Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488
Rev. Colomb. Entomol. vol.39 no.1 Bogotá Jan./June 2013
Ovitraps placed in dwellings and on public paved areas for Aedes aegypti (Diptera: Culicidae) monitoring
Ubicación de ovitrampas en viviendas y en la vía pública para el monitoreo de Aedes aegypti (Diptera: Culicidae)
Nora Burroni1,5 Veronica Loetti2,5, Paula Prunella3 and NicolÁs Schweigmann4,5
1 Ph. D. de la Universidad de Buenos Aires, orientación Ciencias Biológicas, nburroni@ege.fcen.uba.ar.
2 Licenciada en Ciencias Biológicas. vloetti@ege.fcen.uba.ar. Corresponding autor.
3 Grupo de Estudio de Mosquitos, Depto. de Ecología Genética y Evolución, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires. paulaprunella@hotmail.com.
4 Ph. D. de la Universidad de Buenos Aires, orientación Ciencias Biológicas. Investigador del Consejo Nacional de Investigaciones Científicas y Tecnológicas, Buenos Aires. nicolas@ege.fcen.uba.ar.
5 Grupo de Estudio de Mosquitos, Depto. de Ecología Genética y Evolución, Instituto UBA - CONICET de Ecología, Genética y Evolución de Buenos Aires (IEGEBA), Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires.
Received: 23-May-2012 - Accepted: 26-May-2013
Abstract: The oviposition level between ovitraps placed in human dwellings and on public paved areas for the monitoring of Aedes aegypti was compared,and proposes the best ovitrap installation sites on public paved areas in a neighborhood of Buenos Aires City, Argentina. A total of 60 randomly distributed ovitraps were placed in human dwellings (n=38) and public paved areas (n=22), and examined weekly over a ten-week period. The Ovitrap Positivity Index (OPI) and the Egg Density Index (EDI) were used as indicators of oviposition level. The indexes were calculated on a weekly basis. Environmental variables evaluated for public paved areas were type of corridor, tree height and dwelling height. OPI and EDI values were higher for dwellings than for public paved areas. There was a positive correlation between the infestation indices in dwellings and in public paved areas. In the latter there was a positive association between low OPI and EDI values and tall trees, avenue as type corridor, and dwellings with two stories or more, whereas there was a positive association between high values of these indices and short and medium trees, and a negative association with tall trees, avenue, and dwellings with two stories or more. Our results suggest that the sensitivity of ovitraps in monitoring of A. aegypti in public paved areas is lower than in dwellings, and this sensitivity would increase if considering some environmental variables
Key words: Oviposition. Environmental variables. Argentina.
Resumen: Se comparó el nivel de oviposición entre ovitrampas ubicadas en viviendas y la vía pública para el monitoreo de Aedes aegypti, y se propone el mejor sitio de instalación en la vía pública en un barrio de la ciudad de Buenos Aires, Argentina. Un total de 60 ovitrampas distribuidas al azar fueron ubicadas en viviendas (n=38) y la vía pública (n=22), se examinaron semanalmente por diez semanas. El índice de Positividad de Ovitrampas (IPO) y el Indice de Densidad de Huevos (IDH) utilizados como indicadores del nivel de oviposición, fueron calculados semanalmente. Las variables ambientales evaluadas en la vía pública fueron el tipo de corredor, la altura de los árboles y altura de las viviendas. Los valores obtenidos de IPO e IDH fueron más altos en las viviendas que en la vía pública, y se observó una correlación positiva entre los índices de infestación en viviendas y la vía pública. En este último ambiente, se halló una asociación positiva entre valores bajos de IPO e IDH y árboles altos, corredor tipo avenida, y viviendas con dos o más plantas, se observó también que los valores altos de dichos índices se asociaron en forma positiva con árboles medianos o bajos, y negativamente con árboles altos, avenida, y viviendas con dos o más plantas. Nuestros resultados sugieren que la sensibilidad de las ovitrampas en monitoreos de A. aegypti en vía pública es menor que en las viviendas, y esta sensibilidad en vía pública aumentaría si se consideran algunas variables ambientales.
Palabras clave: Oviposición. Variables ambientales. Argentina.
