Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488
Rev. Colomb. Entomol. vol.40 no.1 Bogotá Jan./June 2014
Effect of intercropping on predation of Oncideres ocularis (Coleoptera: Cerambycidae) in Brazilian Acacia mangium plantations
Efecto del cultivo intercalado sobre la depredación de Oncideres ocularis (Coleoptera: Cerambycidae) en plantaciones brasileñas de Acacia mangium
PEDRO G. LEMES1, NORIVALDO DOS ANJOS1, RODOLFO M. SOUZA1 AND ISAAC R. JORGE2
1 D. Sc., D. Sc. Departamento de Entomologia, Universidade Federal de Viçosa, Av. P.H. Rolfs, s/n, Centro, Viçosa, Minas Gerais, Brasil, pedroglemes@hotmail.com. Corresponding author.2 Bel. Forest Engineering. Departamento de Engenharia Florestal, Universidade Federal de Viçosa, Av. P.H. Rolfs, s/n, Centro, Viçosa, Minas Gerais, Brazil
Received: 18-Aug-2013 • Accepted: 4-Jun-2014
Abstract: Oncideres ocularis (Cerambycidae) is a twig girdler beetle with potential to become a pest of Fabaceae forest plantations. The diversity of agroecosystems can affect populations of insect pests and their natural enemies, and intercropping may provide resources such as food, alternative prey and hosts, and shelter for the natural enemies. The objective of this work was to verify if the number of larvae and the action of natural enemies of this twig girdler vary along the girdled branch and with the different plantation methods. Fresh branches of Acacia mangium girdled by O. ocularis were collected at two types of plantations, a monoculture and an intercropping with eucalyptus and Brachiaria spp. The dead and living larvae were removed from the galleries along the branch (basal, middle, and apical sections). The number of larvae was different between sections of the girdled branch for all variables analyzed. The total number of larvae of O. ocularis did not differ between the types of plantation, but larvae survival was significantly higher in the intercropping than in monoculture. The type of plantation also affected the action of predators on larvae in the early instars, besides varying along the branch. In intercropping systems with eucalyptus, acacia, and grasses, the predators may not be able to reduce the population of this twig girdler.
Key words: Branch girdling. Larvae predation. Twig girdler.
Resumen: Oncideres ocularis (Cerambycidae) es un escarabajo "corta palo" que tiene potencial para convertirse en plaga en plantaciones forestales de Fabaceae. La diversidad de los agroecosistemas puede afectar a las poblaciones de insectos plaga y sus enemigos naturales. El cultivo intercalado puede proporcionar recursos, como alimentos, presas alternativas, hospederos y refugio para los enemigos naturales. El objetivo de este trabajo fue verificar si el número de larvas y la acción de los enemigos naturales de este corta palo varían a lo largo de la rama anillada y con los diferentes métodos de plantación. Se colectaron ramas frescas de Acacia mangium anilladas por Oncideres ocularis en dos tipos de plantaciones, un monocultivo y otro intercalado con eucalipto y Brachiaria spp. Las larvas muertas y vivas fueron retiradas de las galerías a lo largo de la rama (secciones basal, media y apical). El número de larvas fue diferente entre las secciones de la rama anillada para todas las variables analizadas. La sección de la rama anillada afectó a todas las variables. Aunque el número total de larvas de O. ocularis no fue diferente entre los dos tipos de plantación, la supervivencia de las larvas fue mayor en el cultivo intercalado que en el monocultivo. El tipo de plantación afectó también a la acción de los depredadores de las larvas en los primeros estadios, además de variar a lo largo de la rama. En los sistemas con eucaliptos, acacias y pastos intercalados, los depredadores pueden ser incapaces de reducir la población de este "corta palo".
Palabras clave: anillamiento de rama, depredación, corta palo.
Introduction
Twig girdlers are beetles belonging to the subfamily Lamiinae (Cerambycidae) that girdle branches and stems of living trees. The females girdle branches to lay their eggs in the incisions made by their jaws in the branches by using bark and wood as padding support. The fallen branches provide suitable conditions of humidity for the larvae development as well as serve as a food source for them (Paulino Neto et al. 2006; Calderón-Cortés et al. 2011; Lemes et al. 2012; Lemes et al. 2013).
