Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488
Rev. Colomb. Entomol. vol.39 no.2 Bogotá July/Dec. 2013
Lethal and sublethal effects of neem oil to the predatory mite Proprioseiopsis neotropicus (Acari: Phytoseiidae)
Toxicidad letal y subletal del aceite de nim sobre el ácaro depredador Proprioseiopsis neotropicus (Acari: Phytoseiidae)
AMANDA C. B. SILVA1,2, ADENIR V. TEODORO1,3, EUGÉNIO E. OLIVEIRA4 and ANILDE G. S. MACIEL1,5
1 Graduate Programme in Agroecology, Maranhäo State University (UEMA), PO Box 09, Säo Luis, MA, Brazil.
2 M. Sc.
3 Ph. D. Embrapa Coastal Tablelands, Av. Beira-Mar 3250, Jardins, PO Box 44, Aracaju, SE, Brazil. adenir.teodoro@embrapa.br. Corresponding author.
4 Ph. D. Federal University of Viçosa, Department of Entomology, Entomology Building Room 309, 36570-000, Viçosa, MG, Brazil.
5 Agronomist.
Abstract: Lethal and sublethal toxicity of the neem oil Bioneem® to the predatory mite Proprioseiopsis neotropicus (Acari: Phytoseiidae) were evaluated by combining lethal doses (LD) with population growth and biological parameter studies. Dose-mortality bioassays were conducted to estimate the lethal doses of the neem oil to P. neotropicus using probit analyses. Afterwards, the instantaneous rate of increase (ri) was used to evaluate the sublethal effects of the neem oil to P. neotropicus based on reproduction and mortality data. The effects were further assessed by comparing some life history parameters of P. neotropicus exposed to a sublethal dose of neem oil. The dose of neem oil which kills 50% of the population (LD50) of the predatory mite P. neotropicus was 7.5 ul/ cm2. The instantaneous rate of increase of the predatory mite P. neotropicus did not vary with increases of dosages of neem oil. Similarly, the LD25 of the neem oil did not affect biological parameters of the predatory mite. Based on our lethal and sublethal approach we conclude that the neem oil Bioneem® is generally selective to the predatory mite P. neotropicus.
Key words: Biological control. Natural enemy. Selectivity.
Resumen: Las toxicidades letal y subletal del aceite de nim, Bioneem®, sobre el ácaro depredador Proprioseiopsis neotropicus (Acari: Phytoseiidae) fueron evaluadas mediante la combinación de dosis letales (DL), el crecimiento demográfico y estudios de los parámetros biológicos. Se llevaron a cabo bioensayos de dosis-mortalidad para estimar las dosis letales del aceite de nim sobre P. neotropicus mediante análisis de probit. Después, la tasa instantánea de crecimiento poblacional (ri) se utilizó para evaluar los efectos subletales del aceite de nim a P. neotropicus basado en la reproducción y datos de mortalidad. Además, los efectos fueron evaluados mediante la comparación de algunos parámetros de la historia de vida de P. neotropicus expuestos a una dosis subletal de aceite de nim. La dosis de aceite de nim que mata al 50% de la población (DL50) del ácaro depredador P. neotropicus fue estimada en 7,5 ul/ cm2. La tasa instantánea de crecimiento poblacional del ácaro depredador P. neotropicus no varió con el aumento de las dosis de aceite de nim. De manera similar, la dosis subletal de aceite de nim no afectó los parámetros biológicos del ácaro depredador. Basado en nuestro enfoque letal y subletal llegamos a la conclusión de que el aceite de nim Bioneen® es generalmente selectivo para el ácaro depredador P. neotropicus.
Palabras clave: Control biológico. Enemigo natural. Selectividad.
Introduction
Many studies have focused on lethal effects of pesticides on target and non-target arthropod species. Only few studies have attempted to understand the side effects of chronic exposure to pesticides on reproduction patterns of natural enemies, which may lead to disruption in population dynamics and influence the impact of pest species on crops. Lethal concentrations or doses are widely used to assess the toxicity of pesticides to arthropods (Stark and Banks 2003; Desneux et al. 2007), but they evaluate only mortality as a toxicity parameter. Arthropod populations exposed to pesticides can be negatively affected by sublethal effects ranging from reduction in oviposition rates, survival and growth rate to impaired foraging behavior and interferences on biological aspects (Stark and Banks 2003; Teodoro et al. 2005, 2009; Desneux et al. 2007; Alzoubi and Cobanoglu 2008; Guedes et al. 2009). Lethal doses combined with growth rates or biological studies have been used to evaluate the toxicity of pesticides to arthropods as they allow a more complete toxicological assessment of such compounds by integrating both lethal and sublethal effects (Teodoro et al. 2005; Desneux et al. 2007; Guedes et al. 2009; Hamedi et al. 2010). The instantaneous rate of increase (rt) is a measure of population growth over a given period of time and it has been successfully used to estimate population increase of arthropods (Walthall and Stark 1997; Stark and Banks 2003). The r is closely correlated with the intrinsic growth rate (rm) with the main advantage that ri discards the need of establishment of life table studies (Walthall and Stark 1997). Positive ri-values indicate population increase, negative ri-values indicate decrease, and ri= 0 indicates a stable population (Walthall and Stark 1997).
