Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488
Rev. Colomb. Entomol. vol.39 no.2 Bogotá July/Dec. 2013
Assemblages of saprophagous muscids (Díptera: Muscidae) in three urban sites of temperate Argentina
Ensambles de múscidos saprófagos (Díptera: Muscidae) en tres sitios urbanos en la Argentina templada
LUCIANO DAMIÁN PATITUCCI1,2, PABLO RICARDO MULIERI1,2, JUAN ALBERTO SCHNACK1,3and JUAN CARLOS MARILUIS1,2
1 Consejo Nacional de Investigaciones Científicas y Técnicas, Buenos Aires, Argentina.
2 Museo Argentino de Ciencias Naturales "Bernardino Rivadavia", Av. A. Gallardo 470, C1405DJR, Buenos Aires, Argentina. lpatitu@yahoo.com.ar. Corresponding author.
3 División Entomología, Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, Paseo del bosque s/n°, 1900 La Plata, Buenos Aires, Argentina.
Received: 12-Jun-2013 - Accepted: 11-Nov-2013
Abstract: Muscidae occupy a great diversity of habitats and trophic niches. In temperate environments, the knowledge of ecological aspects of saprophagous muscids is fragmentary. The aim of this work was to characterize the assemblages of saprophagous muscids regarding their richness and abundance, bait preference, heliophily, and seasonality of species in three sites with different urbanization levels sampled during two years in an area of temperate Argentina. A total of 3,321 specimens belonging to 20 species were collected. The baits and microhabitat preferences, and seasonal fluctuation of species were described. An increase in diversity in terms of evenness and richness in less urbanized sites was observed. Results support the hypotheses about sites with an intermediate urbanization, which contain major landscape heterogeneity, show a higher number of species. The assemblage response regarding the environment types reflects the adaptability to the physical changes along the three sites with different degree of urbanization.
Key words: Biodiversity. Richness. Seasonality. Flies.
Resumen: Las especies de Muscidae ocupan una gran diversidad de hábitats y nichos tróficos. En ambientes templados, el conocimiento de los aspectos ecológicos de múscidos saprófagos es fragmentario. El objetivo de este trabajo fue caracterizar los ensambles de múscidos saprófagos en cuanto a su riqueza y abundancia, la preferencia de cebo, heliofilia, y la estacionalidad de las especies en tres sitios con diferente nivel de urbanización colectados durante dos años en una zona templada de la Argentina. Se colectaron un total de 3.321 ejemplares pertenecientes a 20 especies. Se describen las preferencias de cebo y de microhábitat, y fluctuación estacional de las especies. Se observó un aumento de la diversidad en términos de uniformidad y riqueza en los sitios menos urbanizados. Los resultados apoyan las hipótesis sobre los sitios con una urbanización intermedia, que contienen gran heterogeneidad del paisaje, muestran un mayor número de especies. La respuesta del conjunto con respecto a los tipos de medio ambiente, refleja la capacidad de adaptación a los cambios físicos a lo largo de los tres sitios con diferente grado de urbanización.
Palabras clave: Biodiversidad. Riqueza. Estacionalidad. Moscos.
Introduction
The impact of urbanization on biodiversity has produced changes in species richness and species composition along the urban-rural gradients (McKinney 2002). However, these changes can generate either an increase or a decrease in species richness according to the spatial scale and taxa selected for the study or to the characteristics of the sites (e.g. intensity of urbanization, geographic and climatic variation) (McKin-ney 2008). Several works concentrated on insects argue that species richness decreases with increasing urbanized environments, and that, with the addition of exotic urban-adapted species, the native fauna is replaced with exotic wildlife (Montes 2005; McKinney 2008). In contrast, other authors suggest that urban environments can retain a group of native insects (Bates et al. 2011).
Muscidae is one of the most diverse families within the Diptera. Adult Muscidae, which occupy a great diversity of habitats and trophic niches, could be predators of other insects, hematophagous, pollinators, or saprophagous (necrophagous and coprophagous). Also, this family includes species of public health importance, as well as others affecting farming and some associated with urban environments (Carvalho et al. 2005). Some of them, such as Musca domestica Linnaeus, 1758, or Stomoxys calcitrans Linnaeus, 1758, have medical impact as mechanical vectors of several pathogenic microorganisms, which are associated with human and animal disease (Pape 2009).
The diversification of trophic niches of Muscidae represents a difficulty for a community study (Krüger et al. 20l0) and particularly few studies were focused on the assemblage of saprophagous muscids comparing environments with different degrees of urbanization. Studies developed in tropical (Uribe et al. 2010) and subtropical Neotropics (Linhares 1981; Carvalho et al. 1984) showed an increase of richness and abundance of muscid flies in sites with low degree of urbanization. In temperate environments, the knowledge of the assemblages of saprophagous muscids is fragmentary and refers mainly to the necrophagous fauna (Figueroa-Roa and Linhares 2004; Battän-Horenstein et al. 2010).