The twig girdler species Oncideres ocularis Thomson, 1868 has the potential to become a pest of forest plantations, causing injuries to several trees of Fabaceae. This species has been reported to cause damage to Acacia meanrsii De Wild. and Acacia mangium Willd. in southern and southeastern Brazil (Vulcano and Pereira 1978; Lemes et al. 2013), and to other species of Fabaceae (e.g., A. bonariensis Gillie ex Hook., Mimosa caesalpiniifolia Benth. and Pithecolobium sp.) (Vulcano and Pereira 1978; Lemes 2011).
The branch girdling is considered a keystone process to the structure of the arthropod community composed of predators, parasitoids and xylophages that inhabit these branches (Calderón-Cortés et al. 2011). The most important natural enemies of twig girdlers are Coleoptera predators and Hymenoptera and Diptera parasitoids (Linsley 1959, Costa et al. 1992a; Paulino Neto et al. 2006.). Ants of the genus Camponotus (Formicinae) and Cephalotes (Myrmicinae) prey on eggs and larvae of O. humeralis (Paulino Neto et al. 2006). Predation by birds is also common (Linsley 1959) and is the major cause of mortality of larvae of O. pustulata within the girdled branches (Rodríguez-del-Bosque & Garza-Cedillo 2008). Insects and arthropods cohabiting girdled branches can also act as competitors, acting as an important natural enemies
(Hovore & Penrose 1982; Calderón-Cortés et al. 2011; Lemes et al. 2013). Costa et al. (1992a) studied the distribution of larvae and natural enemies of O. impluviata (Germar, 1824) in girdled branches, and they found that the larvae tend to concentrate between the basal and the middle sections, but predators and parasitoids concentrate in the bas al section. In addition, they found that there is a well-defined stratification of predators group along the branch.
Studies had evaluated the effect of girdling on the structure of plant communities (Caraglio et al. 2001; Romero et al. 2005; Duval & Whitford 2008) and the preference of host plants choice (Ansley et al. 1990; Paulino Neto et al. 2005; Uribe-Mu & Quesada 2006; Paro et al. 2011). But few studies had focused on the influence of plants and environment on the biology and reproduction of twig girdler beetles (Rodríguez- del-Bosque & Cedillo 2008; Rodríguez-del-Bosque 2013).
I t is known that the diversity of agroecosystems can positively or negatively affect populations of insect pests and their natural enemies (Andow 1991; Righi et al. 2013; Sabatier et al. 2013; Zhou et al. 2013) and can influence the adoption of these systems (Tey et al. 2014). One important contribution of intercropping, in the context of integrated pest management, is that it may create a suitable ecological environment within the plantation landscape that provides resources such as food, alternative prey and hosts, and shelter for the natural enemies (Xu et al. 2011; Ahmed et al. 2013). Intercropping may also act by providing visual (leaf shape and color) and/ or chemical camouflage (volatile chemicals released by the plants) that may confound insect orientation to a host-plant, interfering on it visual and olfactory host-finding mechanisms (Smith and McSorley 2000). Some plants can also exhibit repellent or attractive effects on insect populations (Tang et al. 2013). In some cases, the architecture or the surface of the plant may affect the dispersion of those insects, acting as a physical barrier (Compton 2002).
The knowledge about the influence of diversification of the intercropped systems on the population dynamics of those pests is important to understand and improve adoption of biological control (Girma et al. 2000; Cividanes and Barbosa 2001; Saeed et al. 2013). However, the effects of the plants diversification in intercropping on the population dynamics of twig girdlers and their natural enemies remain unknown. In this context, this study investigated whether the number of larvae of O. ocularis and the action of their natural enemies vary along the girdled branch of A. mangium and with the different plantation methods.