Introduction
Aedes aegypti (Linneo, 1752) is the main vector of dengue virus in America. Although this mosquito was considered eradicated from Argentina in 1963 (Carcavallo 1968), in 1986 A. aegypti reinfestationwas detected (Curto et al. 2002), and since then been reported several dengue fever outbreaks in this country (Vezzani and Carbajo 2008). Aedes aegypti was found in urban areas adjacent to Buenos Aires City in 1991 (Campos et al. 1993) and within the city in 1995 (Junin et al. 1995). The introduction of the virus in Buenos Aires was detected in 2007 (MSN 2007), reported in early 2009 an outbreak of indigenous dengue in the Buenos Aires Metropolitan area (Seijo et al. 2009). This fact, together with high infestation levels of vector (Schweigmann et al. 2002; Carbajo et al. 2004) may increase the risk of virus transmission in this city.
The distribution of A. aegypti in Buenos Aires shows a spatio-temporal pattern (Carbajo et al. 2004). Peaks of abundance have been reported in February, March and April and oviposition activity from October to May (Schweigmann et al. 2002). The highest oviposition activity has been registered in neighbourhoods with low-rise houses and a few multi-story buildings, which are located in the periphery of the city (Carbajo et al. 2004).
The use of an efficient tool for the detection of vectors allows a better knowledge of the actual vectorial status. In particular, the oviposition trap (ovitrap) is regarded as one of the most sensitive and cheap tools for surveillance of A. aegypti, especially in situations of low vector density (Jakob and Bevier 1969a; Chadee 1991; PAHO 1994; Focks 2003). Despite the wide use of ovitraps (PAHO 1994), studies on factors affecting their efficiency are scarce. Some criteria have been established for the location of ovitraps based on environmental features (Fay and Eliason 1966; Jakob and Bevier 1969b). More recently, some authors have investigated the relationship between the degree of detection and ovitrap installation sites in residences (Chadee 1991, 1992; Dibo et al. 2005).
Generally, it is accepted that the breeding sites of A. aegypti are placed in the dwellings range (PAHO 1994), reason why in many cities the ovitraps are placed in the dwellings (e.g., Chadee 1991, 1992; Dibo et al. 2005; Lenhart et al. 2005). However, the use of ovitraps for monitoring is hampered if the permission to enter a house is refused (Calvo 2008; Stein and Oria 2002), and this may lead to the failure of control measures against A. aegypti (Chadee 1988). Besides dwellings, these devices have been laid on public paved areas to investigate the spatial distribution of the vector (Chadee 1990; Ai-lenn and Song 2000; Schweigmann et al. 2002; Carbajo et al. 2004, 2006). However, no research has so far been conducted to evaluate the use of ovitraps in this environment.
Therefore, we present the results of a preliminary study whose the objectives were to compare oviposition levels between ovitraps placed in dwellings and on public paved areas for the surveillance of A. aegypti, and to identify the best ovitrap installation sites on public paved areas.
Materials and methods
Study area. The climate of Buenos Aires City (34º35’S 58º29’W) is influenced by the De la Plata River. It is temperate and shows a marked seasonality, with an annual mean precipitation of 1,076 mm and a mean temperature of 17.4 °C (National Weather Service).
The study was performed in the periphery of Buenos Aires City, in the neighbourhood of Villa Pueyrredón, in the northeast of this city. An area of approximately four hectares of this neighborhood was chosen by the project "Abordaje ecosistémico para la prevención y el control del vector del dengue en Uruguay y Argentina" by support of IDRC. Our study was carried out in this area. The study area had a total of 328 buildings: residential a 97.6% (320 houses/328 buildings), commercial a 2.1% (7/328), and only one property empty. No park or square was included in the study area.
Villa Pueyrredón is characterised by one-story houses with large gardens and yards, blocks showing a high proportion of vegetation cover and many trees on the pavements. The population in the neighbourhood is 40,235 inhabitants (INDEC 2001). In Villa Pueyrredón, A. aegypti infestation level showed a House Index (the percentage of houses infested with larvae and/or pupae) and a Breteau Index (the number of positive containers divided by number of houses examined per 100) (Breteau 1954) of 50 and 85.7, respectively, for the period February-April 2006.
Oviposition activity of A. aegypti was monitored weekly with ovitraps during ten consecutive weeks between February and April 2006, no were made vector control interventions with insecticide (chemical or biological) during this period. The ovitraps were set at ground level, in shaded or partially shaded sites close to vegetation. Each ovitrap consisted of a black 330 ml-glass jar containing about 100 ml of tap water and a hardboard paddle (10 x 3.2 x 0.35 cm); it was held in vertical position by a paper clip, with its rough side facing the centre. This wood-based material provides a surface suitable for A. aegypti females to lay eggs (Service 1976). No infusion was added to the traps.
