Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488
Rev. Colomb. Entomol. vol.39 no.2 Bogotá July/Dec. 2013
Toxic effects of virtako on the brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae)
Efectos tóxicos del virtako sobre el saltahojas marrón Nilaparvata lugens (Hemiptera: Delphacidae)
YONG CHEN1, XIAOWA QING1, JIE LIU1, JIE ZHANG2 and RUNJIE ZHANG1,3
1 Ph. D. State Key Laboratory for Biocontrol and Institute of Entomology, Sun Yat-Sen University, Guangzhou 510275, P. R. China.
2 Postdoctor, Institute of Biotechnology and Germplasm Resources, Yunnan Academy of Agricultural Sciences, Kunming, 650223, China.
3 Ph. D. Profesor. Tel./fax: +86 (020) 84110168. lsszrj@mail.sysu.edu.cn. Corresponding author.
Received: 20-Feb-2013 - Accepted: 3-Dic-2013
Abstract: Laboratory assays (Institute of Entomology, Sun Yat-Sen University, Guangzhou, China) were conducted to assess the potential of virtako, a mixture insecticide, which contains 20% chlorantraniliprole and 20% thiamethoxam, against the brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae). The toxic effects of virtako against the nymphal instars of N. lugens indicated that all instars were sensitive to the five concentrations (16, 8, 4, 2, 1 mg/L). The first-second instars were the most susceptible, and the median lethal concentrations [LC50] were 4.76, 1.96 and 0.85 mg/L at 24, 48 and 72 h after treatment, respectively. Fifth-instars were the least susceptible with the LC50 values of 23.76, 7.25 and 3.95 mg/L at 24, 48 and 72 h after treatment, respectively, and were significantly greater than those of the first - second instars. The LC50s of the third - fourth instars were 11.59, 5.72 and 2.17 mg/L at 24, 48 and 72 h after treatment, respectively. These results indicate that virtako might be an effective alternative for the control of BPH N. lugens, by delaying the resistance levels of thiamethoxam.
Key words: Brown planthopper. Toxicity. Laboratory assays. Lethal concentrations.
Resumen: Ensayos de laboratorio (Institute of Entomology, Sun Yat-Sen University, Guangzhou, China) fueron llevados a cabo con el objeto de determinar el potencial de virtako, una mezcla insecticida, que contiene 20% de clorantra-niliprole y 20% de tiametoxam, contra el saltahojas marrón, Nilaparvata lugens (Hemiptera: Delphacidae). Los efectos tóxicos del virtako sobre los estadios ninfales de N. lugens indicaron que todos ellos fueron susceptibles a las cinco concentraciones (16, 8, 4, 2, 1 mg/L). El primero-segundo estadios fueron los más susceptibles, y las concentraciones media letales [CL50] fueron 4,76, 1,96 y 0,85 mg/L a 24, 48 y 72 h, respectivamente, después del tratamiento. El estadio quinto fue el menos susceptible con valores de CL50 de 23,76, 7,25 y 3,95 mg/L a 24, 48 y 72 h, respectivamente, después del tratamiento. Estos fueron significativamente mayores comparados con los estadios primero-segundo. Las CL50s para los estadios tercero - cuarto fueron 11,59, 5,72 y 2,17 mg/L a 24, 48 y 72 h respectivamente, después del tratamiento. Estos resultados indican que virtako puede ser una alternativa efectiva en el control del saltahojas marrón N. lugens, al retardar los niveles de resistencia al tiametoxam.
Palabras clave: Saltahojas marrón. Toxicidad. Ensayos de laboratorio. Concentraciones letales.
Introduction
Insecticides have been used to control many key agricultural pests and have greatly improved agricultural production worldwide. In the integrated pest management (IPM) system, insecticides remain as key and popular weapons as there are quick, efficient, cost-effective and easy to use against pests (Karunaratne et al. 2007; Xue et al. 2010; Perry et al. 2011).
Brown planthopper, Nilaparvata lugens (Stál) (BPH) (Hemiptera: Delphacidae), is one of the most economically important insects of rice in most Asian countries. Both nymphs and adults of the BPH damage rice directly by removing nutrients and indirectly by transmitting rice pathogens, e.g., ragged stunt virus, striped virus and grassy stunt virus (Ghaffar et al. 2011; Gurr et al. 2011). Recently, most Chinese rice growers depend solely on chemicals to manage N. lugens. This continuous and indiscriminate use of one insecticide has resulted in the rapid development of insecticide resistance and exhaustion of most insecticide options in many rice-growing regions. For example, the resistance levels of BPH to imidacloprid in Nanning, Haiyan, Nanjing and Tongzhou populations has reached 200-799 fold compared with the susceptible strain because of continuous use of it to control this pest (Wang et al. 2009).
