Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488
Rev. Colomb. Entomol. vol.38 no.2 Bogotá July/Dec. 2012
Population dynamics of Leptopharsa heveae (Hemiptera: Tingidae) and
Erythmelus tingitiphagus (Hymenoptera: Mymaridae) in rubber tree plants
Dinámica poblacional de Leptopharsa heveae (Hemiptera: Tingidae) y Erythmelus tingitiphagus
(Hymenoptera: Mymaridae) en plantas de caucho
RODRIGO S. SANTOS1, VALMIR A. COSTA2, JOAQUIM M. SILVA3 and SÉRGIO FREITAS4
1 D. Sc. Embrapa Acre - Centro de Pesquisa Agroflorestal do Acre (CPAFAC) - Rod. BR-364, Km 14, CP 321, Rio Branco, AC, Brazil, rodrigo.s.santos@embrapa.br, corresponding author.
2 D. Sc. Instituto Biológico -Agência Paulista de Tecnologia dos Agronegócios (IB/APTA) - Rod. Heitor Penteado, Km3,CP 70, Vila Brandina, 13012-970, Campinas, SP, Brazil, valmir@biologico.sp.gov.br
3 M. Sc. Universidade do Estado do Mato Grosso (UNEMAT) - Rod. BR-158, Km 148, CP 08, 78690-000, Nova Xavantina, MT, Brazil, joaquim.manoel@gmail.com
4 D. Sc. Universidade Estadual Paulista - Faculdade de Ciências Agrárias e Veterinárias (UNESP/FCAV) - Via de Acesso Prof. Paulo Donato Castellane, s/n, 14884-900, Jaboticabal, SP, Brazil, serfre@fcav.unesp.br. = Deceased.
Received: 20-Oct-2011 - Accepted: 24-Oct-2012
Abstract: The tingid Leptopharsa heveae, known as the lace bug, occurs in large populations in rubber tree plantations, limiting the production of latex due to losses of photosynthetic area and weakening of the infested trees. The alternative for the use of insecticides would be the release of biological control agents, but little is known about the natural enemies of L. heveae. The parasitoid Erythmelus tingitiphagus parasitizes eggs of the lace bug in rubber tree plantations. The knowledge of the population dynamics and the peak of occurrence of economically important insect species and their natural enemies are indispensable requirements for the establishment of efficient and rational control strategies. The objective of this work was to study the population dynamics of L. heveae and E. tingitiphagus in rubber tree plantations in the county of Itiquira, MT, Brazil. Mature folioles were collected weekly from the lower stratum of the canopy of the tree clones RRIM 600, PR 255, GT 1, PB 235 and PB 217, from August/2005 to February/2006. The parasitoid was observed during the whole study period. The population peaks of the populations of the L. heveae and E. tingitiphagus in the study area occurred at the end of October and beginning of November. This result demonstrates that measures for the control of L. heveae and population increase of E. tingitiphagus must be adopted before the peak population of this pest in cultivated rubber plantations.
Key words: Biological control. Chalcidoidea. Hevea brasiliensis. Egg parasitoid.
Resumen: El tingido Leptopharsa heveae, conocida como la chinche de encaje, se presenta en grandes poblaciones en las plantaciones de caucho, limitando la producción de látex, debido a las pérdidas en el área fotosintética y el debilitamiento de los árboles infestados. La alternativa al uso de insecticidas sería la liberación de agentes de control biológico, pero poco se sabe sobre los enemigos naturales de L. heveae. El parasitoide Erythmelus tingitiphagus parasita los huevos de la chinche de encaje en plantaciones de caucho. El conocimiento de la dinámica poblacional y el pico de aparición de ciertas especies de insectos económicamente importantes así como sus enemigos naturales son requisitos indispensables para el establecimiento de estrategias de control eficiente y racional. El objetivo de este trabajo fue estudiar la dinámica poblacional de L. heveae y E. tingitiphagus en las plantaciones de caucho en el condado de Itiquira, MT, Brasil. Se recolectaron semanalmente foliólos maduros de la capa inferior de la copa de árboles de los clones RRIM 600, PR 255, GT 1, PB 235 y PB 217, a partir de agosto/2005 a febrero/2006. Se observó el parasitoide durante todo el estudio. Los picos poblacionales de L. heveae y E. tingitiphagus en el área de estudio ocurrió a finales de octubre y principios de noviembre. Este resultado demuestra que las medidas para el control de L. heveae e incremento poblacional de E. tingitiphagus deben ser adoptadas antes del pico poblacional de la plaga en plantaciones de caucho de cultivo.
