Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Colombiana de Entomología
versão impressa ISSN 0120-0488
Rev. Colomb. Entomol. vol.40 no.1 Bogotá jan./jun. 2014
Sección Agrícola
Non-preference for oviposition and antibiosis in bean cultivars to Bemisia tabaci biotype B (Hemiptera: Aleyrodidae)
No preferencia de oviposición y antibiosis en cultivares de frijol por Bemisia tabaci biotipo B (Hemiptera: Aleyrodidae)
ANDERSON GONÇALVES DA SILVA1, ARLINDO LEAL BOIÇA JUNIOR2, PAULO ROBERTO S. FARIAS1, NARA ELISA L. RODRIGUES2, BRUNO HENRIQUE S. DE SOUZA2, DALINE BENITES BOTTEGA2 and ALISSON FERNANDO CHIORATO31 Instituo de Ciencias Agrárias - Universidade Federal Rural da Amazonia - UFRA, Av. Presidente Tancredo Neves, 2501, 660077-530, Belém, PA, Brasil. Ph.D. and professor agroanderson.silva@yahoo.com.br (corresponding author); Ph. D. and professor paulo.farias@ufra.edu.br.
2 Departamento de Fitossanidade - Universidade Estadual Paulista Júlio de Mesquita Filho (FCAV/UNESP), Via Acesso Prof. Paulo Donatto Castellane,s/n - CEP: 14870-000, Jaboticabal-SP, Brasil. Ph. D. and professor aboicajr@fcav.unesp.com.br; Ph. D. nara_elr@yahoo.com.br; Ph. D. souzabhs@gmail.com; Ph. D. daline4@bol.com.br.
3 Instituto Agronómico de Campinas - IAC, Centro de Análise e Pesquisa Tecnológica dos Agronegócios dos Graos e Fibra. Rua Barao de Itapura, 1481 Botafogo, CEP 13001-970 - Campinas, SP,Brasil. Ph. D. pesquisador afchiorato@iac.sp.gov.br.
Received: 21-Mar-2013 • Accepted: 4-Jun-2014
Abstract: Among the pests that cause reduction on common bean yield, the whitefly Bemisia tabaci biotype B (Hemiptera: Aleyrodidae) stands out. The insect causes direct damages due to its feeding on plants and indirect damages by the sugary excretion of honeydew, providing the development of the sooty mould. In addition this species is an important vector of virus such as the golden bean mosaic virus. Thus, this work aimed to study the non-preference for oviposition and antibiosis in bean cultivars to the whitefly. Experiments were carried out in a greenhouse at Jaboticabal, SP, Brazil, from January to March 2012. The following cultivars were used in the assays: IAC-Centauro, IAC-Una, IAC-Formoso, IAPAR-81, IPR-Eldorado, IPR-Siriri (all resistant); and Pérola and IAC-Harmonia (both susceptible), previously screened from field experiments. Cultivars IAC-Harmonia, IPR-Eldorado, IAPAR-81 and IPR-Siriri were the least preferred for oviposition; and the cultivar IAC-Harmonia extended the whitefly life cycle, expressing nonpreference for feeding and or/antibiosis-type resistance.
Key words: Whitefly. Phaseolus vulgaris. Genetic resistance. Categories and levels of resistance.
Resumen: Entre las plagas que causan la disminución de la producción de frijol común, la mosca blanca Bemisiatabaci biotipo B (Hemiptera: Aleyrodidae) es notable. Esta causa daños directos debido a la forma de alimentación e indirectos debido a la excreción de melaza y producción de fumagina, además de ser importante para la transmisión del virus del mosaico dorado del frijol. Los objetivos de esta investigación fueron estudiar la no preferencia para la oviposición y antibiosis en cultivares de frijol de la mosca blanca. Los experimentos se llevaron a cabo en el invernadero del Departamento de Fitosanidad, en Jaboticabal, SP, Brasil, de enero a marzo de 2012. Se utilizaron las cultivares: IAC-Centauro, IAC-Una, IAC-Formoso, IAPAR-81, IPR-Eldorado, IPR-Siriri (resistentes); Pérola y IAC-Harmonia (susceptibles), seleccionados en experimentos de campo. Los cultivares IAC-Harmonia, IPR-Eldorado, IAPAR 81 y IPR-Siriri fueron los menos preferidos para la oviposición; y el cultivar IAC-Harmonia prolongó el ciclo de vida de la mosca blanca, mostrando no preferencia para la alimentación y/o antibiosis.
Palabras clave: Mosca blanca. Phaseolus vulgaris. Resistencia genética. Tipos y grados de resistencia.
Introduction
Among the pest insects that cause economical losses to common bean (Phaseolus vulgaris L.), the whitefly Bemisia tabaci (Genn., 1889) biotype B (Hemiptera: Aleyrodidae) stands out due to its direct damage by feeding on the phloem, weakening the plant by sucking nutrients, in addition to injection of toxins, resulting in physiological problems in bean plants, as well as indirect damages through the excretion of honeydew. This excretion serves as substrate for the growth of saprophyte fungi from genus Capnodium (sooty mould) on leaves, flowers and fruits, preventing gas exchange, such as respiration and photosynthesis and hence decreasing yield. It also complicates pesticide action, resulting in higher production costs (Lima 2001).
However, the most serious damage caused by B. tabaci biotype B is concerning to virus transmission, such as the golden bean mosaic virus (GBMV), one of the major problems for common bean crop in Latin America. This disease causes economical losses that may range from 30 to 100% depending on the cultivation, plant growth stage, population level of the vector, presence of alternative hosts and environmental conditions (Salguero 1993).
Use of pesticides is the main control method adopted by bean growers. However, Horowitz and Ishaaya (1995) reported that in several cases, treatment with conventional insecticides is not efficient mainly because adults are located in the lower surface of leaves and due to fast resistance deployment against the active ingredients. Moreover, successive utilization of pesticides may unbalance the environment and eliminate beneficial arthropods (Prabhaker et al. 1985).