Comparison of oviposition levels between public paved areas and. In an area of approximately 4 ha a total of 60 ovitraps were randomly distributed, 38 ovitraps were placed in dwellings and 22 at the base of trees on public paved areas. In each dwelling (house) was placed one ovitrap in yard or garden. None of the ovitraps placed in public paved areas was installed immediately in front of a house that had an ovitrap inside. Mean distance between ovitraps was 25 ± 12.1 m (mean ± SD). In ovitraps placed on public paved areas, the number of traps lost ranged between 0-3/week. Each week, the hardboard paddles and the water in the ovitraps were replaced, and the jars washed. The number of eggs on each hardboard paddle was counted under stereoscopic microscope. Ovitraps were considered positive (infested by A. aegypti) when at least one egg was detected. Each week four paddles randomly selected were conditioned for eggs hatching and larvae rearing. Fourth instar larvae were identified with taxonomic key (Darsie 1985).
The Ovitrap Positivity Index (OPI) and the Egg Density Index (EDI) were used as indicators of oviposition level. These indexes were calculated on a weekly basis, with OPI= (nº positive ovitraps/total nº examined ovitraps) x 100, and EDI= total nº eggs/total nº positive ovitraps (Gomes 1998).
The Mann-Whitney U test (Zar 1996) was used to compare weekly values of OPI and EDI between dwellings and public paved areas. The Spearman’s non-parametric correlation test (Zar 1996) was used to assess the correlation between OPI and EDI for dwellings and public paved areas.
Environmental variables of public paved areas.The three following environmental variables, located within a radius of 6 m from the ovitrap, were registered for each installation site: a) type of corridor: Street (narrow corridor with scarce vehicles and one-way traffic) or Avenue (wide corridor with heavy two-way traffic); b) tree height: Tall (taller than 6 m; smallest distance between the trunk base and edge of foliage higher than 4 m), Medium (height between 4 and 6 m; smallest distance between the trunk base and foliage edge between 2 to 4 m) or Short (height less than 4 m; smallest distance between the trunk base and edge of foliage less than 2 m); c) height of dwellings: H1 (< 2 stories) and H2 (≥ 2 stories). The relationships between OPI and EDI and each of the variables were studied with correspondence analysis (Gauch 1982), dividing the values of the indices into intervals (OPI: low 0-30%, medium >30-70%, and high >70%; EDI: low 0-10 eggs, medium >10-20 eggs, and high >20 eggs).
The temperature and precipitation data used were obtained from the meteorological station at the Jorge Newbery Airport of Buenos Aires City (NCDC 2006). This meteorological station is located about 8 km of study area. The analysis was based on weekly-accumulated rainfall and mean temperatures, which were calculated from mean daily temperatures.
Results and discussion
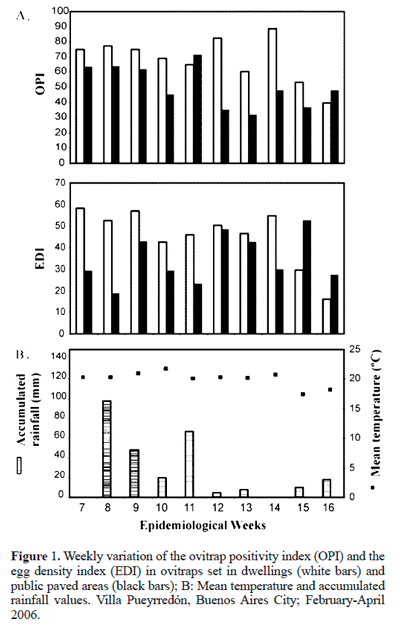
Comparison of oviposition levels between public paved areas and dwellings.The studied period was characterised by a mean weekly temperature of 20 °C (17.4-21.7 °C) and a weekly mean accumulated rainfall of 27.3 mm (Fig. 1).
Of the 330 ovitraps data of dwellings, 65.4% were positive with a total of 10,550 eggs, whereas 50.5% of the 206 ovitraps data of public paved areas were positive with a total of 3,368 eggs.