The aim of this work was to characterize the assemblage of saprophagous muscids regarding its richness and abundance, bait preference, heliophily, synanthropy, and season-ality of species in three sites with different urbanization level in a temperate environment of Argentina.
Materials and methods
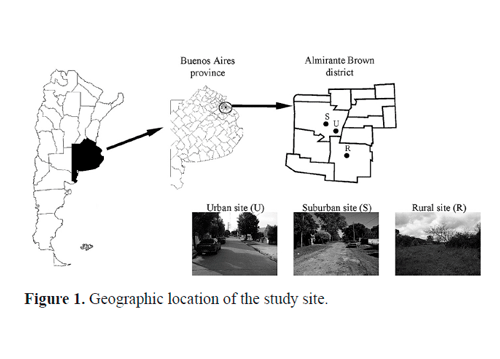
Study sites. Greater Buenos Aires is the urbanized area surrounding the City of Buenos Aires, Argentina. This region has an area of 2,750 km2 and a population of 13,028,000 inhabitants and is composed of 24 districts. Fourteen of these districts are totally urbanized and 10 of them are in the process of urbanization. This study was carried out in Almirante Brown (Fig. 1), a highly urbanized district with rural remnant areas. Almirante Brown is located 30 km south of Buenos Aires City, with an area of 129.33 km2 and a population of 515,556 inhabitants. Originally, this area was dominated by grassland and small patches of xerophilic woods. A high proportion (70%) of this land is currently used for agriculture, livestock, and human settlements. The climate is temperate and humid, with a mean annual precipitation of approximately 1,000 mm. Because of these characteristics, we consider Almirante Brown as a heterogeneous area.
Samplings were performed at three sites along a gradient of urbanization (urban, suburban, and rural site). The urban core of Burzaco city was categorized as the urban site (U) (34°50'15.02"S 58°23' 52.75"W). This site is composed of houses and private gardens, some buildings, and has a high proportion of built surface and paved streets.
The suburban site (S) (34°49'36.90"S 58°24'16.56"W) was located 1 km away from U. This site has a lower density of houses; the houses have large gardens, often surrounded by small patches of exotic trees, the roads are unpaved and the houses have no sewage systems.
Finally, the rural site (R) chosen was Ministro Rivada-via (34°51'29.38"S, 58°23'17.75"W), located 8 km away from U, an area of pastureland and agricultural activities (mainly cattle and poultry farms) and a lower density of un-paved roads. The vegetation of this site is composed mainly of grassland patches and native woods dominated by Celtis tala Gillies (Ulmaceae) and Parkinsonia aculeata Linnaeus (Fabaceae) and dispersed shrubs of Citrus trifoliate Linaeus (Rutaceae) and Baccharis spp. (Asteraceae).
Field samples. Sampling methods for saprophagous Diptera are based mainly on captures made on attracting baits. Here, two kinds of baits: 250 g of rotten cow liver (aged five days at ambient temperature) and 250 g of fresh dog feces (from a single dog, fed with dry dog food) were used. Samples were taken monthly from May 2005 to April 2007, once a month in each site (U, S, R) (72 samples). Seven hourly events of capture of adult flies (10:00 - 16:00) were made with a hand net on each bait. The sampling effort was the same at each site, consisting of four baits in each site: one of rotten cow liver and one of dog feces in a shaded microhabitat, and a similar pair of baits in a sunny microhabitat (totalizing 168 h per bait. Were considered a shaded microhabitat, a place under the shade of trees, and the sunny microhabitat, a place in open pasture or gardens).
Specimens were killed in glass vials with carbon tetrachloride and then stored in the field in labeled envelopes for further study in the laboratory. The flies were identified using an appropriate key (Patitucci et al. 2013). Voucher specimens were pinned and housed in "Museo Argentino de Ciencias Naturales "Bernardino Rivadavia" (MACN)", Buenos Aires, Argentina.
Data analysis. In order to describe the assemblage of sa-prophagous muscids in the gradient studied, the number of dates on which different species were present in samples (frequency), and total individuals caught per species (abundance) were estimated. The degree of representativeness of the richness obtained in the samples through rarefaction curves using the program EstimateS vers. 8.2 (Colwell 2011) was calculated. In order to estimate the number of species that would be found by taking subsequent samples, non-linear regression models to the rarefaction curves were fitted. The choice of an appropriate non-linear model is influenced by the size of the area or the heterogeneity/homogeneity of the habitat (Jiménez-Valverde and Hortal 2003). Each sampling site (U, S, R) were considered as a homogeneous area regarding its environmental characteristics, and the district of Almirante Brown as a whole (U+S+R) was considered as a heterogeneous area. A Clench model ((az) / (1+(bz))) and a Logarithmic B model ((log (1+(abz)))/b) for a heterogeneous area, and an exponential model (a+(b log10(z))) for a homogenous area were applied (Clench 1979; Longino and Colwell 1998; Thompson et al. 2003). The parameters estimated were a and b, sample effort (24 sample dates per site) are z. In addition, the evenness between the environments sampled by a rank-abundance curve was analyzed (Krebs 1999).