Neem (Azadirachta indica A. Juss) based pesticides have been proven to be efficient in controlling a range of agricultural pests and they have been recognized as relatively nontoxic to natural enemies (Goncalves et al. 2001; Venzon et al. 2005, 2008; Isman 2006; Andrade et al. 2012). These botanical pesticides contain azadirachtin, a limonoid triterpene, the main insecticidal component which leads to feeding inhibition, disruption of immature development, lower fecundity and fertility of adults, behavior alterations, cell anomalies and mortality in eggs, larvae and adult stages (Mordue (Luntz) et al. 2005). Although considered generally selective for several natural enemies, some investigations have also shown side effects of neem pesticides on non-target species (Castagnoli et al. 2002; Cordeiro et al. 2010).
Naturally occurring predatory mites of the family Phyto-seiidae are key natural enemies of pest mites (McMurtry and Croft 1997) and they are subjected to the same pesticides exposure in the field as target pest species. However, few comprehensive studies have addressed the lethal and sublethal effects of botanical pesticides to natural enemies (Venzon et al. 2007; Cordeiro et al. 2010). Proprioseiopsis neotropicus (Ehara, 1966) (Acari: Phytoseiidae) is a generalist predatory mite found inhabiting natural vegetation and crop fields in Brazil, Colombia and Ecuador (Moraes et al. 2004; Mineiro et al. 2009). Although no study has specifically addressed the role of P. neotropicus in biological control, it is expected that this predatory mite may contribute in the natural regulation of pest mite populations due to its generalist feeding habit and wide distribution. Here, we aimed at assessing the lethal and sublethal effects of a neem based pesticide to the predatory mite P. neotropicus by combining lethal dose estimates with demographic and biological studies.
Materials and methods
Stock cultures of the predatory mite P. neotropicus were established from mites collected from Acacia mangium Willd 1806 plants maintained free from pesticides in Sao Luís, Ma-ranhao state, Brazil. The predatory mite was reared indoors at uncontrolled temperature, humidity and photoperiod. The mites were confined to a plastic arena (5 cm diameter) floating in distilled water within an open Petri dish (10 cm diameter x 1.5 cm depth). Each arena was centrally perforated by a pin attached to the bottom of the Petri dish by a silicon-based glue. Pollen of castor bean (Ricinus communis L. 1753) was provided as food source every other day.
All experiments were conducted under laboratory conditions (27 ± 5 °C, 10h L: 14 h D photoperiod, and 60 ± 10% relative humidity). Dose-mortality bioassays were carried out to determine the lethal doses of the neem oil Bioneem® (Bioneem, Aracuaí - MG, Brazil) to adult females of P. neotropicus, at the beginning of their reproductive period (7-8 days old). The neem oil was sprayed through a Potter tower (Burkard, Rickmansworth, UK) in plastic arenas (5 cm diameter) at 0.34 bar (34 kPa) pressure with a 2.3 ml spray aliquot which resulted in a residue of 1.7 ± 0.30 mg/cm2 of the pesticide in accordance with recommendations of the International Organization for Biological Control of Noxious Animals and Plants/ West Paleartic Regional Section (IOBC/WPRS) (Hassan et al. 1994). Control arenas were sprayed with distilled water, since distilled water was used to dilute the neem oil. Sprayed arenas were dried in open air for 1 h before 5 adult females (7-8 days old) of P. neotropicus were placed on them. Five replicates (arenas) for each dose were used. The arenas were placed to float in Petri dishes in the laboratory as described for rearing colonies of the predator. The doses were selected after preliminary tests with broad dosage range allowing selection of lower (the highest neem oil dose unable to kill P. neotropicus) and upper (the smallest neem oil dose able to kill 100% of P. neotropicus) mortality responses. The doses used ranged from 1.7 to 10.1 ul of the neem oil/ cm2, which represents 1 to 5.9 folds the dosage recommended for field applications. Mite mortality was assessed after 72 h exposure and the mites were considered dead if unable to move for a distance at least equal to their body length. Dosage-mortality curves were estimated by probit analyses using PROC PROBIT procedure (SAS Institute 2002).