The intrinsic ability that some genotypes possess in comparison to others from the same species, in order to obtain higher yield and/or quality under the same attack of pest population in equal conditions is known as host plant resistance (HPR) (Lara 1991). Screening of plants resistant to B. tabaci biotype B, transmitting geminivirus or causing physiological disorders in cultivated plants, represents an important research tool aiming to diminish injuries and losses caused by this insect (Mcauslane et al. 1994). For HPR use, it is necessary to know morphological and physiological traits of the plant and the insect behavior and biology as well as its relationship with the plant host. These factors are indispensable to the host response to pest action, determining plant resistance or susceptibility (Campos 2003).
Resistance categories described by Painter (1951) were related as non-preference, antibiosis and tolerance. Nonpreference or antixenosis refers to insect behavioral aspects on the plant, which may be non-preference for feeding, oviposition or shelter, and the two formers are the most studied. Regarding antibiosis, direct lethal effects are verified on the different stages of the insect, whereas tolerance is defined as the plant ability to withstand insect attack without significant yield reduction.
Several studies have pointed out resistant cultivars that can be utilized by the growers, thus minimizing B. tabaci biotype B attack on bean crop, especially non-preference for oviposition- and antibiosis-type resistance (Toscano et al. 2002; Campos et al. 2005; Paron and Lara 2005; Jesus et al. 2010; Rodrigues et al. 2012a). In addition, HPR can be associated harmoniously with other control methods, according to studies conducted by Jesus et al. (2009), Costa et al. (2010) and Janini et al. (2011).
In this context, HPR should be used as one more control method within the integrated pest management (IPM) concepts, aiming to attenuate injuries caused by B. tabaci biotype B. Among the main benefits provided by this method adoption, the following are highlighted: reduction of insect population to uninjured levels; non-interference on agroecosystem; is non-pollutant; provides cumulative and persistent effects; and does not demand specific knowledge by the producer (Boiça Júnior et al. 2013). Given the importance of bean crop to Brazilian population and HPR as a control method, the present research aimed to study the non-preference for oviposition and antibiosis in bean cultivars to the whitefly.
Materials and methods
Non-preference for oviposition and antibiosis experiments were carried out in a greenhouse of the Departamento de Fitossanidade at the Faculdade de Ciências Agrárias e Veterinárias - FCAV/UNESP, Jaboticabal, SP, Brazil, from January to March 2012. The following cultivars were used for the tests: IAC-Centauro, IAC-Una, IAC-Formoso, IAPAR-81, IPR-Eldorado, IPR-Siriri (all resistant), Pérola and IAC-Harmonia (all susceptible). These cultivars were previously screened from field experiments in winter, water and dry seasons (Silva 2012).
Non-preference for oviposition
Maintenance colony of Bemisia tabaci biotype B. The whitefly colony was maintained inside cages made by antiaphid screen (2.0 m length x 3.0 m width x 2.0 m height), using kale as the host plant, cultivar Manteiga da Georgia (Brassica oleracea L. var. acephala), grown in 4 L pots. To obtain high infestations of the pest, adults of the whitefly were released into the cages, which were obtained from colonies of the Entomology sector of the Instituto Agronômico de Campinas (IAC), given by Dr. André Luiz Lourenção, and identified as B. tabaci biotype B. Fortnightly, new plants were introduced to replace senescent plants. Cultural practices and irrigation were done when needed.
Free-choice test. Oviposition preference by B. tabaci biotype B was observed on eight common bean cultivars: IAC-Centauro, IAC-Una, IAC-Formoso, IAPAR-81, IPR-Eldorado, IPR-Siriri, Pérola and IAC-Harmonia. They were screened as resistant (six formers) and susceptible (two latter), taking into account the three sowing seasons in experiment conducted in field conditions, and considering the cultivars performance against the incidence of BGMV.
The cultivars were sown in 5 L pots, containing soil and manure in the proportion of 3:1, and 20 days after the emergence one plant per plot was kept after thinning, which received cultural practices and fertilization as recommended for the crop. Twenty-five days after emergence (V4 growth stage – three opened trifoliates), the pots with bean cultivars were distributed at random and circularly inside screened cages (2.0 m base x 1.8 m height) in which 100 non-sexed adults of the whiteflies per plot (treatment) were released in the center (total of 800 adults). Adults were from the maintenance colony, and were reared according to the methodology by Toscano et al. (2002). The number of adults of B. tabaci biotype B released per treatment followed the methodology recommended by Jesus et al. (2011), who reported this density provides adequate oviposition for plant resistance studies on bean cultivars. Five replicates were used for each cultivar.
After 24 hours of the insects release, plants were removed and taken to the Laboratório de Resistência de Plantas a Insetos at FCAV/UNESP. In the laboratory, eggs were counted per cm2 on the abaxial surface of one trifoliate from the upper, middle and lower thirds of the cultivars through a stereoscope. Leaf area of the plants thirds was measured using an electronic leaf area meter, model LI-COR 3100A®.
No-choice test. For the no-choice test, pots with one 25 dayafter emergence plant of each cultivar were individualized into cylindrical cages (40 cm diameter x 60 cm height) coated with voile fabric. Next, 100 non-sexed adults of B. tabaci biotype B per cultivar were released into each cage. At 24 hours after release, the whole leaf area was removed from plants and taken to the laboratory, where the same assessments were performed as described for the free-choice test. Five cages (replicates) were used for each bean cultivar in this assay.
Experimental design and statistical analysis. A completely randomized design was used for both tests in a 8 x 3 (cultivars x plant thirds) split-plot arrangement for the free-choice test, and in a 8 x 3 factorial arrangement for the no-choice test. The assays consisted in five replicates for each cultivar. Data obtained from both assays were first transformed in (x + 1)½ for normalization. Next, data recorded from the freechoice test were subjected to ANOVA of a split-plot arrangement, and data from the no-choice test were subjected to the two-way ANOVA to determine the main effects of cultivars, plants thirds and the interaction of cultivars x plants thirds. Scott-Knott's post-hoc test was used for means separation at the level of 5% probability.