Palabras clave: Control biológico. Chalcidoidea. Hevea brasiliensis. Huevos parasitoide.
Introduction
The genus Hevea (Euphorbiaceae) encompasses 11 species (Watson and Dallwitz 1992), being Hevea brasiliensis (Willd. A. Juss.) Müell Arg., the rubber tree, is the species with the highest reproductive capacity, largest genetic variability and highest latex productivity (Costa 2001, Francisco et al. 2004). On a national level, most of the latex produced comes from the states of São Paulo, Mato Grosso, Bahia and Espírito Santo, with São Paulo being the main producer of natural rubber in Brazil (IAC 2004).
The planting of the rubber tree in monoculture in extensive areas favours the attack of pests. A considerable number of insect and mite species is associated to the rubber trees, and some of them are considered pests due to the frequency and levels of infestation in which they occur (Abreu 1996).
One of the main insect-pests of rubber trees is the lace bug Leptopharsa heveae Drake and Poor, 1935 (Hemiptera: Tingidae), which in large infestations causes premature defoliation, affects the functioning of the plant chlorophyll and favours the penetration of microorganisms through the damage produced in the host tissues (Moreira 1986). Large infestations of seedlings in nurseries reduce the plant height in up to 28% and the plant diameter, and up to 44.5% at the base (Moreira 1986), or reduce latex production up to 30% (Tanzini and Lara 1998). Leptopharsa heveae nymphs and adults are distributed uniformly on the different plant strata and on the abaxial area of the leaves, grouping together in large colonies (Cividanes et al. 2004a). In the state of Mato Grosso, the beginning of the rainy season correlates positively with the population increase of this species (Santos and Freitas 2008). The entomopathogenic fungus Sporothrix insectorum (Tanzini 2002), the predatory Chrysopidae (Scomparin 1997) and the parasitoid Erythmelus tingitiphagus (Soares, 1941) (Hymenoptera: Mymaridae) area are among the main natural enemies of L. heveae (Costa et al. 2003, Santos and Freitas 2008).
Studies of the population dynamics of insect-pests and of their natural enemies are essential to increase the knowledge regarding the occurrence of outbreaking periods and for the establishment of efficient and rational control strategies (Gingrich 1993; Batista Filho et al. 2003; Ronchi-Teles and Silva 2005). The aim of this study was to assess the population dynamics of L. heveae and E. tingitiphagus in five rubber tree plant clones in the county of Itiquira, MT, Brazil.
Material and Methods
The study was conducted between October 2005 and February 2006, in the farm of the “Plantações E. Michelin Ltda.” located in the county of Itiquira, MT, Brazil (17º22’S 54º44’W), in a polyclonal plantation of approximately 1.2 ha, with trees of 22 years of age, average height of 11 to 13 m and spacing of 2.5 x 8 m. The trees have not received any type of phytosanitary treatment against the lace bug since the 14 December 2002.
Completely expanded mature leaves were collected weekly from the trees of the RRIM 600, PB 235, PB 217, PR 255 and GT 1 clones, totalling 18 samples in the period. In each sample were randomly chosen five trees per clone and collected three mature leaves, the lower stratum of the canopy of each one of them, totaling 15 leaves per clone and 75 leaves per sample. The leaves presented symptoms of attack by L. heveae.