To avoid these problems, alternative insecticides must be developed for use against BPH to better manage the development of resistance to old ones. Mixture pesticides could have greater efficacy than individual pesticides in that they could manage several insects at a time, reduce the chemical concentrations needed for control, and play a synergistic action on pest control (Han et al. 1993; Corbel et al. 2003; Peng et al. 2010). Such a strategy has been recognized as an effective method to prolong older classes of insecticides service life, and it is also an acknowledged strategy for managing insecticide resistant populations via multiple attacking (McKenzie 1996; Tao et al. 2006; Ghafoor et al. 2011). Although few studies have involved in the use of mixed pesticides against pests, it has been widely used to combat insect pests in China where more than 2,200 different mixture pesticides are registered for use (Peng et al. 2010).
Virtako is a new mixed insecticide that contains 20% chlorantraniliprole and 20% thiamethoxam, developed in Switzerland during the 2000s by Syngenta. Thiamethoxam is presently one of the most effective chemicals for the control of sucking pests, including aphids, whiteflies, thrips, and some microlepidoptera (Sharma & Lal 2002). Chlorantra-niliprole is a new insecticide belonging to the anthranilic di-amide class. It is considered to be highly toxic to lepidopteran, coleopteran, and dipteran pests in several crops (Xu et al. 2008). Virtako provides the advantages of each of the two insecticides and is also considered to have low mammalian toxicity, high insecticidal activities and broad spectrum to Lepidoptera, Coleoptera, Diptera, Hemiptera and Orthoptera pests. Recently, virtako has been used for control of Cnapha-locrocis medinalis Guenee, and Lissorhoptrus Oryzophilus Kuschel in China (Yang et al. 2010; Geng et al. 2011).
To date, there is little information on the efficacy of virtako against BPH. Detailed information only exists in non-English language (especially Chinese) publications or in institutional reports. Thus, the objective of this study was to determine the effects of virtako on the nymphal stages of brown planthopper using laboratory immersion assays.
Materials and methods
Insects and insecticides. An insecticide-susceptible strain of BPH was maintained on rice seedlings at 26 ± 1 °C with a 16:8 h (L:D) photoperiod for more than three years at the Institute of Entomology, Sun Yat-Sen University, Guangzhou, China, without any exposure to insecticide. Virtako with a purity of 40.0% was obtained from Syngenta AG (Switzerland).
Bioassay. Bioassays were undertaken using the rice-stem dipping method (Zhuang et al. 1999; Wang et al. 2008; Zhang et al. 2010). Virtako was diluted in distilled water to five concentrations (a.i), each of which was half of the previous concentration (16.0, 8.0, 4.0, 2.0, 1.0 mg/L). Rice plants at tillering to booting stage were washed thoroughly and rice stems (about 10 cm long) with roots were cut and air dried to remove excess water. Three rice stems were grouped and dipped into different insecticide solutions for 30 sec. After the rice stems air dried, moistened cotton was used to wrap the rice roots. The treated rice stems were then placed into a 500-ml plastic cup. BPH nymphs were divided into three groups, first - second instars, third - fourth instars, and fifth instars. Thirty nymphs of the same group were introduced into each plastic cup using a vacuum device. All three groups were used as virtako test insects. Each concentration was replicated three times; a distilled water treatment was used as a control. Mortality of the nymphs was recorded every 24h after treatment. Treated insects were maintained at a temperature of 26 ± 1 °C with a 16: 8 h (L: D) photoperiod. The nymphs were considered dead if they failed to move after being gently prodded with a fine brush. Data with 20% mortality in the control treatment were rejected, and the assays were repeated.
Data analysis. Mortality data were subjected to analysis of variance (ANOVA of arcsine, logarithmic and square root transformed percentages). Significant differences were determined by using Tukey's honestly significant difference (HSD) test (P < 0.05) with SPSS software (version 17.0, SPSS Inc., Chicago, IL). Mortality was corrected using Abbott's formula, if necessary. Lethal concentrations (LC30, LC50 and LC90) were calculated using probit analysis; values were expressed as means ± SE (SE) of 3 replicates. The indices of toxicity measurement derived from this analysis were: LC30 = lethal concentration that causes 30% response (mortality) of exposed organisms to the corresponding insecticide.