The OPI ranged from 39.4 to 88.6% (median = 72.0%; lower quartile (LQ): 60.0- upper quartile (UQ): 77.4) for dwellings, and from 31.8 to 71.4% (median = 29.7 eggs; LQ: 36.8- UQ: 63.2) for public paved areas. Between 14 to 16 epidemiological weeks, OPI values for dwellings exceeded approximately from 18 to 135% those for public paved areas, with the former being lower than the latter on two occasions only (9 and 17%, Fig. 1).
The EDI ranged from 6.4 to 43.7 eggs (median = 48.5 eggs; LQ: 42.6- UQ: 54.8) for dwellings and from 12 to 26.6 eggs (median = 29.7 eggs; LQ: 27.4- UQ: 42.9) for public paved areas. In 14 to 16 epidemiological weeks, EDI values for dwellings exceeded approximately from 4 to 179% those for public paved areas, with the former being lower than the latter on two occasions only (41 and 44%, Fig. 1).
There were significant differences in the presence and density of eggs between dwellings and public paved areas, with higher values for dwellings (OPI: U(10,10) = 17; P= 0.013; EDI: U(10,10) = 23; P = 0.041).
There was a significant positive correlation between both indices for dwellings (rs = 0.75; P< 0.05) and public paved areas (rs = 0.74; P< 0.05).
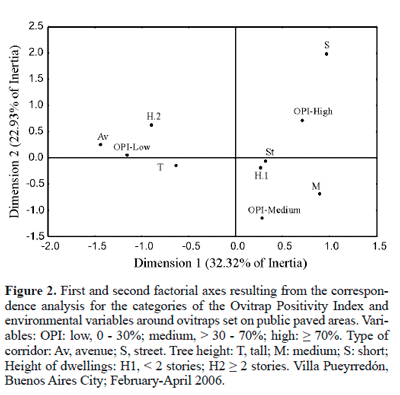
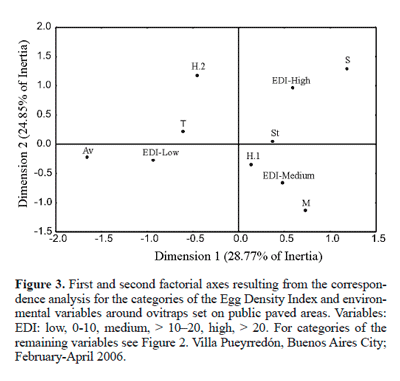
Environmental variables of public paved areas. The correspondence analysis showed that both OPI and EDI were similarly associated with the different categories of the environmental variables. The percentage of the variance explained by the first two factorial axes was 55.2% for OPI (Fig. 2) and 53.6% for EDI (Fig. 3).
For both indices, the category "low" was positively associated with tall trees, avenue, and dwellings with two stories or more, whereas the category "high" was positively associated with short and, to a lower extent, with medium trees; finally, the categories "high" and "medium" were negatively associated with tall trees, avenue and dwellings with two stories or more (Figs. 2 and 3).
The dwellings were better for ovitrap installation than public paved areas based on the comparison of their respective OPI and EDI values. Since our ovitraps not had hay infusion, these would probably be as attractive as ordinary water-holding containers. Thus, probably, ovitraps inside houses had more containers to compete against in comparison with the ones in the public paved areas. Despite that, the ones inside houses collected more eggs. Taking into account that A. aegypti lives in high association with humans, we expected that they blood feed and rest inside houses. Then, we may expect gravid females searching in the immediate surroundings a place to lay eggs. In that case, ovitraps in dwellings would have a higher probability to receive mosquito eggs than the public paved areas. The difference between urban environments should be taken into account when data from ovitraps are used to identify high-priority areas for the implementation of control measures, as proposed by Regis et al. (2008).
A decrease in both indexes was observed in middle of April (epidemiological weeks 15 and 16), when the population density of this mosquito begins to decrease in Buenos Aires City (Carbajo et al. 2004) and coincided with a period of low rainfall (Fig. 1). In addition, the values of the indexes were higher for public paved areas than for dwellings only at these dates. Likely, low rainfall reduce availability of oviposition sites and, since Aedes dispersion is basically oviposition guided (Reiter 2007), the highest oviposition activity on public paved areas during the period of decreased population density may indicate an increased a tendency by females to disperse over a larger area.
The positive relationship between OPI and EDI found for both dwellings and public paved areas is in agreement with that reported by Chadee (1992) and Dibo et al. (2005) for other urban environments in America. A practical application of this positive correlation is to estimate the mean number of eggs per ovitrap without counting them (Mogi et al. 1990).