The leaves were placed in identified paper bags and taken to the laboratory. According to Cividanes et al. (2004a), nymphs and adults of L. heveae are distributed uniformly on the different strata of the plant, not compromising vertical sampling.
In the laboratory, one foliole per leaf was randomly selected, totalling 15 folioles per clone, which were washed with gentle brush stroke, in a 1.5% solution of sodium hypochlorite (2 min) for the removal of residues, including the eggs of other insects that could be found on the foliole surface. The folioles were then washed in distilled water (2 min) and kept on absorbent paper to dry. Afterwards, each foliole was examined under a stereoscopic microscope, and the areas containing eggs of L. heveae were delimited with an indelible marker pen for the determination of the number of eggs/foliole, according to Costa et al. (2003).
The petiole of each foliole was inserted in a plastic tube (tips of a micropipette) containing distilled water, with their opening sealed with Parafilm M®. Afterwards, they were placed in coded plastic bags (12 x 30 cm) inflated with an air compressor, sealed with the aid of an electric sealer and kept under controlled conditions (25 ± 1 ºC, photophase of 12 h).
The occurrence of parasitoids was assessed five days after sampling, and the eggs that remained in the folioles were dissected to check for non-emerged parasitoids. Meteorological data were provided by the Meteorological Station of the “Plantações E. Michelin Ltda.” (17º37’24”S 54º73’87”W).
The treatments consisted of a factorial 5 x 18 (Clones RRIM 600, GT1, PB 217, PB 235 and PR 255 X Collects Seasons) with randomized blocks (each rubber tree consisting of a block). The data were subjected to ANOVA and averages obtained were compared using the test (P x 0.05) (Zar 2009). A test for normality was applied and the total number of L. heveae eggs and of parasitoids were log transformed for data normalization prior to analysis, and the Levene test was applied to check for homocedasticity (Zar 2009). The correlation between the parasitoid and the host occurrence was tested by a Pearson correlation analysis (P x 0.05) (Zar 2009).
Results and Discussion
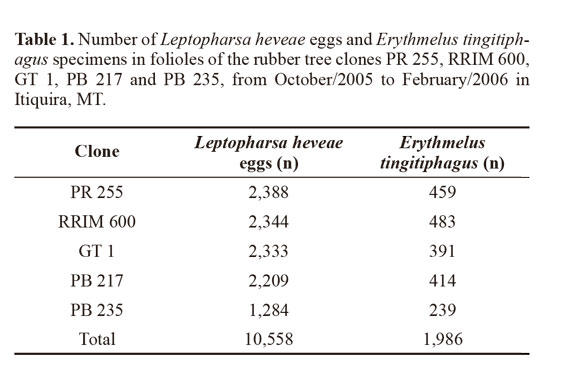
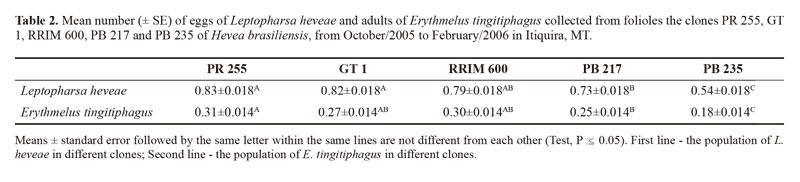
A total of 1,350 folioles were assessed from five clones of the rubber tree in the studied area, and 10558 eggs of L. heveae and 1,986 specimens of the egg parasitoid E. tingitiphagus were sampled (Table 1). Our analyses indicated an interaction between the factors clone type and sampling time influencing the number of eggs of L. heveae sampled: for clone x season (F68;1,260 = 7.38; P 0.001), for clone (F4;1,260 = 46,49; P xx0.001) and for season (F17;1,260 = 61.26; P x 0.001) (Table 2).