The instantaneous rate of increase (ri)was used to evaluate the sublethal effects of the neem oil to the predatory mite P. neotropicus based on reproduction and mortality data. This index is calculated using the equation ri=[ln (Nf/N0)]/ At, where Nf is the final number of live mites (including eggs and immatures), N0 is the initial number of mites, and At is the interval (7 days) elapsed between start and end of the bioassay (Walthall and Stark 1997). Five adult females of P. neotropi-cus as previously described were placed on arenas sprayed with increasing lethal doses (LD5, LD10, LD25, LD50, LD95, LD99) of neem oil, which were based on the dosage-mortality curves previously obtained. Control arenas (LD0) were sprayed only with distilled water. Five replicates for each dose were used. A male predator was added to each arena and replaced whenever it died. One-way ANOVA was conducted to assess the effects of increasing lethal doses of neem oil on the instantaneous rate of increase of P. neotropicus.
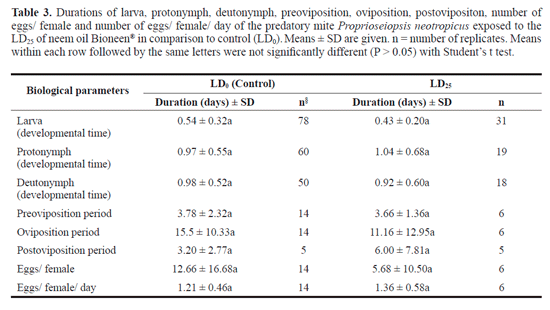
Sublethal effects of the neem oil was further assessed by comparing some biological parameters of the predatory mite P. neotropicus exposed to the LD25 of neem oil and LD0 (control). Females and males of P neotropicus were confined to arenas for 12 hours to obtain eggs of similar age. Arenas (5 cm diameter) were sprayed through a Potter tower with the LD25 of neem oil, i.e. 5.7 Lil of neem oil/cm2 or distilled water (LD0 - control) at the same conditions described above. Preliminary observations showed that eggs that had contact with LD25- sprayed arenas did not hatch; therefore we decided to transfer newly hatched larvae to arenas. Larvae were individually placed on arenas and observed twice a day (8 a.m. and 16 p.m.) until reaching adulthood. Newly molted females and males were observed once a day (8 a.m.) until their death. One male of the stock culture was added to each arena containing a newly molted female and the periods of preoviposition, oviposition, postoviposition, eggs/female and eggs/female/day were recorded. Castor bean pollen was supplied every other day as food source on a small piece of cover glass placed on the arena to avoid direct contact with the neem oil. Cotton threads underneath a small plastic sheet (1.0 x 0.5 cm) served as oviposition place. The periods of immature development, preoviposition, oviposition, postovi-position, eggs/ female and eggs/ female/ day of the predatory mite P. neotropicus exposed to the LD25 of neem oil and the control (LD0) were compared by t tests.
Results
Lethal doses (LD5, LD10, LD25, LD50, LD95, and LD99) of neem oil, which were estimated based on dose-mortality bioassays, are shown in the Table 1. Doses of neem oil below the maximum recommended dosage for field applications (i.e., 15 ml of neem oil/ L of distilled water or 1.7 µl of neem oil/cm2) did not kill the predatory mite P. neotropicus. The completely elimination of P. neotropicus populations was achieved only in dosages 10 fold higher than the recommended dosage for field applications (Table 1).
Reproductive side effects caused by chronical exposure to the neem oil were assessed by calculating the instantaneous rate of increase (ri) of P. neotropicus populations. ri values were not affected by increases on the neem oil doses (F4,20 = 1.80, P = 0.16, Table 2). Positive values of ri indicate population growth of P. neotropicus exposed up to the LD50, i.e., 7.5 Ld of neem µl/cm2 (ri= 0.2 ± 0.03/ day). Predator extinction (Nf = 0) took place only after exposure to the LD95 (14.4 µl/cm2) of neem oil onwards. Comparisons among biological parameters of individuals non-exposed and exposed to a sublethal dose (DL25) were also performed. The duration of the periods of larva (df = 107, t = 1.80, P = 0.07), protonymph (df = 77, t = 0.40, P = 0.68), deutonymph (df = 66, t = 0.42, P = 0.67), preoviposition (df = 18, t = 0.11, P = 0.90), oviposition (df = 18, t = 0.79, P = 0.43), postoviposition (df = 6, t = 0.75, P = 0.47), number of eggs/ female (df = 20, t = 1.14, P = 0.26) and number of eggs/female/day (df = 18, t = 0.60, P = 0.55) of P. neotropicus were not affected by the sublethal dose of the neem oil (Table 3).