Oviposition preference index (OPI). Oviposition preference index (OPI) was calculated using the formula proposed by Fenemore (1980): OPI = [(T-P)/(T+P)] x100, where T = number of eggs on the tested cultivar, and P = number of eggs on the standard cultivar (susceptible). OPI varies from +100 (very stimulant), 0 (neutral) to -100 (total deterrence). The cultivar IAC-Centauro was set as the susceptible standard as it exhibited the highest oviposition means in free-choice and no-choice tests. Classification of cultivars (stimulant/ deterrent) was done from comparison of the mean numbers of eggs on treatments with the pattern cultivar, taking into account the mean standard error (± SE) in order to allow differentiation among cultivars, according to the methodology used by Baldin et al. (2000).
Antibiosis test. Antibiosis assay was conducted in a greenhouse using pots with one 25 day-old plant of each cultivar. One leaflet per plant was individualized and cages made by voile fabric were attached to the leaflet, according to methodology by Campos et al. (2005), and then, 50 non-sexed adults were released inside each cage. Infestation was remained for 24 hours, and next the whitefly adults were withdrawn and the leaflets were examined. We attempted to keep 50 eggs on the abaxial surface of leaves, and the excess of eggs was removed using a pair of tweezers. Each leaflet containing initially 50 eggs represented one replication, totaling five replications per cultivar, and the assay was set in a completely randomized design.
The leaflets were uncovered until the end of the nymphal stage, when the cages were attached to leaves again in order to avoid adults to escape. Observations were done daily and at the same time, evaluating the duration of the incubation period, duration of period and viability of nymphs, duration of development (from egg to adult) and adult longevity (without food). For longevity observation, 25 recentlyemerged adults were collected randomly (five adults from each leaflet) from the cages and placed into glass tubes (2.5 cm diameter x 8.5 cm height), and the number of dead insects was reported daily.
Data recorded from duration of incubation period, duration of the nymphal period, duration from egg to adult, and longevity of adults were transformed in (x + 0.5)½, and data recorded from nymphal viability in arcsine (x + 0.5/100)½ for normalization and then were subjected to the one-way ANOVA. Tukey's post-hoc test was used at 5% probability for means separation, using the software SAS (Sas Insitute 1994).
In order to discriminate the bean cultivars regarding the resistance degrees to B. tabaci biotype B, the biological parameters duration of incubation period, duration of period and viability of nymphs, duration of development from egg to adult and adult longevity were evaluated through the Principal Component Analysis (PCA) (Jackson 1991) to classify the cultivars that showed the maximum similarity and minimum dissimilarity among groups. Results from multivariate (PCA) and univariate (ANOVA) analyses were adopted to discriminate the bean cultivars regarding the resistance degrees to B. tabaci biotype B. For multivariate analysis and graph preparation, the software Statistica version 7.0 (Statsoft 2012) was used.
Results and discussion
Non-preference for oviposition
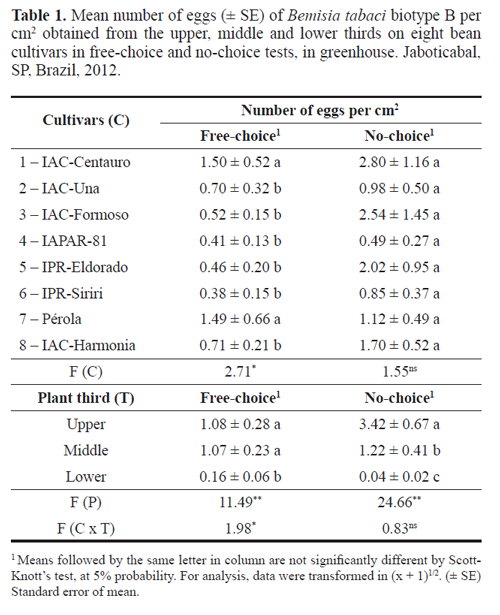
Free-choice and no-choice tests. There were significant differences in the oviposition preference of B. tabaci biotype B among the evaluated cultivars only the in free-choice test (Table 1). The cultivars IPR-Siriri, IAPAR-81, IPR-Eldorado, IAC-Formoso, IAC-Una and IAC-Harmonia highlighted as the least preferred for oviposition, with means ranging from 0.38 to 0.71 eggs per cm2. Overall, these cultivars are early development cultivars which may have interfered on resistance expression. On the other hand, the cultivars IAC-Centauro and Pérola were the most preferred for oviposition, with 1.50 eggs per cm2 (Table 1), and we may infer this cultivar holds traits acting as oviposition stimulant for the whitefly.
In the no-choice test, no significant differences were observed among cultivars. However, there was a trend of higher number of eggs on the cultivar IAC-Centauro (2.80 per cm2), and numerically the lowest mean of eggs were observed on IAPAR-81 (0.49 cm2) (Table 1).
It is reported in literature that the presence of trichomes acts as a stimulant factor for the B. tabaci biotype B oviposition, and the most pilose cultivars are the most infested since these cultivars may provide a more suitable microclimate for oviposition and better protection for the nymphs (Butter and Vir 1989). In addition, females prefer to lay eggs on the base of trichome insertion (Omram and El-khidir 1978). Three types of trichomes are found in bean plants: acicular trichomes (needle-shaped), which are long, erect, distally narrowed and formed by two basal cells and one terminal cell; unciform trichomes (hook-shaped), which are smaller and also possess two basal cells and one terminal cell; and glandular trichomes, which are short. These characteristics were first described by Moutt (1932) cited by Dahlin et al. (1992). Costa et al. (2004) reported that non-preference for oviposition of B. tabaci biotype B on cowpea cultivars may be related to lower content of attractant substances or higher content of repellents, which influence the insect behavior on host selection. These factors may also be associated to the relationship whitefly-common bean.