Materials and methods
Study sites. Fresh branches of A. mangium girdled by O. ocularis were collected at the beginning of November 2009 until February 2010 at two types of plantations: A monoculture of A. mangium trees in the city of Coimbra, State of Minas Gerais, Brazil (20°51' S 42°48' W 720 masl) with 3000 trees planted in 3 × 2 m spacing which were 65 months old at the beginning of the study. The other was a intercropping of hybrid clones of Eucalyptus urophylla S.T. Blake x E. grandis W. Hill ex Maiden, 60 trees of A. mangium and Brachiaria spp., located in Viçosa, State of Minas Gerais (20°45' S 46°51' W 689 masl). In this second system, the trees, which were 34 months old, were planted in 12 × 2 m spacing and the grasses were established in bands between the contour lines occupied by the trees. Trees of A. mangium had the objective of fixing nitrogen in the soil to promote the growth of the grasses.
Laboratory study. Adult insects that were causing the girdling of the branches in these locations were collected and sent for specific determination to the taxonomist Prof. Dr. Ubirajara Martins from the Museum of Zoology, University of São Paulo (MZUSP), where they are preserved.
The branches collected were used in the laboratory to quantify the larval survival and their natural enemies, according to methodology adapted from Costa et al. (1992b). Dead and alive larvae, were removed from the galleries along the branch (basal, middle and apical sections). The living larvae were placed in test tubes (120 × 12 mm) containing moistened and compressed A. mangium sawdust. Larvae that died, but were not preyed, were individualized in separate plates to detect possible parasitoids and entomopathogenic fungi. The predation was quantified considering the presence of preyed larvae remains in the galleries or traces that they were preyed (e.g. presence of excrement, and a larval head capsule), predator and larvae in the same gallery and/or predator alone in the larvae gallery.
Experimental design. It was completely randomized with a factorial arrangement: type of plantation and the branch section (basal, middle, and apical) with 22 repetitions. The dependent variables in this study were as follows: the total number of larvae (dead and living larvae), preyed larvae, parasitized larvae and total number of dead larvae (sum of the number of preyed, parasitized, and dead due to unknown reasons larvae). The assumptions of normality and homoscedasticity were assessed by the tests of Kolmogorov and Lilliefors and Levene. The factorial analysis of variance to evaluate the effects of section of the branch, type of plantation, and the interaction of the two factors in each of the variables and the means were compared by Tukey's test (α = 5%). All tests were performed using the software Statistica 9.0 (Stat Soft. Inc., 2009).
Results
It was not possible to identify the cause of death for the larvae not killed by predators. Thus, only the number of living larvae and preyed larvae were discussed in this work. The number of larvae was different between the sections of the girdled branch, for all analyzed variables (Table 1). The type of plantation has no effect on the number of dead larvae and the total number of larvae (Table 1). In addition, the interaction between the type of plantation and branch section (basal, middle, and apical) has influence on the number of preyed larvae (Table 1).