The features of the immediate environment are important in the selection of oviposition sites by A. aegypti (e.g., Christophers 1960; Focks 2003). For example, the oviposition pattern observed in dwellings may vary according to ovitrap location (Dibo et al. 2005; Chadee 1991, 1992). In this sense, the analysis performed in this paper relating some surrounding environmental variables with ovitrap positivity and number of A. aegypti eggs can contribute to establish the best ovitrap installation sites on public paved areas. The correspondence analysis revealed that the highest oviposition activity of A. aegypti was positively associated with short and medium trees. The fact that the foliage of these trees is closer to the ovitrap than that of tall ones may create conditions (air temperature, relative humidity, shade, etc) favourable for vector activity. It is generally accepted that temperature and relative humidity affect the survival of A. aegypti adults (Christophers 1960). However, there is no consensus on whether females prefer shady sites for oviposition; this was observed by some authors (Kittayapong and Strickman 1993; Tun-Lin et al. 1995; Espinoza Gómez et al. 2001), but not by Chadee (1991, 1992), and was considered by Vezzani et al. (2005) as a function of the spatial scale. On the other hand, the correspondence analysis suggests that ovitraps placed under tall trees, closer to avenues, and surrounded by houses with two or more stories are less attractive to A. aegypti females for laying eggs. The low values of the positivity index are likely to be related to tall trees, which may not provide suitable conditions for oviposition. Likewise, the avenues, namely wide corridors with a large number of pedestrians and heavy vehicular traffic, may act as a hindrance to A. aegypti oviposition.
The negative association between oviposition activity of A. aegypti and high-rise buildings is concordant with the pattern observed by Carbajo et al. (2004) at the scale of Buenos Aires City. These authors suggested that the low infestation levels in areas with high-rise buildings are related to scanty vegetation, low availability of containers and/or low connectivity within these areas. Due to the scarce number of high buildings in the neighbourhood of Villa Pueyrredón, our study had few houses with two stories or more (5/22). Thus, it’s unlikely that would affect female dispersal. More detailed studies are necessary to evaluate if houses with two stories or more may be indicated a lower resource availability indirectly.
In brief, our study suggests that ovitraps on public paved areas show a lower oviposition level than in dwellings. Additionally, different environmental variables of public paved areas that serve to improve the detection of oviposition activity were identified.
Acknowledgments
This work was supported by the International Development Research Center- Canada (IDRC), through the Project Nº 101814-002 (2005-2007) grants. We thank the residents of the neighbourhood of Villa Pueyrredón, who facilitated the work by allowing income to their homes, and logistically supporting the field work.
Literature cited
Ai-leen, G. T.; Song, R. J. 2000. The use of GIS in ovitrap monitoring for dengue control in Singapore. Dengue Bulletin 24: 110-116. [ Links ]
Calvo, N. A. 2008. Variación espacial y temporal de la abundancia de Aedes aegypti (Diptera: Culicidae) en dos áreas contiguas de un barrio de la Ciudad Autónoma de Buenos Aires, Licenciate Thesis, Universidad de Buenos Aires. 62 p. [ Links ]
Campos, R. E.; Maciá A.; García, J. J. 1993. Fluctuaciones estacionales de culícidos (Diptera) y sus enemigos naturales en zonas urbanas de los alrededores de La Plata, provincia de Buenos Aires. Neotrópica 39 (101-102): 55-66. [ Links ]
Carbajo, A.; GÓmez S.; Curto, S.; Schweigmann, N. 2004. Variación espaciotemporal del riesgo de transmisión de dengue en la ciudad de Buenos Aires. Medicina (Buenos Aires) 64 (3): 231-234. [ Links ]