Significant differences were found for plants thirds in both free-choice and no-choice tests (Table 1). In the freechoice test, the upper and middle thirds were equally preferred for oviposition, with means of 1.08 and 1.07 eggs per cm2, respectively, differing from the lower third (0.16 eggs per cm2). In the no-choice test, the highest number of eggs was observed on the upper third (3.43 eggs per cm2), differing from the middle third (1.22 eggs per cm2), which on the other hand, differed from the lower third (0.04 eggs per cm2) (Table 1). Similar results were obtained by Rodrigues et al. (2012b), while studying the life history and non-preference for oviposition of B. tabaci biotype B on cowpea cultivars. The authors reported the whitefly prefers to lay eggs on the abaxial surface of leaves from the upper part of plant canopy.
The whitefly probably estimates the age and quality of the host plant through stylet insertion into the plant tissue before selecting the oviposition site (Vendramim et al. 2009), nevertheless, without ingesting sap in order to find a favorable chemical or morphological constitution depending on the plant age (Walker and Perring 1994) and stimuli involved between the insect and plant (Lara 1991). Likewise, Campos et al. (2005) reported the highest number of eggs laid by the whitefly on the apex leaf on cotton. According to Van Lenteren and Noldus (1990), preference for the youngest parts of the bean plant may be related to the highest concentration of nutrients (amino acids), which are promptly available for the sucking insects. In addition, younger leaves have thinner and softer cuticle, as well as a higher amount of water. Therefore, these characteristics may facilitate the whitefly oviposition (Eichelkraut and Cardona 1989) and eggs hydration (Gill 1990), providing a higher survivorship of the nymphs.
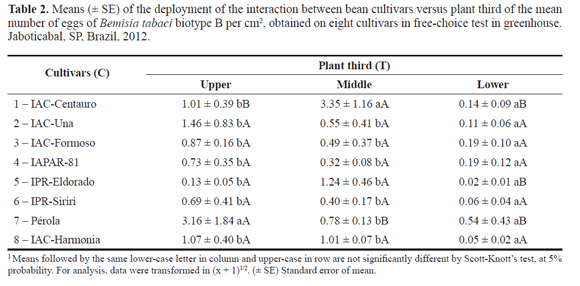
For the interaction of cultivar x plant third, significant difference was observed only in the free-choice test (Table 2). Although numerically, means of number of eggs per cm2 were higher on the upper third of bean plants of most cultivars. We observed through the deployment of the interaction significant differences only in the cultivars that had over 3 eggs per cm2. The cultivar Pérola stood out with 3.16 eggs per cm2 on the upper third, differing from the middle and lower thirds. On the cultivar IAC-Centauro, preference was observed for the middle third (3.35 eggs per cm2) and differed significantly from the other plant thirds (Table 2).
Because they were the most preferred for oviposition (over 3 eggs per cm2), the cultivars IAC-Centauro and Pérola also differed significantly from the other cultivars regarding the plant thirds separately. As results, Pérola exhibited the higher number of eggs on the upper third, and the cultivar IAC-Centauro on the middle third. There were no differences for the lower third among the bean cultivars, which had low infestation (Table 2). Berlinger (1986) underlines that physical traits of leaf surfaces, such as pilosity, presence of adherent glandular trichomes and leaf format are aspects affecting oviposition preference by the whitefly. In addition, pubescence is one of the factors that allows B. tabaci biotype B oviposition preference on the lower surface of leaves, but, other traits, e.g. number of leaves and foliar area, are also important for host selection (Simmons 1994).
For beans, specifically, feeding and oviposition non-preference are the most prevalent resistance-types, in addition to antibiosis. There are few reports of tolerance in common beans against the whitefly. A study conducted by Oriani et al. (2005) showed that, among other factors, resistance in common bean against B. tabaci biotype B is related to the presence of arcelin or acicular trichomes. The presence of trichomes acts as stimulant for the oviposition of B. tabaci biotype B on beans, and the pilose cultivars were more infested as previously reported by Peña et al. (1992), Peña et al. (1993), Oriani and Lara (2000) and Oriani et al. (2005). Also, trichomes are stimulant in other crops such as soybean (Valle and Lourenção 2002), tomato (Toscano et al. 2002), and cotton (Chu et al. 2001). Therefore, this feature should be avoided in breeding programs of common bean against the whitefly.
Further studies are needed to unveil the resistance mechanisms present in the least preferred cultivars IPR-Siriri, IAPAR-81, IPR-Eldorado, IAC-Formoso, IAC-Una and IAC-Harmonia, especially in free-choice test, in order to head investigations to transfer or increase these resistancerelated traits to new cultivars.
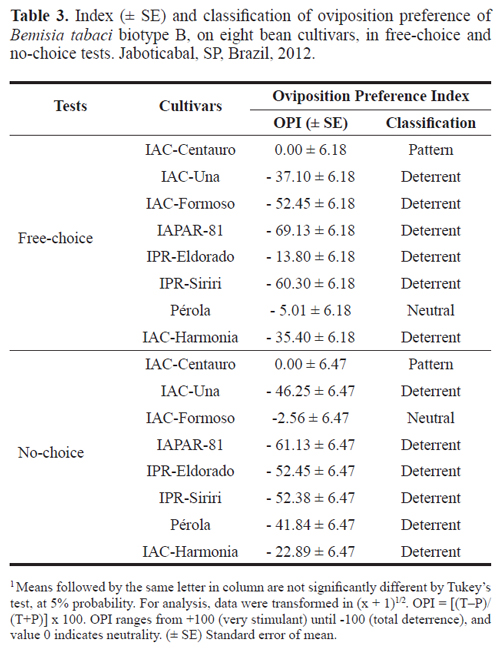
Oviposition preference index (OPI). In the free-choice test, all cultivars were deterrent for B. tabaci biotype B oviposition (Table 3), except Pérola, which behaved as neutral. In the no-choice test, all cultivars were classified as deterrent but IAC-Formoso, which were neutral (Table 3). According to Lara (1991), the presence of deterrence is of great importance in resistant entries, by reducing feeding and/or oviposition of the insect on that genotype.