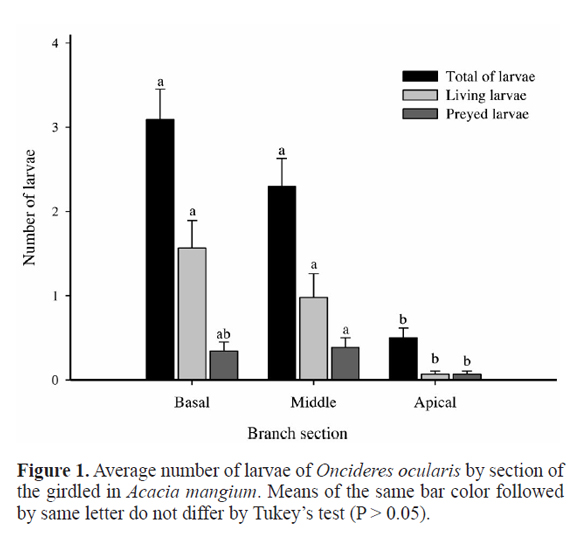
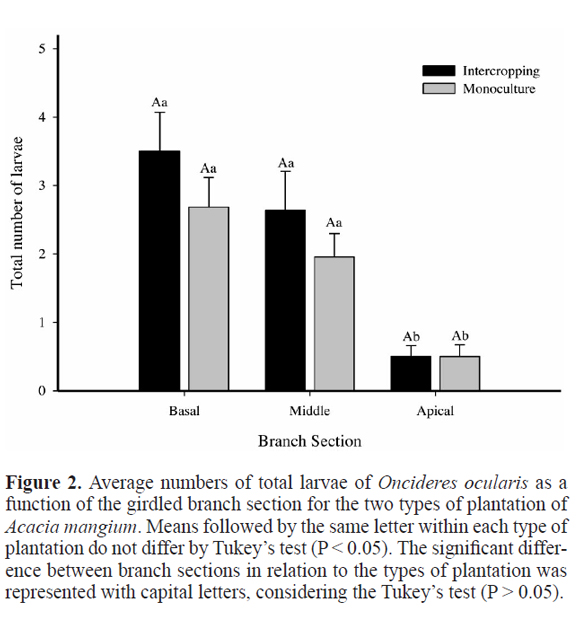
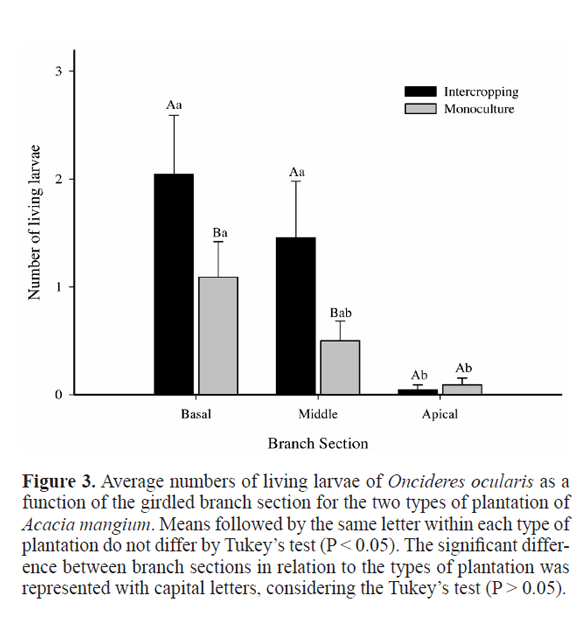
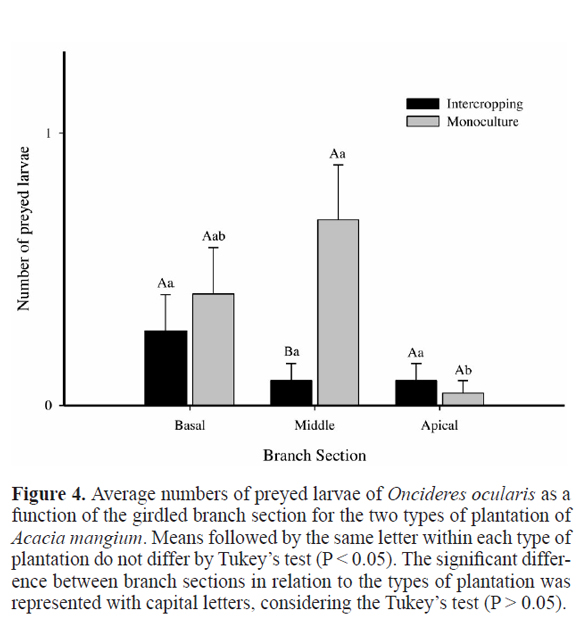
The total number of larvae (Figs. 1 and 2) and the number of living larvae of O. ocularis (Figs. 1 and 3) showed the same distribution pattern along the branches of A. mangium, whereas in the apical section these parameters were lower. However, regarding the distribution of the number of preyed larvae, the apical section differed only from the middle section, which in turn did not differ from basal section (Figs. 1 and 4).