LC50 = median lethal concentration that causes 50% response (mortality) of exposed organisms to the corresponding insecticide.
LC90 = lethal concentration that causes 90% response (mortality) of exposed organisms to the corresponding insecticide.
Results
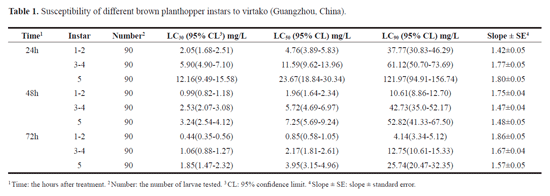
Laboratory test results showed that different instars of BPH had different levels of susceptibility to virtako (Table 1). The fifth instars were the least susceptible to virtako than the first -second and the third - fourth instars with an observed toxicity by stage being fifth instars < third - fourth instars < first - second instars. At 72h after treatment, The LC50 value was 3.95 mg/L (x2 = 0.52; df = 3; P > 0.05) for fifth instars, 2.17 mg/L (X2 = 3.60; df = 3; P > 0.05) for third - fourth instars, and 0.85 mg/L (x2 = 4.90; df = 3; P > 0.05) for first - second instars.
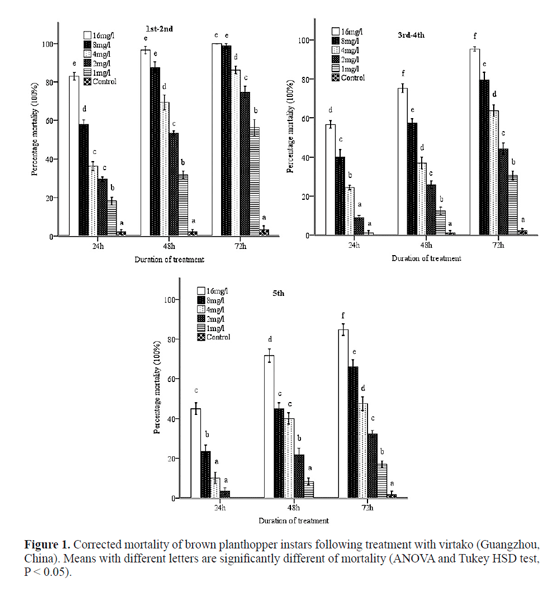
Greater numbers of nymphs were killed at a faster rate with the higher concentrations (16 mg/l and 8 mg/l) than at the median (4 mg/l and 2 mg/l) and lower (1 mg/l) concentrations of virtako (Fig. 1), while mortality in the control (treated with distilled water) remained low. No significant differences (P > 0.05) in mortality of the first - second instars treated with 4 and 2 mg/L were observed at 24 h after treatment, but significant differences (P < 0.05) was observed by 48 and 72 h after treatment. Significant differences (P < 0.05) in mortality were observed between 16 and 8 mg/1 24 h after treatment, but no significant differences (P > 0.05) was observed by 48 and 72 h after treatment. However, significant differences (P < 0.05) in mortality were observed with other concentrations of virtako or the control. At 24 h after treatment, mortality in the third-fourth instars treated with 2 and 1 mg/L was no significant difference (P > 0.05). However, significant differences (P < 0.05) in mortality were observed with other concentrations of virtako or the control. For fifth instars, at 72 h after treatment, significant differences (P < 0.05) in mortality were observed with other concentrations of virtako or the control.
Discussion
In the laboratory bioassays, the 72-h LC50 value of virtako against 3-4 instars of N. lugens was 2.17 mg/L, much higher than 96-h LC50 value of thiamethoxam (0.11 mg/L) against 3 instars, which was reported by Wang et al. (2009). These different toxic effects on BPH are due to the combination of highly toxic thiamethoxam and the less toxic chlorantranilip-role in virtako. The use of brown planthopper populations from distinctly different geographic areas and different instars may be also influence the results.