Total richness and diversity index (Shannon and Simpson) of the three sites was calculated. A first approach to describe the associations of different species of saprophagous muscids with the different combinations of bait types, sites and microhabitats was obtained by using Correspondence Analysis (CA). The CA is an exploratory technique to graphically represent qualitative data (urban-rural gradient, microhabitats and types of bait) with quantitative data (number of specimens captured). In addition, this analysis uses the "inertia" (represented on an axis) as a tool that measures the weight for the associations between qualitative and quantitative variables. The abundance of saprophagous muscids was transformed with log(x+1). Only 12 dominant species (more than 15 specimens sampled) were considered for this analysis. Subsequently, the abundance of each species with the variables studied (type of bait and microhabitat) by using the chi-square test was compared (Zar 1996).
The spatial distribution of saprophagous muscids was analyzed along the urban-rural gradient by using two different methods. First, the Synanthropy Index (SI) (Nuorteva 1963) for the dominant species present in the sample was calculated. This index provides a range of values between +100 (species present only in an urbanized environment) and -100 (species present only in an environment without human intervention). Second, the proportional abundance (p = nspi / N total) obtained for each species between the three sites, using a test for independent proportions was compared (Fleiss 1981).
Results
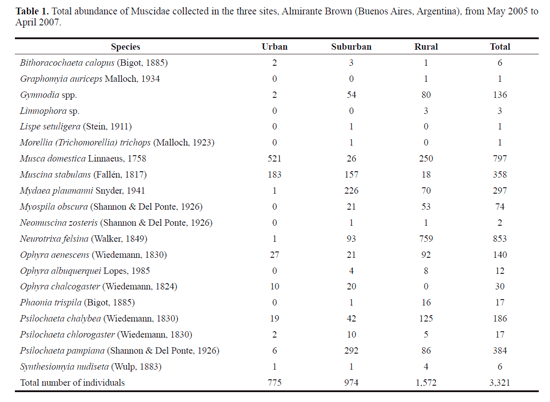
Assemblage structure, richness, and diversity. A total of 3,321 specimens of Muscidae belonging to 20 species were collected. Neurotrixa felsina (Walker, 1849) and Musca domestica were the most abundant species. On the other hand, Muscina stabulans (Fallen, 1817) and Psilochaeta pampiana (Shannon & Del Ponte, 1926), which were captured on 49 and 43 samples respectively, were those with highest frequency. Ophyra aenescens (Wiedemann, 1830), although recorded in low frequency (seven sampling dates), showed a relative high abundance (140 specimens). In general terms, 47.34% of the specimens were collected in the rural environment, while 29.33% were recorded in the suburban environment and 23.34% in the urban environment. Only seven species (two found in the rural site, two in the suburban site, and three in the three sites) were considered rare species (species collected with ≤ six specimens in the total sample). Finally, each of three species (Graphomya auriceps Malloch, 1934, Lispe setuligera (Stein, 1911) and Morellia (Trichomorellia) trichops) (Malloch, 1923) were represented by a single specimen (Table 1).
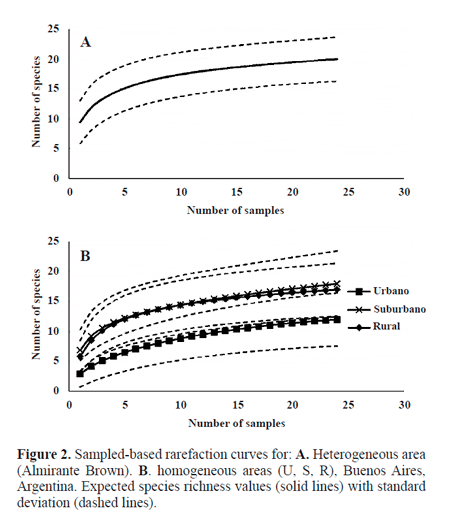
When the number of sampling units was increased, we observed a plateau of the rarefaction curve for the U+S+R (heterogeneous area). This curve showed asymptotic values when reaching 20 species. The fitting models (Clench: R2 = 0.98, a = 12.86, b = 0.62; Logarithmic B: R2 = 0.99, a = 138.08, b = 0.13) predicted that if we tripled the sampling effort, we could capture three additional species (Figure 2). Considering each site as a homogeneous area, the rarefaction curves showed high similarity between the S (R2 = 0.99, a = 6.65, b = 7.99) and R (R2 = 0.99, a = 6.29, b = 7.94) sites, so tripling the sampling effort, would add a number of three or four species. On the other hand, the number of species of the U site (R2 = 0.99, a = 1.86, b = 7.17) was lower than that of the S and R sites, although by tripling the sampling effort the expected number of species was also about 3 or 4 (Fig. 2).