Carbajo, A.; Curto, S.; Schweigmann, N. J. 2006. Spatial distribution pattern of oviposition in the mosquito Aedes aegypti in relation to urbanization in Buenos Aires: southern fringe bionomics of an introduced vector. Medical and Veterinary Entomology 20 (2): 209-218. [ Links ]
Carcavallo, R.U.; Martínez, A. 1968. Fiebre amarilla, vectores y cadena epidemiológica. pp. 105-144. En Carcavallo, R. U.; Martínez, A. Entomoepidemiología de la República Argentina. XXIII comunicaciones científicas de las fuerzas armadas, Buenos Aires, 341 p. [ Links ]
Chadee, D. D. 1988. Effects of "closed" houses on the Aedes aegypti eradication programme in Trinidad. Medical and Veterinary Entomology 2 (2): 193-198. [ Links ]
Chadee, D. D. 1990. Aedes aegypti surveillance in Tobago, West Indies (1983-88). Journal of the American Mosquito Control Association6 (1): 148-150. [ Links ]
Chadee, D. D. 1991. Seasonal incidence and vertical distribution patterns of oviposition by Aedes aegypti in an urban environment in Trinidad, W.I. Journal of the American Mosquito Control Association 7 (3): 383-386. [ Links ]
Chadee, D. D. 1992. Seasonal incidence and horizontal distribution patterns of oviposition by Aedes aegypti in an urban environment in Trinidad, W.I. J. Journal of the American Mosquito Control Association 8 (3): 281-284. [ Links ]
Christophers, S. R. 1960. Aedes aegypti (L.) the yellow fever mosquito. Its life history, binomics and structure, Cambridge University Press, London, 739 p. [ Links ]
Curto, S. I.; Boffi R.; Carbajo, A. E.; Plastina, R.; Schweigmann, N. 2002. Reinfestación del territorio argentino por Aedes aegypti. Distribución geográfica (1994-1999). pp. 127-137. In: OD Salomón, Actualizaciones en Artropodología Sanitaria Argentina, Fundación Mundo Sano, Buenos Aires, 302 p. [ Links ]
Darsie, R.F. Jr. 1985. Mosquitoes of Argentina. Part I, keys for identification of adult females and fourth stage larvae in English and Spanish (Diptera, Culicidae). Mosquito Systematic 17: 153-253. [ Links ]
Dibo, M. R.; Chiaravalloti-Neto, F.; Battigaglia, M.; Mondini, A.; Favaro, E.; Barbosa, A.; Glasser, C. 2005. Identification of the best ovitrap installation sites for gravid Aedes (Stegomyia) aegypti in residences in Mirassol, state of São Paulo, Brazil. Memórias do Instituto Oswaldo Cruz 100 (4): 339-343. [ Links ]
Espinoza Gómez, F.; Hernández Suárez, C. M.; Coll Cárdenas, R. 2001. Factores que modifican los índices larvarios de Aedes aegypti en Colima, México. Revista Panamericana de Salud Pública10 (1): 6-12. [ Links ]
Fay, R. W.; Eliason, D. A. 1966. A preferred oviposition site as a surveillance method for Aedes aegypti. Mosquito News 26 (4): 531-535. [ Links ]
Focks, D. A. 2003. A review of entomological sampling methods and indicators for dengue vectors. Special Program for Research and Training in Tropical Diseases (TDR), UNICEF, UNDP, World Bank, World Health Organization. Available from: http://whqlibdoc.who.int/hq/2003/TDR_IDE_DEN_03.1.pdf Review date: 20 May 2012. [ Links ]
Gauch, H. G. 1982. Multivariate Analysis in Community Ecology, Cambridge Unversity Press, London, 298 p. [ Links ]
Gomes, A. C. 1998. Medidas dos níveis de infestação urbana para Aedes (Stegomyia) aegypti e Aedes (Stegomyia) albopictus em programa de vigilancia entomológica. Informe Epidemiológico do SUS (Brazil) 7(3): 49-57. [ Links ]
INDEC - Instituto Nacional de Estadísticas y Censos 2001. Censo Nacional de Población, Hogares y Viviendas 2001, Serie 2 - Resultados generales, N° 1 Ciudad de Buenos Aires. Published in CD-ROM ISBN 950-896-305-0. [ Links ]
JaKob, W. L.; Bevier, G. A. 1969a. Application of ovitraps in the U.S. Aedes aegypti eradication program. Mosquito News 29 (1): 55-62. [ Links ]
JaKob, W. L.; Bevier, G. A. 1969b. Evaluation of ovitraps in the U.S. Aedes aegypti eradication program. Mosquito News29: 650-653. [ Links ]