Discussion
The combination of dose-response with population growth and biological parameter bioassays were used to evaluate the toxicity of the neem oil Bioneem® to the predatory mite P. neotropicus. This predatory mite showed highly tolerant to action of the neem oil. Predatory mites of the family Phyto-seiidae, as P. neotropicus, are important natural enemies of pest mites, but their efficiency is contingent on a combination of a myriad of factors, including botanical pesticide use. Neem based pesticides have been used against a variety of agricultural pests and have been broadly recognized as selective to non-target natural enemies (Goncalves et al. 2001; Venzon et al. 2005, 2008; Isman 2006; Andrade et al. 2012), although very few studies have addressed the potential side-effects of these botanical insecticides (Castagnoli et al. 2002; Cordeiro et al. 2010).
Toxicity of pesticides to arthropods has been widely evaluated by comparing the lethal doses of these compounds (Stark and Banks 2003; Desneux et al. 2007). As observed in previous studies using neem based pesticides (Cote et al. 2002), our results for mortality show that the dosage of Bi-oneem® (1.7 Ld /cm2) recommended for field applications was unable to kill P. neotropicus, suggesting that neem oil might have considerable selectivity to this predator. The estimated LD50 of the neem oil for P. neotropicus was over four times higher than the dose recommended for field application and the completely extinction occurred only after exposures to the LD99 (18.9 Lil/cm2) of neem oil, which represent situations rather unlikely to happen in any crop system. However, such analysis might be done with considerable cautions. Since the major effects of neem-based insecticides normally take a while to occur, e.g. the field degradation of azadirachtin varies from one week to three months (Stark and Walter 1995; Sundaram 1996; Mordue (Luntz) et al. 2005), this lower tox-icity could be due to the fact that 72 h exposure would be insufficient to evaluate the real side effects of neem oil to P. neotropicus and to compute all the effects derived from the non-lethal exposures, which may prevail over lethal ones under field conditions. Additionaly, we assessed only one potential exposure pathway (residual bioassay) and the possibility of P. neotropicus being more severely affected by other exposure pathways (e.g. ingestion of preys that have been exposed to neem or direct exposure through spraying) can not be rulled out.
Organisms exposed to pesticides are known to suffer with sub-lethal effects (Stark and Banks 2003; Desneux et al. 2007; Teodoro et al. 2005, 2009; Guedes et al. 2009) that are, therefore, not apparent on mortality bioassays. Here, the combination of lethal doses estimates with studies of sub-lethal effects on growth rate and some life history parameters allowed a more accurate evaluation of the impact of neem oil to the predatory mite P. neotropicus. The instantaneous rate of increase (ri) of P. neotropicus, which was evaluated over a period of 7 days and reflects sub-lethal effects on population growth, did not respond to increases on lethal doses up the LD50 (7.5 Lil of neem oil/cm2) of neem oil (Table 2). This reinforces the assumption of selectivity of the neem oil to this natural enemy. Additionally, all of these populations presented similar positive values of ri, indicating population growth of P. neotropicus and confirming low toxicity of neem oil to this natural enemy. Predator extinction took place only after exposures to the LD95 of neem oil onwards. Additional sublethal studies evaluating some biological parameters of the predatory mite P. neotropicus exposed to LD25 of neem oil and LD0 (control) were in line with population growth results (Table 3). The LD25, which was over three times higher than the recommended field concentration of the neem oil, did not affect the biological parameters of the predatory mite (Table 3). However, it is important to stress that eggs of P. neotropicus that had contact with LD25 - sprayed arenas did not hatch, suggesting ovicidal activity of the neem oil. More studies are necessary to shed light on the mechanisms underlying the ovicidal activity of neem oil.