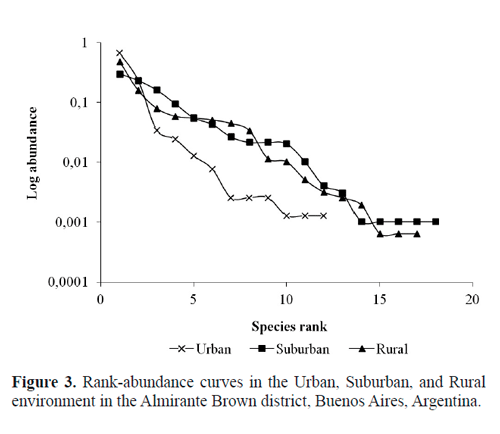
The rank-abundance curves showed that suburban and rural sites had the highest diversity. The slopes of both curves represented similar evenness profiles and exhibited a higher evenness than that at the urban site. Indeed, the urban site was dominated by few species (M. domestica, M. stabulans) (Fig. 3). The highest value of total richness was obtained for S (18 species), followed by R (17 species) and U (12 species) sites. The diversity indexes showed similar results for S (Shannon = 1.98; Simpson = 0.81), R (Shannon = 1.76; Simpson = 0.72), and U (Shannon = 0.98; Simpson = 0.49).
Seasonality, bait preference, and spatial distribution.
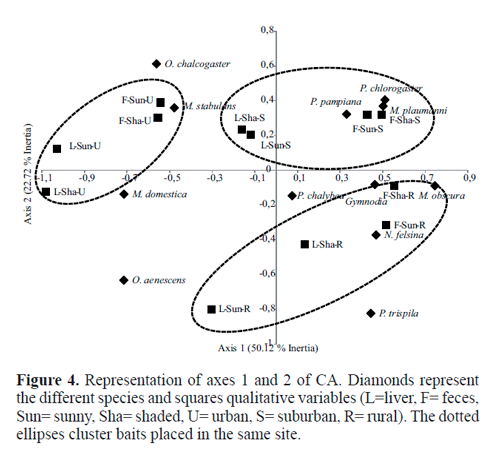
Correspondence analysis showed the association between the abundance of species and the three sites. The first two axes explained most of the variability (72.84% of the total inertia). An association between cosmopolitan species (M. domestica, M. stabulans) with the urban site (U) was observed. Native species were mostly captured on baits placed in sites with lower degree of urbanization (S and R). Mydaea plaumanni Snyder, 1941, P. pampiana, and Psilo-chaeta chlorogaster (Wiedemann, 1830) were associated with the suburban site (S), whereas N. felsina and M. obscura were associated with the rural site (R). On the other hand, the group of species in the positive values of axis 1 were mostly captured on feces (N. felsina, M. plaumanni, Myospila obscura (Shannon & Del Ponte, 1926), P. pampiana), whereas those in the negative values of this axis were captured on rotten liver. The genus Ophyra did not show association with a particular site, although its position in the diagram relative to the first axis indicated its association with rotten liver. By contrast, the PCA diagram did not show a b separation of species and sites in relation to the shaded or sunny microhabitats (Fig. 4).
Captures of species showed different patterns in relation to the bait type and microhabitat. Musca domestica was the only species showing preference to the liver and sunny microhabitat, while the remaining liver-associated species (M. stabulans, Phaonia trispila (Bigot, 1885), and Ophyra spp.) were captured mostly on liver baits placed in shaded microhabitats. The species belonging to the genus Ophyra showed similar patterns of captures associated with liver. However, non-significant differences in the case of Ophyra chalcogaster (Wiedemann, 1824) and Ophyra albuquerquei Lopes, 1985 may be due to their low frequencies of captures. A major fraction of the species analyzed showed preference for the feces. Regarding the species of the genus Psilochaeta, P. chalybea (Wiedemann, 1830) and P. chlorogaster were more abundant in sunny microhabitats, while P. pampiana was associated with the shaded microhabitats. Similarly, N. felsina, M. obscura and M. plaumanni were associated with the shaded microhabitats. Gymnodia spp. showed higher abundance on feces placed in sunny microhabitats (Fig. 5).
In addition to the multivariate analysis provided by the CA, the relationship of each species and sites was evaluated by mean of Synanthropy Index (SI), and comparison of proportions (Fig. 6).
All species showed significant differences in their relative frequencies along the three sites. Two species, M. stabu-lans (SI = +68.01) and M. domestica (SI = +35.63), showed a higher presence in the urban site. In contrast, Neurotrixa felsina (SI = -83.41), M. obscura (SI = -57.43) and P. trispila (SI = -91.17) had higher proportional abundances in the rural site. The presence of M. plaumanni (SI = +14.81) was higher in the suburban site. Finally, Gymnodia spp. (SI = -37.5) showed similar proportions for suburban and rural sites. However, in the CA, this genus showed an association with the rural site. The genus Psilochaeta showed different preferences: Psilochaeta chalybea (SI = -45.69) was present in all three sites but resulted in higher proportion in the rural site, whereas P. pampiana (SI = +17.18) and P. chlorogaster (SI = +11.76) showed higher proportions in the suburban site.