In recent years, the BPH has caused severe damage to rice plants in most east and south-east Asian countries (Matsumura et al. 2008). Insecticides have been used extensively to control N. lugens, resulting in the development of resistance to many insecticides (Endo and Tsurumachi 2001; Yoo et al. 2002; Zhang et al. 2010). A mix of two unrelated insecticides may be more effective for managing insect pests than the alternate use of single insecticides (Corbel et al. 2003), which reduced the amount of pesticides to the target insects and was an important strategy to suppress the resistance level (Yin 2010; Zhang et al. 2010). Thiamethoxam and chlorantranilip-role belong to different groups of insecticides and have different pesticidal mechanisms. Thiamethoxam has been used for controlling N. lugens for a long time and resulted in a gradual decrease of efficacy against the pest, with the resistance levels in Ningbo, Hangzhou and Shaoguan populations ranging from 9.4 to 15.8 fold compared with the susceptible strain (Wang et al. 2009; Liu et al., 2010). Furthermore, the BPH was not the target pest of chlorantraniliprole (Xu et al. 2008). In our study, virtako affected various nymphal instars of brown planthopper (first - second, third - fourth, and fifth) at all five concentrations (16, 8, 4, 2, 1 mg/L). Higher rates of mortality were obtained with higher concentrations. Vir-tako caused 100% mortality of the first - second instars and nearly 100% mortality of the third - fourth and fifth instars at 16 mg/L at 72 h after treatment. In general, the increase of mortality was related to both time and virtako concentrations. Thus virtako may be used as an alternative insecticide to delay the resistance levels of thiamethoxam to control N. lugens. Virtako appears to be a potential candidate for the further management of BPH in rice production.
Acknowledgements
This study was supported by the National Science and Technology Support Project (200SBADA5B05) and Guangdong Province Science, Technology Support Project (2007A020100004-4) and Science and Technology Program of Yunnan province (2012FD069). Our thanks are also given to Dr. Ke Shen (Yunnan Academy of Agricultural Sciences, Kaiyuan, 661600, China) for providing help with the language used.
Literature cited
CGRBEL, V.; RAYMGND M.; CHANDRE F.; DARRIET F.; HGÜ-GARD, J. M. 2003. Efficacy of insecticide mixtures against larvae of Culex quinquefasciatus (Say) (Diptera: Culicidae) resistant to pyrethroids and carbamates. Pest Management Science 60: 375-3S0. [ Links ]
ENDG, S.; TSÜRÜMACHI, M. 2001. Insecticide susceptibility of the brown planthopper and the white-back planthopper collected from southeast Asia. Journal of Pesticide Science 26: S2-S6. [ Links ]
GENG, P.; YANG, L.; HU, M. Y.; AN, G. D. 2011. Efficacy of 40% chlorantraniliprole. thiamethoxam WDG against rice pests. Guangdong Agricultural Sciences 3: 63-64, 73. [ Links ]
GHAFFAR, M. B.; PRITCHARD, J.; LGYD, B. F. 2011. Brown pl-anthopper (N. lugens Stal) feeding behaviour on rice germplasm as an indicator of resistance. PLoS ONE 6(7): e22137. [ Links ]
GHAFGGR, A.; KHAN, M. S.; MAHMGGD, A.; AHMAD, F.; HASSAN, M.; ANEES, A. R. 2011. Resistance status of He-licoverpa armigera (Hub) against mixture of Profenofos and Indoxacarb (1:1) insecticides at Faisalabad, Pakistan. World Applied Sciences Journal 13(1): 90-94. [ Links ]
GÜRR, G. M.; LIÜ, J.; READ, D. M. Y.; CATINDIG, J. L. A.;CHENG, J. A.; LAN, L. P.; HEGNG, K. L. 2011. Parasitoids of Asian rice planthopper (Hemiptera: Delphacidae) pests and prospects for enhancing biological control by ecological engineering. Annals of Applied Biology 15S: 149-176. [ Links ]
HAN, X. L.; QIAN, C. F.; CHEN, F. H. 1993. China agricultural encyclopedia. China Agricultural Press. Beijing. China. 126 p. [ Links ]
KARÜNARATNE, S. H. P. P.; WEERAKGGNL, K. C.; NÜNGALIYADDA, L.; MANÜWEERA, G.K. 2007. Susceptibilityof rice insect pests and their natural enemies to commonly used insecticides. Journal of the National Science Foundation of SriLanka 35 (2): 97-102. [ Links ]