Several studies report that botanical pesticides like the neem oil are efficient against pest mites and have low toxicity to natural enemies (e.g. Brito et al. 2006). Indeed, population growth and biological parameters of the predatory mite P. neotropicus were not affected by both increasing doses and the LD25 of the neem oil in this study, indicating no potential impairments of the biological control provided by this natural enemy. Therefore, it is expected that neem oil would not significantly affect populations of the predatory mite P. neotropicus. Likewise, Venzon et al. (2005) did not found negative effects of concentrations up to 0.1 g a.i/l of neem on survival of the predatory mite Iphiseiodes zuluagai Denmark & Muma, 1972 (Acari: Phytoseiidae). Further studies focusing on behavioral responses of P. neotropicus treated with neem oil will contribute to a better understanding of the sublethal effects caused by this botanical pesticide. Based on our combined lethal and sublethal approach we conclude that the neem oil Bioneen® is generally selective to the generalist predatory mite P. neotropicus.
Acknowledgements
We thank Marcal Pedro Neto from Federal University of To-cantins for the mite species identification, the National Council for Scientific and Technological Development (CNPq) and the Federal Agency for Support and Evaluation of Graduate Education (CAPES) for funding.
Literature cited
ALZOUBI, S.; COBANOGLU, S. 2008. Toxicity of some pesticides against Tetranychus urticae and its predatory mites under laboratory conditions. American-Eurasian Journal of Agricultural & Environmental Sciences 3 (1): 30-37. [ Links ]
ANDRADE, L. H.; OLIVEIRA, J. V.; BREDA, M. O.; MARQUES, E. J.; LIMA, I. M. M. 2012. Effects of botanical insecticides on the instantaneous population growth rate of Aphis gossypii Glover (Hemiptera: Aphididae) in cotton. Acta Scientiarum. Agronomy 34 (2): 119-124. [ Links ]
BRITO, H. M.; GONDIM JUNIOR, M. G. C.; OLIVEIRA, J. V.; CÁMARA, C. A. G. 2006. Toxicidade de formulacöes de nim (Azadirachta indica A. Juss.) ao ácaro-rajado e a Euseius alatus De Leon e Phytoseiulus macropilis (Banks) (Acari: Phytoseiidae). Neotropical Entomology 35 (4): 500-505. [ Links ]
CASTAGNOLI, M.; ANGELI, G.; LIGUORI, M.; FORTI, D.; SI-MONI, S. 2002. Side effects of botanical insecticides on predatory mite Amblyseius andersoni (Chant). Journal of Pest Science 75 (5): 122-127. [ Links ]
CORDEIRO, E. M. G.; CORREA, A. S.; VENZON, M.; GUEDES, R. N. C. 2010. Insecticide survival and behavioral avoidance in the lacewings Chrysoperla externa and Ceraeochrysa cubana. Chemosphere 81 (10): 1352-1357. [ Links ]
COTE, K. W.; LEWIS, E. E.; SCHULTZ, P. B. 2002. Compatibility of acaricide residues with Phytoseiulus persimilis and their effects on Tetranychus urticae. HortScience 37 (6): 906-909. [ Links ]
DESNEUX, N.; DECOURTYE, A.; DELPUECH, J. M. 2007. The sublethal effects of pesticides on beneficial arthropods. Annual Review of Entomology 52 (1): 81-106. [ Links ]
GONCALVES, M. J.; OLIVEIRA, J. V.; BARROS, R.; LIMA, M. 2001. Extratos aquosos de plantas e o comportamento do ácaro verde da mandioca. Scientia Agricola 58 (3): 475-479. [ Links ]
GUEDES, R. N. C.; MAGALHÄES, L. C.; COSME, L. V. 2009. Stimulatory sublethal response of a generalist predator to per-methrin: hormesis, hormoligosis, or homeostatic regulation? Journal of Economic Entomology 102 (1): 170-176. [ Links ]
HAMEDI, N.; FATHIPOUR, Y.; SABER, M. 2010. Sublethal effects of fenpyroximate on life table parameters of the predatory mite Phytoseius plumifer. BioControl 55 (2): 271-278. [ Links ]