In both assays, there was an increase of the OPI when the condition was switched from the no-choice to free-choice test (Table 3). The expressive augment of oviposition deterrence on Pérola in the no-choice test is highlighted as this cultivar was neutral in the free-choice test. Similar results were also reported for IAC-Formoso. This cultivar was classified as deterrent in the free-choice test, since the expectation was an increase on deterrence (OPI = -52.45), and in the no-choice test it behaved as neutral (OPI = -2.56) (Table 3). According to Blua et al. (1995), behavioral changes of the whitefly is attributed to several factors that modify insect preference as the confinement conditions are different for free-choice and no-choice tests.
Baldin et al. (2005), while evaluating the resistance in tomato genotypes against B. tabaci biotype B, found that the OPI calculated in free-choice and no-choice tests classified all genotypes as oviposition deterrent to the whitefly when compared to the susceptible pattern IAC-Santa Clara. The same authors still emphasized that results obtained with the genotypes LA-716, PI 134418 and PI 134417 are similar from those found by Toscano et al. (2002) and Fancelli et al. (2003), reporting expression of non-preference for oviposition- type resistance in these genotypes against the whitefly.
For most experiments, we underline that the genotypes with deterrence traits were not commercial cultivars, but wild entries or breeding lines with agronomical characteristics of low yield, whereas for the present work oviposition deterrence was reported for high yielding cultivars.
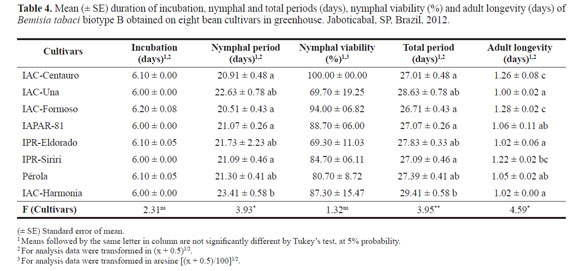
Antibiosis test. There were no significant differences in the mean duration of eggs incubation (Table 4), lasting nearly six days after oviposition for all cultivars evaluated. Similar results were obtained by Lima and Lara (2004) on soybean plants (6.4 to 6.6 days), by Baldin et al. (2005) on tomato genotypes (about 6 days) and by Rodrigues et al. (2012b) on cowpea cultivars (5.53 to 6.72 days), demonstrating that this biological parameter is apparently little affected by the host plant.
For the nymphal period, we observed that cultivar IACHarmonia extended the biological development of the whitefly (23.41 days), differing significantly from the results obtained for IAC-Centauro, IAC-Formoso, IAPAR-81 and IPR-Siriri, which had duration of the biological development around 21 days (Table 4). These data suggest the expression of non-preference for feeding and/or antibiosis in IAC-Harmonia, which, according to Lara (1991), is characterized by the longer period nymphs require to complete the immature stage comparatively to a susceptible plant (Table 4). The other cultivars were intermediate.
Shorter duration of nymphal periods than those obtained in the present study were found by Oriani and Lara (2000) while assessing the antibiotic effects of bean genotypes possessing arcelin on B. tabaci biotype B in Jaboticabal, SP, Brazil, who observed duration varying from 11.0 to 15.4 days. It is important to mention that the study of the authors was car ried out in the dry season, i.e. the hottest period of the year, which may have favored the insect development. In the same work, when the water season was evaluated the results were similar to our study, with means of 23 days for the duration of nymphal period.
Nymphal viability had amplitude between 69.30% on the cultivar IPR-Eldorado and 100% on IAC-Centauro, however, there was no significant difference among cultivars (Table 4). These percentages are considered high when compared to other studies with common bean or other plant species in the literature. Fancelli et al. (2003) obtained 31.2 to 86.9% nymphal viability on tomato genotypes, Campos (2003) found 30.7 to 64.2% on cotton and Rodrigues et al. (2012b) observed from 50.0 and 90.0% nymphal viability on cowpea cultivars. These results prove this biological parameter is variable depending on the host plant.
With respect to the total period (duration from egg to adult), results were influenced by the nymphal period, with significant differences. The cultivar IAC-Harmonia highlighted as the most harmful to B. tabaci biotype B development, evidenced by the elongation of its life cycle (29.41 days), and differed from IPR-Siriri (27.09 days), IAC-Centauro (27.01 days) and IAC-Formoso (26.71 days), which were the most suitable for the whitefly development. Duration of the total period on these cultivars was about 2.5 days shorter than on IAC-Harmonia (Table 4).
For this parameter, Oriani and Lara (2000), while studying the antibiotic effects of nine bean cultivars with or without arcelin in greenhouse conditions, observed that B. tabaci biotype B total cycle was between 21 and 37 days in experiments conducted in the water and dry seasons in Jaboticabal, SP, Brazil, respectively. Studies conducted by Rodrigues et al. (2012b) on cowpea showed that the cultivar Sempre Verde was the most adequate for the whitefly development, and the cultivars BRS Urubuquara and IPA-206 expressed non-preference for feeding and or/antibiosis-type resistance.
Adults of the whitefly lived less when their nymphs were fed the cultivars IAC-Una (1.0 day), IPR-Eldorado (1.02 days) and IAC-Harmonia (1.02 days), differing significantly from IAC-Formoso (1.28 days) and IAC-Centauro (1.26 days) (Table 4). Most studies in literature do not report differences among treatments for adults longevity, mainly because experiments were evaluated without adults feeding (Oriani et al. 2008; Rodrigues et al. 2012b).
It is important to note that the cultivar IAC-Harmonia, which stood out as the least suitable for whitefly development by extending its life cycle, was considered susceptible in the water season, with the highest infestation in comparison to other 18 cultivars. In addition, this cultivar exhibited the highest incidence of the BGMV in field conditions (Silva 2012).