LIÜ, X. G.; ZHAG, X. H.; WANG, Y. H.; WE, J. J.; SHEN, J. L.; KGNG, J.; CAG, M. Z.; ZHGÜ, W. J.; LÜG, C. H. 2010. Dynamic changes of resistance to fipronil and neonicotinoid insecticides in brown planthopper, Nilaparvata lugens (Homoptera: Delphacidae). Chinese Journal of Rice Science 24 (1): 73-S0. [ Links ]
MATSÜMÜRA, M.; TAKEÜRCHI, H.; SATGH, M.; MGRIMÜ-RA, S. S.; GTÜKA, A.; WATANABE, T.; THANH, D. V. 200S. Species-specific insecticide resistance to imidacloprid and fipro-nil in the rice planthoppers Nilaparvata lugens and Sogatella furcifera in East and South-east Asia. Pest Management Science 64 (11): 1115-1121. [ Links ]
McKENZIE, J. A. 1996. Applying the theory, the better management of resistance and pests: Ecological and Evolutionary Aspects of Insecticide Resistance. Academic Press. The Austin. pp. 149-173. [ Links ]
PENG, Y., SHAO, X. L.; HOSE, G. C.; LIU, F. X.; CHEN, J. 2010. Dimethoate, fenvalerate and their mixture affect Hyly-phantes graminicola (Araneae: Linyphiidae) adults and their unexposed offspring. Agricultural and Forest Entomology 12: 343-351. [ Links ]
PERRY, T.; BATTERHAM, P.; DABORN, P. J. 2011. The biology of insecticidal activity and resistance. Insect Biochemistry and Molecular Biology 4: 411-422. [ Links ]
SHARMA, D. R.; LAL, O. P. 2002. Bio-efficacy of thiamethoxam in comparison to recommended insecticides against leafhopper and white fly of brinjal (Solanum melongena L.). Journal of Entomological Research 26 (3): 257-262. [ Links ]
TAO, L. M.; YANG, J. Z.; ZHUANG, P. J.; TANG, Z. H. 2006. Effect of a mixture of iprobenfos and malathion on the development of malathion resistance in the mosquito Culex pipiens pallens Coq. Pest Management Science 62: 86-90. [ Links ]
WANG, Y. H.; CHEN, J.; ZHU, Y. C.; MA, C. Y.; HUANG Y.; SHEN, J.L. 2008. Susceptibility to neonicotinoids and risk of resistance development in the brown planthopper, Nilaparvata lugens (Stal) (Homoptera: Delphacidae). Pest Management Science 64: 1278-1284. [ Links ]
WANG, Y. H.; WU, S. G.; ZHU, Y. C.; CHEN, J.; LIU, F. Y.; ZHAO, X. P.; WANG, Q.; LI, Z.; BO, X. P.; SHEN, J. L. 2009. Dynamics of imidacloprid resistance and cross-resistance in the brown planthopper Nilaparvata lugens. Entomologia Experimentalis et Applicata 131: 20-29. [ Links ]
XU, SH. C.; YU, Y. F.; WANG, X. J.; WAN, Q. 2008. Rynaxypyr, a new insecticide and its research & development in application. Modern Agrochemicals 7(5): 8-11. [ Links ]
XUE, J.; BAO, Y. Y.; LI, B. l.; CHENG, Y. B.; PENG, Z. Y.; LIU, H.; XU, H. J.; ZHU, Z. R.; LOU, Y. G.; CHEN, J. A.; ZHANG, C. X. 2010. Transcriptome analysis of the brown planthopper Nilaparvata lugens. PLoS ONE 5(12): e14233. [ Links ]
YANG, L.; ZHANG, Y. B.; HU, M. Y.; GENG, P. 2010. Toxic effects of virtako on Cnaphalocrocis medinalis. Jiangsu Agricultural Sciences 5: 158-160. [ Links ]
YIN, S. M. 2010. Insecticide resistance. Pest control Newsletter 20: 1. [ Links ]
YOO, J. K.; LEE, S. W.; AHN, Y. J.; NAGATA, T.; SHONO, T. 2002. Altered acetylcholinesterase as a resistance mechanism in the brown planthopper (Homoptera: Delphacidae) Nilaparvata lugens Stal. Applied Entomology Zoology 37: 37-41. [ Links ]
ZHANG, J.; YUAN, F. H.; LIU, J.; CHEN, H. D.; ZHANG, R. J. 2010. Sublethal effects of nitenpyram on life-table parameters and wing formation of Nilaparvata lugens (Stal) (Homoptera: Delphacidae). Applied Entomology Zoology 45 (4): 569-574. [ Links ]
ZHUANG, Y. L.; SHEN, J. L.; CHEN, Z. 1999. The influence of triazophos on the productivity of the different wing-form brown planthopper, Nilaparvata lugens (Stal). Journal of Nanjing Agricultural University 22: 21-24. [ Links ]
Suggested citation:
CHEN, YONG; XIAOWA QING; JIE LIU; JIE ZHANG and RUNJIE ZHANG. 2013. Toxic effects of virtako on the brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae). Revista Colombiana de Entomología 39 (2): 197-200.