Ophyra aenescens (SI = -38.92) was captured in the three sites but showed a higher proportion in the rural site, whereas O. chalcogaster (SI = +66.66) showed preference for the urban site.
Muscidae species differed in monthly abundance fluctuations. Among those species associated with rural environments, N. felsina, M. plaumanni, and Gymnodia spp. showed peaks of abundance in winter (June-September) and spring (September-December), while M. obscura had highest abundance in late spring and early summer (November-January). Phaonia trispila did not show a definite pattern of its abundance during the study period. On the other hand, the syn-anthropic species M. domestica and M. stabulans showed a typical pattern of abundance associated with the warmer months. Musca domestica was captured only in the summer months (December-April) with higher peaks of abundance in the rural site during February 2007, while M. stabulans was captured throughout the sampling period, increasing its abundance in October and December of both years (Fig. 7).
Species of the genus Psilochaeta showed different patterns of abundance. Psilochaeta chalybea and P. pampiana were present during the end of winter and spring months. Psi-lochaeta chlorogaster showed its highest abundance in winter (May-August), but with lower abundance levels. The genus Ophyra showed similar seasonal patterns for its species, with peaks of abundance occurring during summer months (December-March) (Fig. 8).
Discussion
Assemblage structure, richness, and diversity. A first approximation to study a community is to estimate the representativeness of the samples obtained by rarefaction curves (Go-telli and Colwell 2001). In our work, the curves of the assemblage of saprophagous muscids seemed to reach asymptotic values, thus indicating good representativeness of samples.
When extrapolating values of richness through predictive estimators, both the heterogeneous area (Almirante Brown) and the homogeneous areas (U, S, R) showed similar results. In all the areas, the predictive estimators indicated that if we tripled the sampling effort (six years), a small fraction of additional species could be obtained. This fraction of richness may be influenced by the presence of rare species or by the capture method, in concordance with observations made by Thompson et al. (2003). Also, some species were captured at low abundance because they probably belong to other trophic niches (not saprophagous), and their presence in the samples does not reflect their actual abundance. Two examples may be Bithoracochaeta calopus (Bigot, 1885), whose adults are predators (Rodríguez-Fernández et al. 2006), and Grapho-myia auriceps Malloch, 1934 or Morellia (Trichomorellia) trichops (Malloch, 1923), species considered as floral visitors (Pombal and Morellato 1995; Keiper et al. 2002). In both cases, the species obtain protein food on baits.
The differences in evenness observed along the three sites may reflect the sensitivity of saprophagous muscids to changes in the environment. We observed an increase in diversity in terms of evenness and richness in the suburban and rural sites. Several studies agree that native wildlife affected by urbanization find refuge in patches or remnants of native vegetation (McKinney 2002). For a better knowledge of the effects of urbanization process on Muscidae community, a broad scale study would necessary in order to corroborate the trend obtained. On the other hand, the low evenness present in the urban site, with dominance of synanthropes as M. domestica and M. stabulans, could reflect how the urbanization affects the native saprophagous fauna and generates optimal conditions for those species with cosmopolitan distribution. These cosmopolitan species exploit food and shelter provided by humans, which allows them to reach huge population densities.
The total richness of the assemblage of saprophagous muscids of Buenos Aires (r = 20) was similar to that obtained in subtropical environment (r = 19 in Linhares 1981; r = 27 in Carvalho et al. 1984) and tropical environment (r = 19 in Uribe et al. 2010). On the other hand, a higher richness was observed compared to studies in a temperate environment in Chile (r = 6 in Figueroa-Roa and Linhares 2004), probably because this work was focused only in necrophagous fauna.
The assemblage studied presented slightly higher values of diversity and richness in the suburban site, as observed in subtropical and tropical environments (Carvalho et al. 1984; Uribe et al. 2010), and differing from that observed by Figueroa-Roa and Linhares (2004), which obtained similar values in the three sites studied. Our observations support the hypotheses that sites with an intermediate urbanization, which contain major landscape heterogeneity, show a higher number of species. This kind of environments could contain several types of resources supporting the proliferation of flies adapted to different environments (Connell 1978; Blair 2001; McKinney 2002). Conversely, the abundance of sapropha-gous muscids was higher in the rural site and decreased towards the urban core. The same patter was obtained in subtropical environments (Linhares 1981; Carvalho et al. 1984). In contrast, the urban site had the highest value in Colombia (Uribe et al. 2010), and the suburban had the highest value in Chile (Figueroa-Roa and Linhares 2004). The reason for the differences between studies may be related to the different methodologies used in each study. The reason for the differences between studies may be related to the different methodologies used in each work (exposure time attractant baits, sampling duration, type of bait, etc.), therefore is difficult to establish a general pattern of abundance.