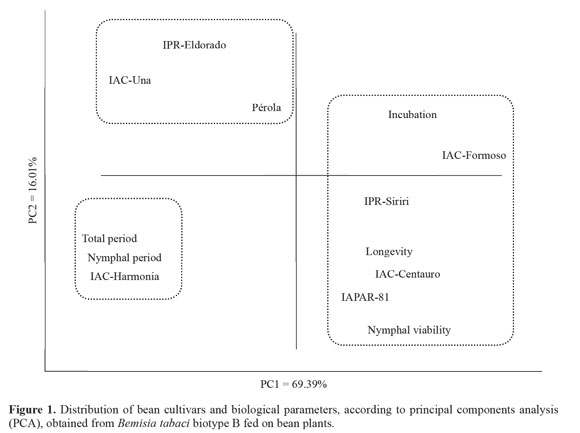
Considering the biological parameters concomitantly through the multivariate Principal Component Analysis, the first principal component (PC1) concentrated 61.39% of the entire variability from the original variables, and the parameters that most influenced this factor were nymphal period (- 0.93), total period (- 0.91) and adults longevity (0.90). The second principal component (PC2) concentrated 16.08% of the variability presented in the original biological parameters, and the most influent variable was nymphal viability (-0.67) (Fig. 1).
Analyzing the distribution of the bean cultivars following the PCA obtained from the B. tabaci biotype B biological parameters, values of duration of nymphal period and total period were the factors that most influenced the analysis by isolating the cultivar IAC-Harmonia in the third quadrant (Fig. 1). Thus, based on the distribution of the cultivars from the whitefly biological aspects in univariate (ANOVA) and multivariate (PCA) analyses, and according to criteria established by Kaiser (1960), it is possible to classify the cultivars regarding the resistance degrees: IAC-Harmonia was classified as moderately resistant; IAC-Una, IPR-Eldorado and Pérola, as susceptible; and IAC-Centauro, IAPAR-81 and IPR-Siriri, are highly susceptible.
The biological parameters period of incubation and nymphal viability were the factors that least influenced the differentiation and classification of the bean cultivars for the resistance degrees to B. tabaci biotype B (Fig. 1), and these parameters also did not differ significantly in the univariate (ANOVA) analysis (Table 4).
It is important to emphasize that multivariate analyses, amongst them the Principal Component Analysis, have been used as an important tool for Entomology, especially Host Plant Resistance, as they are methods that take into account the assessment of the insect biological parameters altogether. Principal Component Analysis provides the formation of groups with genotypes that behaved similarly under the pest attack, allowing the researcher differentiate the genotypes concerning the resistance degrees.
Conclusions
The cultivars IAC-Harmonia, IPR-Eldorado, IAPAR-81, and IPR-Siriri were the least preferred when adults of the whitefly were given a choice for oviposition. In addition, the cultivarIAC- Harmonia extended the life cycle of the whitefly, and holds moderate non-preference for feeding and/or antibiosistype resistance. These cultivars, especially IAC-Harmonia, can potentially be grown in the field aiming to suppress populations of the whitefly.
Acknowledgements
To Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the Doctorate scholarship granted to the first author and to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the scholarships granted to the second, third and seventh authors. Appreciation is also given to Instituto Agronômico de Campinas (IAC) for supplying the seeds of bean cultivars assessed in the present work and to Prof. Dr. Gener Tadeu Pereira from Departamento de Ciências Exatas of FCAV/UNESP for the assistance with the statistical analysis.
Literature cited
BALDIN, E. L. L.; TOSCANO, L. C.; LIMA, A. C. S.; LARA, F. M.; BOIÇA JUNIOR, A. L. 2000. Preferência para oviposição de Bemisia tabaci biótipo "B" por genótipos de Cucurbita moschata e Cucurbita maxima. Boletin de Sanidad Vegetal Plagas 26 (3): 409-413. [ Links ]
BALDIN, E. L. L.; VENDRAMIM, J. D.; LOURENÇÃO, A. L. 2005. Resistência de genótipos de tomateiro à mosca-branca Bemisia tabaci (Gennadius) biótipo B (Hemiptera: Aleyrodidae). Neotropical Entomology 34 (3): 435-441. [ Links ]
BERLINGER, M. J. 1986. Host plant resistance to Bemisia tabaci. Agriculture, Ecosystems and Environment 17 (1): 69-82. [ Links ]
BLUA, M. J.; YOSHIDA, H. A.; TOSCANO, N. C. 1995. Oviposition preference of two Bemisia species (Homoptera: Aleyrodidae). Environmental Entomology 24 (1): 88-93. [ Links ]
BOIÇA JÚNIOR, A. L.; SOUZA, B. H. S.; LOPES, G. S.; COSTA, E. N.; MORAES, R. F. O.; EDUARDO, W. I. 2013. Atualidades em resistência de plantas a insetos. pp. 207-224. In: Busoli, A. C.; Alencar, J. R. D. C. C.; Fraga, D. F.; Souza, L. A.; Souza, B. H. S.; Grigolli, J. F. J. (Eds.). Tópicos em Entomología Agrícola –VI. Jaboticabal: Gráfica Multipress. 327 p. [ Links ]
BUTTER, N. S.; VIR, B. K. 1989. Morphological basis of resistance in cotton to the whitefly Bemisia tabaci. Phytoparasitica 17 (4): 251-261. [ Links ]
CAMPOS, O. R. 2003. Resistência de genótipos de algodoeiro a mosca branca Bemisia tabaci (Gennadius 1889) biótipo B (Hemiptera: Aleyrodidae). Thesis, Faculdade de Ciências Agronômicas, Universidade Estadual Paulista, 69 p. [ Links ]