The clones with the largest number of eggs of L. heveae were PR 255, GT 1 and RRIM 600, while the clone PB 235 had the lowest number (Table 2). Leptopharsa heveae has shown non-preference for oviposition for a number of different clones of H. brasiliensis (Lara and Tanzini 1997), but the mechanisms leading to a lower oviposition on the clone PB235 remain unknown. Nevertheless, the reduced number of eggs observed on this clone suggests this clone as a good candidate for the identification of sources of resistance to L heveae to be further exploited in genetic improvement programs of H. brasiliensis. Characteristics such as color of the leaves, presence of trichomes, temperature, and plant nutrition were cited as factors in determining preference of insects by their host plants (Mirmohammadi et al. 2009; Oriani and Vendramim 2010; Castillo-López et al. 2010; Ugine 2012).
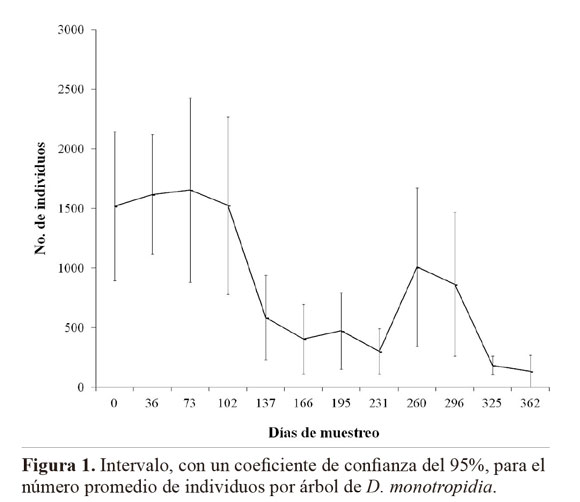
The largest number of eggs of L. heveae was observed in October and November 2005 regardless the clone assessed, coinciding with the beginning of the rainy season (Table 2; Fig. 1). The lowest number of eggs of L. heveae was observed in February 2006, coinciding with the end of the pest cycle in the field in the area (Santos and Freitas 2008) (Fig. 1). The increase in the number of parasitoids observed in November 2005 and the climatic conditions probably contributed to the decline in the population level of L. heveae observed from the end of October and onwards (Fig. 1).
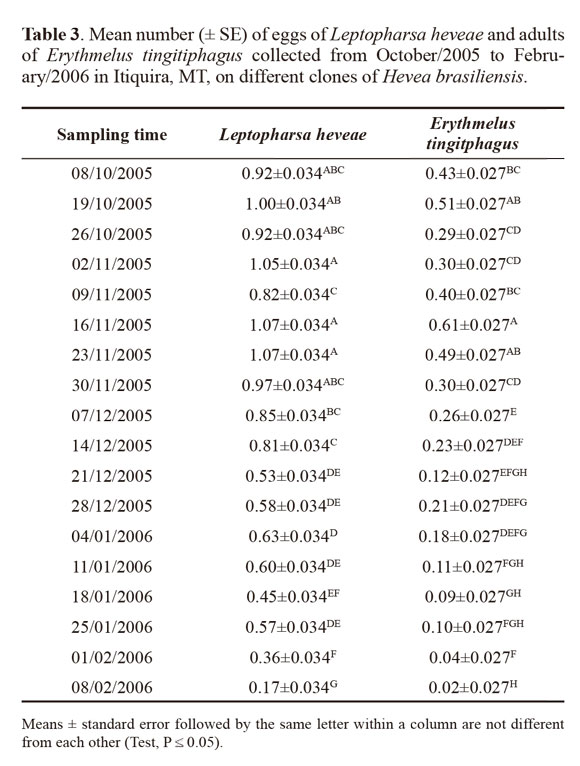
The occurrence of E. tingitiphagus was also significantly affected by the interaction between the tree clone and sampling period: for clone x season (F68;1,260 = 4.04; P x 0.001), for clone (F4;1,260 = 13.47; P x 0.001) and for season (F17;1,260 = 38.45; P x 0.001. As expected due to the host association with the different tree clones, the largest numbers of E. tingitiphagus were collected in PR255, RRIM 600 and GT1 and the lowest in PB 235 (Table 2). Parasitoid occurrence peaked in October and November 2005, while the lowest number of specimens was sampled in February of 2006 (Fig. 1, Table 3). The age of the eggs (Van Duken et al. 1986, Oliveira et al. 2003), the biotic potential of the pest (Caldas et al. 1999, Villas Bôas et al. 2002) and parasitoid (Salvadori 1999; Pratissoli et al. 2004), and the phenology of the studied clones, are all factors known to affect natural enemy efficiency.