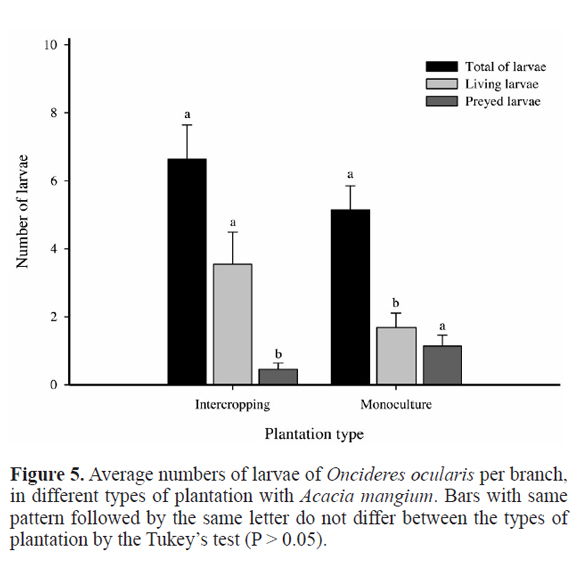
The total number of larvae of O. ocularis did not differ between the two types of plantation with A. mangium (Fig. 5), but larvae survival (number of living larvae) was higher in the intercropping than in the monoculture (Fig. 5).
There was a significant variation in the number of living larvae within and between the type of plantation. The number of living larvae in the basal and middle sections was higher in intercropping than in the monoculture, but lower in the apical section. The variation pattern of living larvae was similar between plantation methods (Fig. 3). The number of preyed larvae of O. ocularis did not change along the branch sections in the intercropping (Fig. 4).
In the monoculture, the number of preyed larvae on the apical section was lower than in the middle section, but it did not differ from that of the basal section, which in turn did not differ from the middle section. However, the number of larvae of O. ocularis preyed in the middle section in the monoculture was higher than in the intercropped plantation (Fig. 4). In fact, the predation on larvae of O. ocularis was higher in the monoculture than in the intercropping (Figure 5). Predation represented 12.1% and 28.4% of the total deaths recorded in the intercropping (n = 66) and in the monoculture (n = 162), respectively.
Discussion
The cause of death of larvae that were not killed by predators was not found, however, parasitoids of the genus Cenocoelius (Hymenoptera: Braconidae) were found in larvae of O. impluviata, from the third to the fifth instars, in plantations of M. scabrella (Costa et al. 1992b). Several parasitic Hymenoptera belonging to the families Chalcedectidae, Pteromalidae, Eupelmidae, Eurytomidae, and Braconidae were found on the larvae of O. rhodosticta (Polk and Ueckert 1973). Parasitic wasps were also found parasitizing larvae of O. humeralis and Oncideres albormaginata chamela (Paulino Neto et al. 2006; Calderón-Cortés et al. 2011). Three different species of parasitoids wasps were obtained from O. impluviata larvae, but in small number, leading to consider the biological control of twig girdlers with parasitoids a very difficult task (Baucke 1958). Although there are records of entomopathogenic fungi as Beauveria bassiana and Metarhizium anisoplae in some Cerambycidae pests (Peng et al. 2011; Meyers et al. 2013; Ugine et al. 2013), there is no record of these fungi in twig girdler beetles. The cause of death can also be attributed to mechanical injury caused by the wind or the fall of the girdled branch (Rogers 1977).
The distribution of the number of larvae of O. ocularis along the girdled branches in A. mangium was similar in both types of plantation indicating the existence of an oviposition pattern apart from the system adopted. This pattern was observed for O. impluviata in a monoculture of M. scabrella, with the greatest number of the larvae concentrated in the basal and middle sections and fewer in the apical third (Costa et al. 1992a). These insects avoid laying their eggs on the edges, ensuring that their offspring have wood in any direction they bored (Paulino Neto et al. 2006; Lemes et al. 2013). This pattern is probably because the volume of wood in the apical third of the branch is very small, making the full development of the larvae difficult (Rice 1989; Lemes et al. 2013).