CAMPOS, Z. R.; BOIÇA JUNIOR, A. L.; LOURENÇÃO, A. L.; CAMPOS, A. R. 2005. Fatores que afetam a oviposição de Bemisia tabaci (Genn.) biótipo B (Hemiptera: Aleyrodidae) em algodoeiro. Neotropical Entomology 34 (5): 823-827. [ Links ]
CHU, C. C.; FREEMAN, T. P.; BUCKNER, J. S.; NATWICK, E. T.; HENNEBERRY, T. J.; NELSON, D. 2001. Silverleaf whitefly colonization and trichome density relationships on upland cotton cultivars. Southwestern Entomologist 25 (4): 237-242. [ Links ]
COSTA, N. P.; SANTOS, T. M.; BOIÇA JUNIOR, A. L. 2004. Preferência para oviposição de Bemisia tabaci biótipo-B em genótipos de caupi. Acta Scientiarum 26 (2): 227-230. [ Links ]
COSTA, G. M; BOIÇA JUNIOR, A. L.; JESUS, F. G.; CHAGAS FILHO. 2010. Efeito do uso de óleos vegetais, associados ou não a inseticida, no controle de Bemisia tabaci (Genn.) e Thrips tabaci (Lind.), em feijoeiro, na época das águas. Bioscience Journal 26 (1): 15-23. [ Links ]
DAHLIN, R. M.; BRICK, M. A.; OGG, J. B. 1992. Characterization and density of trichomes on three common bean cultivars. Economic Botany 46 (3): 299-304. [ Links ]
EICHELKRAUT, K.; CARDONA, C. 1989. Biologia, cria massal y aspectos ecológicos de la mosca blanca Bemisia tabaci (Gennadius) (Homoptera: Aleyrodidae), com plaga del frijol comum. Turrialba 39 (1): 55- 62. [ Links ]
FANCELLI, M.; VENDRAMIM, J. D.; LOURENÇÃO, A. L.; DIAS, C. T. S. 2003. Atratividade e preferência para oviposição de Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) biótipo B em genótipos de tomateiro. Neotropical Entomology 32 (2): 319-328. [ Links ]
FENEMORE, P. G. 1980. Oviposition of potato tuber moth, Phthorimaea operculella Zell. (Lepidoptera: Gelechiidae); identification of host-plant factors influencing oviposition response. New Zealand Journal of Zoology 7 (3): 435-439. [ Links ]
GILL, R. J. The morphology of whiteflies. 1990. pp. 13-46. In: Gerling, D. (Ed.). Whitefly: their bionomics, pest status management. Newcastle: Intercept, Andover. 348 p. [ Links ]
HOROWITZ, A. R.; ISHAAYA, I. 1995. Chemical control of Bemisia - management and application. pp. 537-556. In: Gerling, D.; Mayer, R. T. (Eds.). Bemisia: Taxonomy, biology, damage, control and management. Newcastle: Intercept Ltd. Andover, Hants, UK. [ Links ]
JACKSON, J. E. 1991. A user's guide to principal components. New York: Wiley. 569 p. [ Links ]
JANINI, J. C.; BOICA JUNIOR, A. L.; JESUS, F. G.; SILVA, A. G.; CARBONEL, S. A. M.; CHIORATO, A. F. 2011. Effect of bean genotypes, insecticides, and natural products on the control of Bemisia tabaci (Gennadius) biotype B (Hemiptera: Aleyrodidae) and Caliothrips phaseoli (Hood) (Thysanoptera: Thripidae). Acta Scientiarum, Agronomy 33 (3): 445-450. [ Links ]
JESUS, F. G.; BOICA JUNIOR, A. L.; JANINI, J. C.; SILVA, A. G.; CARBONEL, S. A. M.; CHIORATO, A. F. 2009. Interação de variedades, óleo de nim e inseticida no controle de Bemisia tabaci (Gennadius) biotipo B (Hemiptera:Aleyrodidae) e Caliothrips phaseoli (Hood) (Thysanoptera:Thripidae) na cultura do feijoeiro. Boletín de Sanidad Vegetal. Plagas 35 (2): 491-500. [ Links ]
JESUS, F. G.; BOIÇA JUNIOR, A. L; CARBONELL, S. A. M; STEIN, C. P.; PITTA, R. M.; CHIORATO, A. F. 2010. Infestação de Bemisia tabaci biótipo B e Caliothrips phaseoli em genótipos de feijoeiro. Bragantia 69 (3): 637-648. [ Links ]
JESUS, F. G.; BOIÇA JUNIOR, A. L.; PITTA, R. M.; CAMPOS, A. P.; TAGLIARI, S. R. 2011. Fatores que afetam a oviposição de Bemisia tabaci biótipo b (Hemiptera: Aleyrodidae) em feijoeiro. Bioscience Journal 27 (2): 190-195. [ Links ]
KAISER, H. F. 1960. The application of electronic computers to factor analysis. educational and psychological measurement. Thousand Oaks 20: 141-151. [ Links ]
Lara , F. M. 1991. Princípios de resistência de plantas a insetos. São Paulo: Ícone. 336 p. [ Links ]
LIMA, A. C. S. 2001. Resistência de genótipos de soja [Glycine Max (L.) Merrill] à mosca branca, Bemisia tabaci (Gennadius, 1889) biótipo B (Hemiptera: Aleyrodidae). Thesis, Faculdade de Ciências Agrárias e Veterinárias, Universidade Estadual Paulista. 56 p. [ Links ]
LIMA, A. C. S.; LARA, F. M. 2004. Resistência de genótipos de soja à mosca-branca Bemisia tabaci (Genn.) biótipo B (Hemiptera: Aleyrodidae). Neotropical Entomology 33 (1): 71-75. [ Links ]
McAUSLANE, H. J.; JOHNSON, F. A.; KNAUFT, D. A. 1994. Population levels and parasitism of Bemisia tabaci (Gennadius) (Homoptera: Aleyrodidae) on peanut cultivars. Environmental Entomology 23 (5): 1203-1210. [ Links ]
OMRAM, H. H.; EL-KHIDIR, E. 1978. On the preference of Bemisia tabaci Genn. (Homoptera, Aleyrodidae) on various cotton cultivars in Cukurova, Turkey. Agriculture, Ecosystems & Environment 17 (3): 83-88. [ Links ]