Junín, B.; Grandinetti, H.; Marconi, J. M.; Carcavallo, R. U. 1995. Vigilancia del Aedes aegypti (L.) en la Ciudad de Buenos Aires. Entomología y Vectores 2: 71-75. [ Links ]
Kittayapong, P.; Strickman, D. 1993. Distribution of container-inhabiting Aedes larvae (Diptera: Culicidae) at a dengue focus in Thailand. Journal of Medical Entomology 30(3): 601-606. [ Links ]
Lenhart, A. E.; Walle, M.; Cedillo, H.; Kroeger, A. 2005. Building a better ovitrap for detecting Aedes aegypty oviposition. Acta Tropica 96 (1): 56-59. [ Links ]
MSN - Ministerio de Salud de la Nación 2007. Dirección de Epidemiología. Actualización de la Situación Dengue Argentina y Mercosur. Available from: http://www.msal.gov.ar/htm/site/sala_situacion/PANE-LES/boletines/07-bonr28.xls Review date: 1 Jun 2007. [ Links ]
Mogi, M.; Choochote, W.; Khamboonruang, C.; Suwanpanit, P. 1990. Applicability of prersence-absence and sequential sampling for ovitrap surveillance of Aedes (Diptera: Culicidae) in Chian Mai, Northern Thailand. Journal of Medical Entomology 27: 509-514. [ Links ]
NCDC - National Climate Data Center 2006 - data base on the Internet National Environmental Satellite, Data and Information Service (NESDIS) . U. S. Department of Commerce Available from: http://www.ncdc.noaa.gov/CDO/cdodateoutmod.cmd Review date: 9 Nov 2006. [ Links ]
PAHO - Pan American Health Organization 1994. Dengue and dengue haemorragic fever in the americas: Guidelines for prevention and control, scientific publication N° 548, PAHO, Washington, D. C, 98 p. [ Links ]
Regis, L.; Monteiro A. M.; de Melo Santos, A. M. V.; Silveira, Jr J. C.; Furtado, A. F.; Acioli, R. V.; Santos, G. M.; Nakazawa, M.; Carvalho, S. A.; Ribeiro, Jr P. J.; de Souza, W. V. 2008. Developing new approaches for detecting and preventing Aedes aegypti population outbreaks: basis for surveillance, alert and control system. Memórias do Instituto Oswaldo Cruz103 (1): 50-59. [ Links ]
REITER, P. 2007. Oviposition, Dispersal, and Survival in Aedes aegypti: Implications for the Efficacy of Control Strategies. Vector-Borne and Zoonotic Diseases 7 (2): 261-273. [ Links ]
Schweigmann, N.; Orellano, P.; Kuruc, J.; Vera, M. T.; Vezzani, D.; Méndez, A. 2002. Distribución y abundancia de Aedes aegypti (Diptera: Culicidae) en la ciudad de Buenos Aires. pp. 155-160. In OD Salomón, Actualizaciones en Artropodología Sanitaria Argentina, Publicación Monográfica 2, Buenos Aires, 302 p. [ Links ]
Seijo, A.; Romer, Y.; Espinosa, M.; Monroig, J.; Giamperetti, S.; Ameri, D.; Antonelli, L. 2009. Brote de dengue autóctono en el área metropolitana Buenos Aires experiencia del hospital de enfermedades infecciosas F. J. Muñiz. Medicina (Buenos Aires) 69 (6): 593-600. [ Links ]
Service, M. W. 1976. Mosquito Ecology. Field Sampling Methods, Applied Science Publishers Ltd., London, 583 p. [ Links ]
Stein, M.; Oria, G. I. 2002. Identificación de criaderos de Aedes aegypti (Diptera: Culicidae) y cálculo de índices de infestación en la provincia de Chaco. pp. 161-166. In: Salomón, O. D.Actualizaciones en Artropodología sanitaria Argentina, Publicación Monográfica 2, Buenos Aires, 302 p. [ Links ]
Tun-Lin, W.; Kay, B. H.; Barnes, A. 1995. Understanding productivity, a key to Aedes aegypti surveillance. American Journal of Tropical Medicine and Hygiene 53 (6): 595-601. [ Links ]
Vezzani, D.; Carbajo, A. E. 2008. Aedes aegypti, Aedes albopictus, and dengue in Argentina: current knowledge and future directions Memórias do Instituto Oswaldo Cruz 103 (1): 66-74. [ Links ]
Vezzani, D.; Rubio, A.; Velázquez, S. M.; Schweigmann, N.; Wiegand, T. 2005. Detailed assessment of microhabitat suitability for Aedes aegypti (Diptera: Culicidae) in Buenos Aires, Argentina. Acta Tropica 95 (2): 123-131. [ Links ]
Zar, J. H. 1996. Biostatistical Analysis, Prentice Hall, New Jersey, 662 p. [ Links ]