In the studied clones, the increase in the number of eggs of L. heveae and of specimens of E. tingitiphagus occurred at the beginning of the rainy season in the region (October), diminishing by February 2006 (Fig. 1). The natural senescence of the clones started from the month of June, reaching its maximum intensity during July and August. Plants would flush again in September and reach their maximum foliage level by mid October. Regrowth, together with the increase in soluble nitrogen in the leaf tissues due to the increase in water availability from the first rains could have favoured the peak occurrence of L. heveae in the period from October to November in the studied clones. The increase in the number of parasitoids is related to the increase in the number of hosts available from October to November (rPR 255 = 0.82; rGT 1 = 0.85; rRRIM 600 = 0.70; rPB 217 = 0.91; rPB 235 = 0.86 com P x 0.05, according to Pearson´s Correlation Analysis).
The results of the population dynamics coincide partially with those obtained by Batista Filho et al. (2003) in Pindorama/SP, who recorded the largest number of nymphs in March, followed by December and January, while adults concentrated in December, January and February, and by those obtained by Cividanes et al. (2004b), also in Pindorama/SP, who observed population peaks of nymphs and adults in March, of adults in June and of nymphs in October. The divergence of results could be related to differences in the meteorological conditions prevailing in the studied locations and to the developmental stage of the tinged which were sampled; in this study, eggs were sampled to conduct the population survey of L. heveae. According to Manian and Udaiyan (1992) and Verma (1999), meteorological factors can cause alterations in the population density of tingid species.
The population growth dynamics of insect pest species and their population peaks are key for developing well sounded strategies for pest control through the use of natural enemies (entomopathogenic fungi, predators and parasitoids), either by the inoculative or inundative release of natural enemies. The intensive occurrence of the egg parasitoid E. tingitiphagus indicates that this species is a promising biocontrol agent of L. heveae to be included in the development of integrated pest management of this pest in rubber tree plantations.
Acknowledgements
We are grateful to the “Plantações Michelin Ltda.” firm for financing the project; to the supervisor Fernando da Silva Fonseca for his suggestions and help, and to the employees Braz da Silva and Silmar Dias Ferreira for help in conducting the experiment; to Dr. Reinaldo José Fazzio Feres (IBILCE/UNESP, São José do Rio Preto, SP) for revision and his suggestions regarding the manuscript and Luciana Maira de Sales Pereira for the English revision.