Seasonality, bait preference, and spatial distribution. Human activities cause disturbances in the natural habitat, disrupting wildlife and creating new niches that can be occupied by native and/or exotic species. In the analysis of gradients of urbanization, we found saprophagous muscids in all the environments studied, but their distribution within the three sites differed between species. Species of cosmopolitan distribution considered exotic to the Neotropical region, such as M. domestica, M. stabulans, and O. chalcogaster, were associated with more urbanized environments (Carvalho et al. 1984; Costa et al. 2000; Patitucci et al. 2010; Uribe et al. 2010). Intermediate environments (e.g. suburban site) may offer a wide range of resources that allow the conservation of a large number of species (McKinney 2002). This variety of resources allows the coexistence of endemic species such as M. plaumanni, M. obscura, O. aenescens, O. albuquer-quei, P. pampiana, and P. chlorogaster with the exotic M. domestica and O. chalcogaster. Finally, environments with fewer disturbances showed a greater number of native species and a very low abundance or complete absence of alien species. These environments showed greater abundance of N. felsina, M. obscura and P. chalybea, all species native to the Neotropical region. The introduction of alien species along with increasing urbanization results in displacement of native species to the less urbanized environments (McKinney 2002), a situation which may be evidenced by observing the fauna in the suburban site where native species coexist with exotic ones.
Several studies have analyzed the use of certain resources such as breeding substrate or food source (organic matter decomposition) by saprophagous muscids (D'Almeida and Almeida 1998; Krüger et al. 2003). In this study, we observed a clear dissociation in saprophagous muscids regarding the bait on which they were captured. Only M. domestica, M. stabulans, and Synthesiomyia nudiseta (Wulp, 1883) and three species of Ophyra showed a preference for the rotten liver. Some of these species considered necrophagous (Skidmore 1985) were captured in sunny microhabitats, while the remaining ones were associated with shaded microhabitats, trends also observed in other works (Mendes and Linhares 1993; Figueroa-Roa and Linhares 2004; Pati-tucci et al. 2010). On the other hand, most species showed preference for the feces in shaded microhabitats. Among these species are N. felsina, which breeds in cattle dung (Hernández 1989), species of the genus Psilochaeta, which are considered as coprophilous (Carvalho et al. 1984), and M. plaumanni and M. obscura, whose habitat requirements are almost unknown. This tendency of adult coprophagous muscids to avoid environments with sun exposure could be related to a decrease in the drying time of the substrate, and resource utilization by the larvae as a substrate for breeding (Blackith and Blackith 1993).
The fluctuations of the different species in temperate areas are associated with the marked temperature fluctuation throughout the year. In this work, we observed that although some species such as M. plaumanni and M. obscura and the species of the genus Psilochaeta were present throughout the year, they showed a peak of activity in the intermediate seasons (autumn and spring). As previously noted for M. stabulans (Patitucci et al. 2010), M. domestica and the species of the genus Ophyra were more abundant in summer (Linhares 1981; Carvalho et al. 1984; Costa et al. 2000). Neurotrixa felsina was the only one with a peak of abundance in winter. Although some species of the genera Ophyra and Psilochaeta share living habits with their congeners, they show differences in their distribution in the gradient of urbanization. Regarding Ophyra, O. albuquerquei and O. aenescens occurred in environments with lower levels of urbanization, while O. chalcogater was present in environments with a greater degree of human intervention. This distribution pattern is consistent with that observed by Costa et al. (2000) for these three species, while in Colombia the species O. aenescens, presented an association with more urbanized environments (Uribe et al. 2010). Regarding Psilochaeta, the presence of P. pampiana and P. chlorogaster was significant in the suburban site, in accordance with that observed by Carvalho et al. (1984). Moreover, P. chalybea was more abundant in the rural site, as in Brazil (Carvalho et al. 1984), while in Chile its presence was higher in the intermediate site (Figueroa-Roa and Linhares 2004). The comparative analysis of co-generic species could be a good tool to evaluate the processes of urbanization.
Conclusions
Communities respond differently to environmental conditions, especially when habitats are disturbed (Schowalter 2006). In particular, a number of factors may be determining the composition of an assemblage of saprophagous muscids. From the analysis made here we see an assemblage response regarding the environment types, which reflects the adaptability to the physical changes along the three sites with different degree of urbanization. We can define the following groups of species according to their response to human activities as proposed by Blair (2001) and McKinney (2002). The first group consists of "urban exploiters", referred to species bly dependent on human resources. We can fit principally three liver-attracted species with this characteristic: M. domestica, M. stabulans and O. chalcogaster. Another characteristic shared by these species was their peak of abundance during the summer, probably related to alterations in the physical environment (e.g. higher temperature) present in the urban core (Shea and Chesson 2002). The second group is "urban adapters" or "edge species", which are associated with an intermediate degree of urbanization, and are considered facultative because they can use both natural and urbanized resources. In this group we can locate muscids, mainly coprophagous. Species that can be grouped here are Gymnodia spp., M. plaumanni, N. felsina, P. chlorogaster, P. pampiana, and O. aenescens. Finally, those sensitive species displaying negative responses to changes due to urbanization are referred as "urban avoiders". In this group we recognize almost exclusively coprophagous muscids associated with shaded places, with their abundance peak at intermediate seasons (although some necrophagous species are also present). This group could include M. obscura, P. chalybea and O. albuquerquei, which are species that tend to disappear with the advance of urbanization. The proper characterization of species of an assemblage of saprophagous muscids may be a control tool to establish the state of disturbance of the environment and biodiversity conservation.