Despite the number of living larvae of O. ocularis were higher in the intercropped area with A. mangium than in the monoculture, it is not possible with the data obtained to infer that the development and survival of the twig girdler were completely affected by the type of plantation, because there may be different species of natural enemies and other Cerambycidae cohabiting the girdled branches over the time, which may also influence the development of girdlers (Paine et al. 2001; Calderón-Cortés et al. 2011). The branches were collected soon after the adults of O. ocularis girdled the branches in both areas; thus, the larvae were collected only in the early instars. The first instar larvae have a higher rate of parasitism in relation to others (Rogers 1977). On the other hand, the action of the predators of larvae of O. impluviata was accentuated after the fourth instar, when they become more easier targets to predators such as larvae of beetles of the family Cleridae, which accounted for 80% of predation, besides the predation by birds on mature larvae and pupae (Costa et al. 1992b). Over time, the bark of the girdled branch becomes thinner with the feeding of larvae, facilitating bird predation (Rodríguez-Del-Bosque and Garza-Cedillo 2008). The purpose of A. mangium in the intercropping system is not produce wood but accumulate soil nitrogen, and the productivity is low in this system, which may have led to an increase in the number of Cerambycidae (pests and non-pests) in these trees (Raje et al. 2012). The action of natural enemies also may depend on the availability of alternative preys (Bickerton and Hamilton 2012). Therefore, it is possible that in the intercropping, the abundance of alternative prey is so big that the O. ocularis larvae predation decreases on the girdled branches. On the other hand, the population density is dependent on the densities of natural enemies, which can lead to a higher abundance of natural enemies, predators in this case, in the monoculture than in the intercropping plantations (Bastos et al. 2003).
The higher level of predation on the monoculture found here contradicts those results observed in other cultures. The addition of aromatic plants enhanced the predators' activities on an apple orchard (Beizhou et al. 2012). Ricinus communis L. intercropped with cluster bean (Cyamopsis tetragonoloba (L.) Taub.), cowpea (Vigna unguiculata L.), or black gram (Vigna mungo L.) resulted in a reduction of pests of this crop caused by a buildup on the natural enemies' population (Rao et al. 2012). A lower incidence of Ips subelongatus (Coleoptera: Curculionidae) was observed in dahurian larch (Larix gmelinii (Rupr.) Rupr.) plantation mixed with trees of birch (Betula sp.) compared to the monoculture (Li et al. 2013). The damage caused by the cherry slug (Caliroa cerasi L.) (Hymenoptera: Tenthredinidae) was reduced in stands of Prunus avium L. mixed with other species when compared to the monoculture (Loewe et al. 2013). However, an increase in the density of generalist insects was verified in intercropping systems (Bastos et al. 2003). In this case, a possible explanation for this variation between the types of plantation may be related to the type of spacing adopted. In the monoculture, the tree density is higher with lesser sunlight penetration and higher accumulation of leaves on the ground, creating favorable conditions for the development of predators. In the intercropping, the trees are more widely spaced with greater sunlight penetration and few areas of refuge for predators. This could also explain the greater number of living larvae in the intercropping in relation to that in the conventional plantation.
Another factor that could explain the lower survival and higher predation rates in monoculture compared to intercropped plantation would be that both are young plantations. The number of insects in monocultures can be similar to those of natural forests in the first eight years and begin to fall after 20 years (Meng et al. 2012). The young monocultures may have a high diversity of Cerambycidae, but they cannot shel ter so many species permanently, because of the changes in habitat conditions (Meng et al. 2013). Therefore it is possible that over time the monoculture becomes a less favorable habitat, and the survival and predation rates start to decrease in the monoculture compared to mixed stands. Furthermore, the abundance of predators is not always related to the increasing of diversity of tree species. These predators may exhibit preference for stands composed of certain species of trees, and it can overcome the effects of tree diversity (Vehviläinem et al. 2008). Therefore, is possible that predators are being more attracted by a monoculture of A. mangium than by a mixed plantation with eucalyptus and grasses.