HASSAN, A. S.; BIGLER, F.; BOGENSHUTZ, H.; BOLLER, E.; BRUN, J.; CALIS, J. N. M.; COREMANS-PELSENEER, J.; DUSO, C.; GROVE, A.; HEIMBACH, U.; HELVER, N.; HOK-KANEN, H.; LEWIS, G. B.; MANSUR, F.; MORETH, L.; POLGAR, L.; SAMSOE-PETERSEN, L.; SAUPHANOR, B.; STAUBLI, A.; STERK, G.; VAINIO, A.; VAN DE VEIRE, M.; VIGGIANI, G.; VOGT, H. 1994. Results of the sixth join pesticide testing programme of the IOBC/WPRS - working group ''Pesticides and Beneficial Organisms''. Entomophaga 39 (1): 107-119. [ Links ]
ISMAN, M. B. 2006. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annual Review of Entomology 51 (1): 45-66. [ Links ]
McMURTRY, J. A.; CROFT, B. A. 1997. Life-styles of phytoseiid mites and their roles in biological control. Annual Review of Entomology 42 (1): 291-321. [ Links ]
MINEIRO, J. L. C.; RAGA, A.; SATO, M. E.; LOFEGO, A. C. 2009. Acaros associados ao cafeeiro (Coffea spp.) no estado de Säo Paulo, Brasil. Parte I. Mesostigmata. Biota Neotropica 9 (1): 37-46. [ Links ]
MORAES, G. J.; McMURTRY, J. A.; DENMARK, A. H.; CAMPOS, C. B. 2004. A revised catalog of the mite family Phytoseiidae. Zootaxa 434 (1): 1-494. [ Links ]
MORDUE (LUNTZ), A. J.; MORGAN, E. D.; NISBET, A. J. 2005. Azadirachtin, a natural product in insect control. pp. 117-135. In: Gilbert, L. I.; Iatrou, K.; Gill, S. S. (Eds.). Comprehensive molecular insect science. Elsevier, Oxford, UK. [ Links ]
SAS Institute. 2002. SAS/STAT User's Guide, v.8. SAS Institute, Cary, NC, USA. [ Links ]
STARK, J. D.; BANKS, J. E. 2003. Population-level effects of pesticides and other toxicants to arthropods. Annual Review of Entomology 48 (1): 505-519. [ Links ]
STARK, J. D.; WALTER, J. F. 1995. Neem oil and neem oil components affect the efficacy of commercial neem insecticides. Journal of Agricultural and Food Chemistry 43 (2): 507-512. [ Links ]
SUNDARAM, K. M. S. 1996. Azadirachtin biopesticide: a review of studies conducted on its analystical chemistry, environmental behavior and biological effects. Journal of Environmental Science Health B 31 (4): 913-948. [ Links ]
TEODORO, A. V.; FADINI, M. A. M.; LEMOS, W. P.; GUEDES, R. N. C.; PALLINI, A. 2005. Lethal and sub-lethal selectivity of fenbutatin oxide and sulfur to the predator Iphiseiodes zuluagai (Acari: Phytoseiidae) and its prey, Oligonychus ilicis (Acari: Tetranychidae), in Brazilian coffee plantations. Experimental and Applied Acarology 36 (1): 61-70. [ Links ]
TEODORO A. V.; PALLINI, A.; OLIVEIRA, C. 2009. Sub-lethal effects of fenbutatin oxide on prey location by the predatory mite Iphiseiodes zuluagai (Acari: Phytoseiidae). Experimental and Applied Acarology 47 (4): 293-299. [ Links ]
VENZON, M.; ROSADO, M. C.; FADINI, M. A. M.; CIOCIOLA JR. A. I.; PALLINI, A. 2005. The potential of NeemAzal for the control of coffee leaf pests. Crop Protection 24 (3): 213-219. [ Links ]
VENZON, M.; ROSADO, M. C.; PALLINI, A.; FIALHO, A.; PEREIRA, C. J. 2007. Toxicidade letal e subletal do nim sobre o pulgao-verde e seu predador Eriopsis conexa. Pesquisa Agropecuaria Brasileira 42 (5): 627-631. [ Links ]
VENZON, M.; ROSADO, M. C.; MOLINA-RUGAMA, A. J.; DU-ARTE, V. S.; DIAS, R.; PALLINI, A. 2008. Acaricidal efficacy of neem against Polyphagotarsonemus latus (Banks) (Acari: Tarsonemidae). Crop Protection 27 (3-5): 869-872. [ Links ]
WALTHALL, W. K.; STARK, J. D. 1997. Comparison of two population-level ecotoxicological endpoints: the intrinsic (rm) and instantaneous (ri) rates of increase. Environmental Toxicology and Chemistry 16 (5): 1068-1073. [ Links ]
Suggested citation:
SILVA, AMANDA C. B.; ADENIR V. TEODORO; EUGÈNIO E. OLIVEIRA and ANILDE G. S. MACIEL. 2013. Lethal and sub-lethal effects of neem oil to the predatory mite Proprioseiop-sis neotropicus (Acari: Phytoseiidae). Revista Colombiana de Entomología 39 (2): 221-225.