (3): 457-461. [ Links ]