ORIANI, M. A. G.; LARA F. M. 2000. Antibiosis effects of wild bean lines containing arcelin on Bemisia tabaci (Genn.) biotype B (Homoptera: Aleyrodidae). Anais da Sociedade Entomológica do Brasil 29 (3): 573-582. [ Links ]
ORIANI, M. A. G.; VENDRAMIM, J. D.; BRUNHEROTTO, R. 2005. Atratividade e não-preferência para oviposição de Bemisia tabaci (Genn.) biótipo B (Hemiptera, Aleyrodidae) em genótipos de feijoeiro. Neotropical Entomology 34 (1): 105-111. [ Links ]
ORIANI, M. A. G.;VENDRAMIM, J. D.; BRUNHEROTTO, R. 2008. Aspectos biológicos de Bemisia tabaci (Genn.) Biótipo B (Hemiptera, Aleyrodidae) em 6 genótipos de feijoeiro. Neotropical Entomology 37 (2):191-195. [ Links ]
PAINTER, R. H. 1951. Insect resistance in crop plantas. McMilan, New York. 520 p. [ Links ]
PARON, M. J. F. O.; LARA, F. M. 2005. Relação entre tricomas foliares de genótipos de feijoeiro comum, Phaseolus vulgaris L. e resistência a Diabrotica speciosa Germar, 1824 (Coleoptera: Chrysomelidae). Ciência e Agrotecnologia 29 (4): 894- 898. [ Links ]
PEÑA, E. A.; PANTOJA, A.; BEAVER, J. 1992. Determinación de la pubescencia de cuatro genotipos de habichuela, Phaseolus vulgaris L. Journal of Agriculture of the University of Puerto Rico 76 (4): 71-82. [ Links ]
PEÑA, E. A.; PANTOJA, A.; BEAVER, J.; ARMSTRONG, A. 1993. Oviposicion de Bemisia tabaci Genn. (Homoptera, Aleyrodidae) en cuatro genotipos de Phaseolus vulgaris L. (Leguminosae) con diferentes grados de pubescencia. Folia Entomologica Mexicana 87: 1-12. [ Links ]
PRABHAKER, N.; COUDRIET, D. L.; MEYER-DIRK, D. E. 1985. Insecticide resistance in the sweetpotato-whitefly Bemisia tabaci (Homoptera: Aleyrodidae). Journal of Economic Entomology 78 (4): 748-752. [ Links ]
RODRIGUES, N. E. L.; BOTTEGA, D. B.; SOUZA, B. H. S.; CHIORATO, A. F.; SILVA, A. G.; BOICA JUNIOR, A. L. 2012a. Infestation of Bemisia tabaci B biotype on bean cultivars. Annual Report of the Bean Improvement Cooperative 55 (1): 195-196. [ Links ]
RODRIGUES, N. E. L.; BOICA JUNIOR, A. L.; FARIAS, P. R. S. 2012b. Antibiose e não preferência para oviposição de Bemisia tabaci (Genn.) biótipo B (Hemiptera: Aleyrodidae) por cultivares de Vigna unguiculata (L.) Walp. Arquivos do Instituto Biológico 79 (1): 25-31. [ Links ]
SALGUERO, V. 1993. Perspectivas para el manejo del complejo mosca blanca-virosis. pp. 20-26. In: TALLER, del cenroamericano y del caribe sobre moscas blancas, Turrialba, Costa Rica. 1992. Las moscas blancas (Homoptera: Aleyrodidae) em America Central y Caribe: Memória. Turrialba: CATIE. (Informe Técnico 205). [ Links ]
SAS/STAT 1994. User's Guide, Version 6, Fourth Edition, vol. 1 e 2. SAS Institute Inc., Cary, NC. [ Links ]
SILVA, A. G. 2012. Resistência de cultivares de feijoeiro, dinâmica populacional de Bemisia tabaci (Genn., 1889) (Hemiptera: Aleyrodidae) e incidência de mosaico dourado. Thesis, Faculdade de Ciências Agrárias e Veterinárias de Jaboticabal, Universidade Estadual Paulista, Jaboticabal, 95 p. [ Links ]
SIMMONS, A. M. 1994. Oviposition on vegetables by Bemisia tabaci (Homoptera: Aleyrodidae): temporal and leaf surface factors. Environmental Entomology 23 (2): 381-389. [ Links ]
STATSOFT 2012. STATISTICA. (Data Analysis Software System and User's Manual). Version 10. StatSoft Inc., Tulsa. [ Links ]
TOSCANO, L. C.; BOIÇA JUNIOR, A. L.; MARUYAMA, W. I. 2002. Non preference of whitefly for oviposition in tomato genotypes. Sciencia Agricola 59 (4): 677-681. [ Links ]
VALLE, G. E.; LOURENÇÃO, A. L. 2002. Resistência de genótipos de soja a Bemisia tabaci (Genn.) Biótipo B (Hemiptera: Aleyrodidae). Neotropical Entomology 31 (2): 285-295. [ Links ]
VAN LENTEREN, J. A.; NOLDUS, L. P. J. J. 1990. Whitefly-plant relationships: behavioral and ecological aspects pp. 47-89. In: GERLING, D. (Ed.). Whiteflies: their bionomics, pest status and management. Andover: Intercept. 348 p. [ Links ]
VENDRAMIM, J. D.; SOUZA, A. P.; ONGARELLI, M. G. 2009. Comportamento de oviposição da mosca-branca Bemisia tabaci (Genn.) (Hemiptera: Aleyrodidae) biótipo B em tomateiro. Neotropical Entomology 38 (1): 126-132. [ Links ]
WALKER, G. P.; PERRING, T. M. 1994. Feeding and oviposition behavior of whiteflies (Homoptera: Aleyrodidae) interpreted from AC electronic feeding monitor waveforms. Annals of the Entomological Society of American 87 (3): 363-374. [ Links ]
Suggested citation:
DA SILVA, A. G.; BOIÇA JUNIOR, A. L.; FARIAS, P. R. S.; RODRIGUES, N. E. L.; DE SOUZA, B. H. S.; BOTTEGA, D. B.; CHIORATO, A. L. 2014. Non-preference for oviposition and antibiosis in bean cultivars to Bemisia tabaci biotype B (Hemiptera: Aleyrodidae). Revista Colombiana de Entomología 40 (1): 7-14. Enero-julio 2014. ISSN: 0120-0488.