Acknowledgements
We are grateful to anonymous referees for their useful comments. We wish to thanks the owners of "Estancia Don Mario", and family Nievas-Flores, for their permissions to take samples. We thank to Laura Mulieri for her material support in fieldwork. Financial support for this work was partially provided by CONICET and FONCyT (PICT 2008 N°0094)
Literature cited
BATES, A. J.; SADLER, J. P.; FAIRBRASS, A. J.; FALK, S. J.; HALE, J. D.; MATTHEWS, T. J. 2011. Changing bee and hoverfly pollinator assemblages along an urban-rural gradient. PLoS ONE 6 (8): 1-11. [ Links ]
BATTÁN-HORENSTEIN, M.; LINHARES, A. X.; ROSSO DE FERRADAS, B.; GARCIA, D. 2010. Decomposition and dip-teran succession in pig carrion in Central Argentina: ecological aspects and their importance in forensic science. Medical and Veterinary Entomology 24 (1): 16-25. [ Links ]
BLACKITH, R. E.; BLACKITH, R. M. 1993. Differential attraction of calyptrate flies (Diptera) to faeces. Journal of Natural History 27 (3): 645-655. [ Links ]
BLAIR, R. B. 2001. Birds and butterflies along urban gradients in two ecoregions of the U.S. pp. 33-56. In: Lockwood, J. L.; Mckinney, M. L. (Eds.). Biotic homogenization. Kluwer Academic, New York, USA. 297 p. [ Links ]
CARVALHO, C. J. B. DE; ALMEIDA, J. R. DE; JESUS, C. B. DE. 1984. Dípteros sinantrópicos de Curitiba e arredores (Paraná, Brasil). I. Muscidae. Revista Brasileira de Entomologia 28 (4): 551-560. [ Links ]
CARVALHO, C. J. B. DE; COURI, M. S.; PONT, A. C.; PAMPLONA, D.; LOPES, S. M. 2005. A catalogue of the Muscidae (Diptera) of the Neotropical region. Zootaxa 860: 1-282. [ Links ]
CLENCH, H. 1979. How to make regional list of butterflies: Some thoughts. The Journal of the Lepidopterists' Society 33 (4): 216231. [ Links ]
COLWELL, R. K. 2011. EstimateS Version 8.2.: Statistical estimation of species richness and shared species from samples (Software and User's Guide). Freeware for Windows and Mac OS. Available in: http://viceroy.eeb.uconn.edu/Colwell/#Software. [Review date: 11 March 2012] [ Links ].
CONNELL, J. H. 1978. Diversity in tropical rain forest and coral reefs. Science 199 (4335): 1302-1310. [ Links ]
COSTA, P. R. P.; FRANZ, R. L.; VIANNA, E. E. S.; RIBEIRO, P. B. 2000. Synantropy of Ophyra spp. (Diptera, Muscidae) in Pelotas, Rs, Brazil. Revista Brasileira de Parasitologia Veterinária 9 (2): 165-168. [ Links ]
D'ALMEIDA, J. M.; ALMEIDA, J. R. DE. 1998. Trophic niches on Diptera Calyptratae, in Rio de Janeiro, RJ, Brazil. Revista Brasileira de Biologia 58 (2): 563-570. [ Links ]
FIGUEROA-ROA, L.; LINHARES, A. X. 2004. Synantropy of Muscidae (Diptera) in the City of Valdivia, Chile. Neotropical Entomology 33 (5): 647-651. [ Links ]
FLEISS, J. L. 1981. Statistical methods for rates and proportions. 2nd ed. John Wiley and Sons, New York, USA. 321 p. [ Links ]
GOTELLI, N. J.; COLWELL, R. K. 2001. Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecology Letters 4 (4): 379-391. [ Links ]
HERNÁNDEZ, M. C. 1989. Dípteros coprófagos: Neurotrixa felsina (Walker) (Diptera, Muscidae). Descripción de estadios larvales. Revista de la Sociedad Entomologica Argentina 47 (1-4): 95-99. [ Links ]
JIMÉNEZ-VALVERDE, A.; HORTAL, J. 2003. Las curvas de acumulación de especies y la necesidad de evaluar la calidad de los inventarios biológicos. Revista Ibérica de Aracnología 8: 151-161. [ Links ]
KEIPER, J. B.; WALTON, W. E.; FOOTE, B. A. 2002. Biology and ecology of higher Diptera from freshwater wetlands. Annual Review of Entomology 47: 207-232. [ Links ]
KREBS, C. J. 1999. Ecological methodology. Addison-Wesley Educational, Menlo Park, Canada. 620 p. [ Links ]