The pattern of distribution of preyed larvae along the branches was also different for each area. In other words, in the intercropping, was a homogeneous distribution, while in the monoculture, it was heterogeneous (Fig. 4). This pattern was similar to O. impluviata in a monoculture of M. scrabella, with a higher concentration of predation in the basal section in relation to the middle section, and no predation observed in the apical section (Costa et al. 1992a). This pattern was probably because the predators were not good fliers (Thysanoptera, Dermaptera, and adult Cleridae) (Linsley 1959) or had terrestrial behavior (some ants and Cleridae larvae) chasing for their prey in the section that were located closer to the ground (Costa et al. 1992a; Paulino Neto et al. 2006).
Because no predator was observed or collected, the spatial and temporal diversity of these agents and the effect of diversification of the agrosystem in interfering with the survival of larvae remain unknown. The temporal occurrence of parasitoids may not be coincident with the predators and not be distributed uniformly along the branch (Costa et al. 1992b). However, it is possible to infer that in the early instars of O. ocularis larvae, the type of plantation with A. mangium has effect on the survival of larvae and predation.
Acknowledgements
The authors are thankful to Dr. Ubirajara Martins for the insect identification, and to colleagues from the Laboratory of Integrated Management of Defoliators Beetles of Universidade Federal de Viçosa by the support given throughout this study; And also to "Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)" for financial support and to Global Edico Services revised and edited this manuscript.
Literature cited
AHMED, S.; KHAN, M.A.; QASAM, M. 2013. Effect of intercropping of maize in citrus orchards on citrus leaf miner infestation and population of its natural enemies. Pakistan Journal of Agricultural Sciences 50: 91-93. [ Links ]
ANDOW, D. A. 1991. Vegetation diversity and arthropod population response. Annual Review of Entomology 36: 561-586. [ Links ]
ANSLEY, R. J.; MEADORS, C. H.; JACOBY, P. W. 1990. Preferential attraction of the twig girdler, Oncideres cingulata texana Horn, to moisture stressed mesquite. Southwestern Entomologist 15: 469- 474. [ Links ]
BASTOS, C. S.; GALVÃO, J. C. C.; PICANÇO, M. C.; CECON, P. R.; PEREIRA, P. R. G. 2003. Incidência de insetos fitófagos e de predadores no milho e no feijão cultivados em sistema exclusivo e consorciado. Ciência Rural 33: 391-397. [ Links ]
BAUCKE, O. 1958. Biologia e controle do "Serrador da Acácia-negra" Oncideres impluviata (Germ., 1842) (Cerambycidae-Lamiinae- Onciderini). DSc Thesis, Escola de Agronomia "Eliseu Maciel", Pelotas, Brazil. [ Links ]
BEIZHOU, S.; JIE, Z.; WIGGINS, N. L.; YUNCONG, Y.; GUANGBO, T.; XUSHENG, S. 2012. Intercropping with aromatic plants decreases herbivore abundance, species richness, and shifts arthropod community trophic structure. Environmental Entomology 41: 872-879. [ Links ]
BICKERTON, M. W.; HAMILTON, G. C. 2012. Effects of intercropping with flowering plants on predation of Ostrinia nubilalis (Lepidoptera: Crambidae) eggs by generalist predators in bell peppers. Environmental Entomology 41: 612-620. [ Links ]
CALDERÓN-CORTÉS, N.; QUESADA, M.; ESCALERA-VÁSQUEZ, L. H. 2011. Insects as stem engineers: Interactions mediated by the twig-girdler Oncideres albomarginata chamela enhance arthropod diversity. PLoS One 6: 1-9. [ Links ]