Literature cited
ABREU, J. M. de. 1996. Aspectos bioecológicos e controle das principais pragas da seringueira no Brasil. Ilhéus, CEPLAC/CEPEC. 21 p. [ Links ]
BATISTA FILHO, A.; LAMAS, C.; LEITE, L. G.; ALMEIDA, J. E. M.; COSTA, V. A.; MARTINS, L. M. 2003. Flutuação populacional do percevejo de renda Leptopharsa heveae em Pindorama, SP. Arquivos do Instituto Biológico (Brasil) 70: 435-439. [ Links ]
CALDAS, C. B. H.; REDAELLII L. R.; DIEFENBACH, L. M. G. 1999. Parâmetros reprodutivos de Corecoris dentiventris Berg (Hemiptera: Coreidae) em cultura de fumo (Nicotiana tabacum). Anais da Sociedade Entomológica do Brasil 28: 595-600. [ Links ]
CASTILLO-LÓPEZ, J. L.; CANO-SANTANA, Z.; OYAMA, K. 2010. Preferencias y supervivencia de Lophoceramica pyrrha, un noctuido gregario constructor de refugios, en dos plantas hospederas. Dugesiana17 (2): 229-236. [ Links ]
CIVIDANES, F. J.; FONSECA, F. S.; SANTOS, T. M. 2004a. Distribuição de Leptopharsa heveae em seringal no Estado de São Paulo. Pesquisa Agropecuária Brasileira 39: 1053-1056. [ Links ]
CIVIDANES, F. J.; FONSECA, F. S.; GALLI, J. C. 2004b. Biologia de Leptopharsa heveae Drake e Poor (Heteroptera: Tingidae) e a relação de suas exigências térmicas com a flutuação populacional em seringueira. Neotropical Entomology 33: 685-691. [ Links ]
COSTA, R. B. 2001. Melhoramento e conservação genética aplicados ao desenvolvimento local - o caso da seringueira (Hevea sp.). Interações (Campo Grande, Brasil) 1: 51-58. [ Links ]
COSTA, V. A.; PEREIRA, C. de F.; BATISTA FILHO, A. 2003. Observações preliminares sobre o parasitismo de ovos de Leptopharsa heveae (Hemiptera: Tingidae) em seringueira em Pindorama, SP. Arquivos do Instituto Biológico 70: 205-206. [ Links ]
FRANCISCO, V. L. F. S.; BUENO, C. R. F.; BAPTISTELLA, C. S. L. 2004. A cultura da seringueira no Estado de São Paulo. Informações Econômicas (Brasil) 34: 31-42. [ Links ]
GINGRICH, R. E. 1993. Biological control of tephritid fruit flies by inundative releases of natural enemies. In: Aluja, M.; Liedo, P. (Eds). Fruit flies, biology and management. New York: Springer-Verlag. 492 p. [ Links ]
INSTITUTO AGRONÔMICO DE CAMPINAS - IAC. 2004. Programa seringueira: importância da cultura. Available in: http://www.iac.sp.gov.br/centros/centro_cafe/seringueira. [Review da-te: 16 July 2012]. [ Links ]
LARA, F. M.; TANZINI, M. R. 1997. Nonpreference of the lace bug Leptopharsa heveae Drake e Poor (Heteroptera, Tingidae) for Rubber Tree Clones. Anais da Sociedade Entomológica do Brasil 26: 429-434. [ Links ]
MANIAN, S.; UDAIYAN, K. 1992. Altitudinal distribution of the Lantana lace bug, Teleonemia scrupulosa Stal., in the Anaimalai hills (Western Ghats), India. Tropical Pest Management 38: 93-95. [ Links ]
MIRMOHAMMADI, S.; ALLAHYARI, H.; NEMATOLLAHI, M. R.; SABOORI, A. 2009. Effect of host plant on biology and life table parameters of Brevicoryne brassicae (Hemiptera: Aphididae). Annals of Entomological Society of America 102: 450-455. [ Links ]
MOREIRA, I. P. S. 1986. Biologia da Leptopharsa heveae (Drake e Poor, 1935) e seus danos nas mudas de Hevea brasiliensis (Müell, 1932). Silvicultura (Brasil) 11: 47. [ Links ]