KRÜGER, R. F.; RIBEIRO, P. B.; CARVALHO, C. J. B. DE. 2003. Desenvolvimento de Ophyra albuquerquei Lopes (Diptera, Muscidae) em condicöes de laboratòrio 1. Revista Brasileira de Entomologia 47 (4): 643-648. [ Links ]
KRÜGER, R. F.; CARVALHO, C. J. B. DE; RIBEIRO, P. B. 2010. Assembly rules in muscid fly assemblages in the grasslands bi-ome of Southern Brazil. Neotropical Entomology 39 (3): 345353. [ Links ]
LINHARES, A. X. 1981. Synanthropy of Muscidae, Fanniidae and Anthomyiidae (Diptera) in the city of Campinas, Säo Paulo, Brazil. Revista Brasileira de Entomologia 25 (4): 231-243. [ Links ]
LONGINO, J. T.; COLWELL, R. K. 1998. Biodiversity assessment using structured inventory: capturing the ant fauna of a tropical rainforest. Ecological Applications 7: 1263-1277. [ Links ]
MCKINNEY, M. L. 2002. Urbanization, biodiversity and conservation. BioScience 52 (10): 883-890. [ Links ]
MCKINNEY, M. L. 2008. Effects of urbanization on species rich-ness:A review of plants and animals. Urban Ecosyst 11 (2): 161176. [ Links ]
MENDES, J.; LINHARES, A. X. 1993. Atratividade por iscas, sa-zonalidade e desenvolvimento ovariano em várias espécies de Muscidae (Diptera). Revista Brasileira de Entomologia 37 (2): 289-297. [ Links ]
MONTES, J. 2005. Culicidae fauna of Serra da Cantareira, Säo Paulo, Brazil. Revista do Saúde Publica 39 (4): 578-584. [ Links ]
NUORTEVA, P. 1963. Synanthropy of blowflies (Dipt., Calliphoridae) in Finland. Annales Entomologici Fennici 29: 1-49. [ Links ]
PAPE, T. 2009. Chapter 4, Economic Importance of Diptera; p. 65-78. In: BROWN, B.V.; BORKENT, A.; CUMMING, J. M.;WOOD, D. M.; WOODLEY, N. E.; ZUMBADO, M. Manual of Central America Diptera, Volumen 1. Research Press, Ottawa, Canada. 714 p. [ Links ]
PATITUCCI, L. D.; MULIERI, P. R.; MARILUIS, J. C.; SCHNACK, J. A. 2010. The population ecology of Muscina stabulans (Diptera: Muscidae) along an urban-rural gradient in Buenos Aires, Argentina. Neotropical Entomology 39 (3): 441-446. [ Links ]
PATITUCCI, L. D.; MULIERI, P. R.; OLEA, M. S.; MARILUIS, J. C. 2013. Muscidae (Insecta: Diptera) of Argentina: revision of Buenos Aires province fauna, with a pictorial key to species. Zootaxa 3702 (4): 301-347. [ Links ]
POMBAL, E. C. P.; MORELLATO, P. C. 1995. Polinizacäo por moscas em Dendropanax cuneatum Decne. and Planch. (Araliaceae) em floresta semidecídua no sudeste do Brasil. Revista Brasileira de Botánica 18 (2): 157-162. [ Links ]
RODRÍGUEZ-FERNÁNDEZ, J. I.; CARVALHO, C. J. B. DE; MOURA, M. O. 2006. Estrutura de assembléias de Muscidae (Diptera) no Paraná: uma análise por modelos nulos. Revista Brasileira de Entomología 50 (1): 93-100. [ Links ]
SHEA, K.; CHESSON, R 2002. Community ecology theory as a framework for biological invasions. Trends in Ecology & Evolution 17: 170-176. [ Links ]
SKIDMORE, P. 1985. The biology of the Muscidae of the world. Series Entomologica. 1st ed. Junk Publishers, Dordrecht, Netherlands. 550 p. [ Links ]
THOMPSON, G. G.; WITHERS, P. C.; PIANKA, E. R.; THOMPSON, S. A. 2003. Assessing biodiversity with species accumulation curves; inventories of small reptiles by pit-trapping in Western Australia. Austral Ecology 28 (4): 361-383. [ Links ]
URIBE, M. N.; WOLFF, M.; CARVALHO, C. J. B. DE. 2010. Synanthropy and ecological aspects of Muscidae (Diptera) in a tropical dry forest ecosystem in Colombia. Revista Brasileira de Entomología 54 (3): 462-470. [ Links ]
ZAR, J. H. 1996. Biostatistical analysis. 3rd ed. Prentice Hall, Englewood Cliffs, USA. 662 p. [ Links ]
Suggested citation:
PATITUCCI, LUCIANO DAMIÁN; PABLO RICARDO MULIERI; JUAN ALBERTO SCHNACK and JUAN CARLOS MARILUIS. 2013. Assemblages of saprophagous muscids (Diptera: Muscidae) in three urban sites of temperate Argentina. Revista Colombiana de Entomología 39 (2): 291-300.