OLIVEIRA, H. N. de; PRATISSOLI, D.; ZANUNCIO, J. C.; SERRÃO, J. E. 2003. Influência da idade dos ovos de Oxydia vesulia no parasitismo de Trichogramma maxacalii. Pesquisa Agropecuária Brasileira 38: 551-554. [ Links ]
ORIANI, M. A. G.; VENDRAMIM, J. D. 2010. Influence of trichomes on attractiveness and ovipositional preference of Bemisia tabaci (Genn.) B biotype (Hemiptera, Aleyrodidae) on tomato genotypes. Neotropical Entomology 39: 1002-1007. [ Links ]
PRATISSOLI, D., OLIVEIRA, H. N. de; VIEIRA, S. M. J.; OLIVEIRA, R. C. de; ZAGO, H. B. 2004. Efeito da disponibilidade de hospedeiro e de alimento nas características biológicas de Trichogramma galloi Zucchi (Hymenoptera: Trichogrammatidae). Revista Brasileira de Entomologia 48: 101-104. [ Links ]
RONCHI-TELES, B., SILVA, N. M. da. 2005. Flutuação populacional de espécies de Anastrepha Schiner (Diptera: Tephritidae) na região de Manaus, AM. Neotropical Entomology 34: 733-741. [ Links ]
SALVADORI, J. R. 1999. Controle biológico de pulgões de trigo: o sucesso que perdura. Passo Fundo, Embrapa. (Online Technical Press, 27). Available in: http://www.cnpt.embrapa.br/biblio/p_co27.htm. [Review date: 16 July 2012]. [ Links ]
SANTOS, R. S.; FREITAS S. de. 2008. Parasitismo de Erythmelus tingitiphagus (Soares) (Hymenoptera: Mymaridae) em ovos de Leptopharsa heveae Drake and Poor (Hemiptera: Tingidae), plantios de seringueira (Hevea brasiliensis Müell. Arg.). Neotropical Entomology 37: 571-576. [ Links ]
SCOMPARIN, C. H. J. 1997. Estudo dos crisopídeos (Neuroptera, Chrysopidae) em seringueira (Müell. Arg.), aspectos biológicos e potencial no controle biológico de Leptopharsa heveae Drake e Poor (Hemiptera, Tingidae). 173f. Master Science Dissertation, UNESP, Faculdade de Ciências Agrárias e Veterinárias, Jaboticabal. [ Links ]
TANZINI, M. R.; LARA, F. M. 1998. Biologia do percevejo-de-renda-da-seringueira Leptopharsa heveae Drake e Poor (Heteroptera: Tingidae). Ecossistema (Brasil) 23: 65-67. [ Links ]
TANZINI, M. R. 2002. Controle do percevejo-de-renda-da-seringueira (Leptopharsa heveae) com fungos entomopatogênicos. Doctoral thesis. 140f. USP, Escola Superior de Agricultura "Luiz de Queiroz", Piracicaba. [ Links ]
UGINE, T. A. 2012. Developmental times and age-specific life tables for Lygus lineolaris (Heteroptera: Miridae), reared at multiple constant temperatures. Environmental Entomology 41: 1-10. [ Links ]
VAN DUKEN, M. J.; KOLE, M.; VAN, LENTEREN, J. C.; BRAND, A. M. 1986. Host-preference studies with Trichogramma evanescens Westwood (Hymenoptera: Trichogrammatidae) for Mamestra brassica, Pieris brassicae and Pieris rapae. Journal of Applied Entomology 101: 64-85. [ Links ]
VERMA, S. C. 1999. Population buil-up of Teleonemia scrupulosa Stal. on Lantana camara Linn. in mid hill sub-humid zone of Himachal Pradesh (India). Journal of Entomology Research 23: 373-375. [ Links ]
VILLAS BÔAS, G. L.; FRANÇA, F. H.; MACEDO, N. 2002. Potencial biótico da mosca-branca Bemisia argentifolli a diferentes plantas hospedeiras. Horticultura Brasileira 20: 71-79. [ Links ]
ZAR, J. H. 2009. Biostatical analysis. New Jersey, Prentice Hall. 943 p. [ Links ]
WATSON, L.; DALLWITZ, M. J. 1992. The families of flowering plants: descriptions, illustrations, identification, and information retrieval. Available in: http://delta-intkey.com [Review date: 16 July 2012]. [ Links ]