CARAGLIO, Y.; NICOLINI, E.; PETRONELLI, P. 2001. Observations on the links between the architecture of a tree (Dicorynia guianensis Amshoff) and Cerambycidae activity in French Guiana. Journal of Tropical Ecology 17: 459-463. [ Links ]
CIVIDANES, F. J.; BARBOSA, J. C. 2001. Efeitos do plantio direto e da consorciação soja-milho sobre inimigos naturais e pragas. Pesquisa Agropecuária Brasileira 36: 235-241. [ Links ]
COMPTON, S. G. 2002. Sailing with the wind: Dispersal by small flying insects. pp. 113-133. In: Bullock, J. M.; Kenward, R. E.; Hails, R. S. (Eds.). Dispersal Ecology. Blackwell Publishing, Malden, MA. [ Links ]
COSTA, E. C.; LINK, D.; PEDROSA-MACEDO, J. H. 1992a. Distribuição das posturas, de larvas e de inimigos naturais de Oncideres impluviata (Germar, 1824) (Col., Cerambycidae). Ciência Florestal 2: 59-66. [ Links ]
COSTA, E. C.; LINK, D.; PEDROSA-MACEDO, J. H. 1992b. Eficiência e período de atividade dos inimigos naturais de Oncideres impluviata (Germar, 1824) (Col.; Cerambycidae). Ciencia Florestal 2: 49-58. [ Links ]
DUVAL, B. D.; WHITFORD, W. G. 2008. Resource regulation by a twig-girdling beetle has implications for desertification. Ecological Entomology 33: 161-166. [ Links ]
GIRMA, H.; RAO, M. R.; SITHANANTHAM, S. 2000. Insect pests and beneficial arthropod populations under different hedgerow intercropping systems in semiarid Kenya. Agroforestry Systems 50: 279-292. [ Links ]
HOVORE, F. T.; PENROSE, R. I. 1982. Notes on Cerambycidae coinhabiting girdles of Oncideres pustulata LeConte (Coleoptera: Cerambycidae). The Southwestern Naturalist 27: 23-27. [ Links ]
LEMES, P. G. 2011. Bioecologia de Oncideres ocularis Thomson (Col.: Cerambycidae) M. Sc. dissertation.Viçosa (MG): Universidade Federal de Viçosa. [ Links ]
LEMES, P. G.; AFONSO, R.; ANJOS, N.; SARMENTO, R. A.; LEITE, P. J. B.; CORONETTI, J. A. 2012. First host record of Oncideres mirim Martins and Galileo, 1996 (Coleoptera: Cerambycidae) on Acacia mangium Willd. (Fabaceae). The Coleopterists Bulletin 66: 173-176. [ Links ]
LEMES, P. G.; ANJOS, N.; JORGE, I. R. 2013. Bioecology of Oncideres ocularis Thomson (Coleoptera: Cerambycidae) on Acacia mangium (Fabaceae). Journal of Kansas Entomological Society 86: 307-317. [ Links ]
LI, J.; SHI, J.; LUO, Y.; HELIOVAARA, K. 2013. Responses of monophagous Ips subelongatus Motschulsky (Coleoptera: Curculionidae) and polyphagous Lymantria dispar L. (Lepidoptera: Lymantriidae) to tree species mixture. Entomological News 123: 5-14. [ Links ]
LINSLEY, E. G. 1959. Ecology of Cerambycidae. Annual Review of Entomology 4: 99-138. [ Links ]
LOEWE, M.; MARTA GONZÁLEZ, O.; BALZARINI, M. 2013. Wild cherry tree (Prunus avium L.) growth in pure and mixed plantations in South America. Forest Ecology and Management 306: 31-41. [ Links ]
MENG, L. Z.; MARTIN, K.; WEIGEL, A.; LIU, .J. X. 2012. Impact of rubber plantation on carabid beetle communities and species distribution in a changing tropical landscape (southern Yunnan, China). Journal of Insect Conservation 16: 423-432. [ Links ]
MENG, L. Z.; MARTIN, K.; WEIGEL, A.; YANG, X. D. 2013. Tree diversity mediates the distribution of longhorn beetles (Coleoptera: Cerambycidae) in a changing tropical landscape (Southern Yunnan, SW China). PLoS ONE 8: e75481. doi:10.1371/journal. pone.0075481. [ Links ]
MEYERS, J. M.; STEPHEN, F. M.; HAAVIK, L. J.; STEINKRAUS, D. C. 2013. Laboratory and field bioassays of the effects of Beauveria bassiana Vuillemin (Hypocreales: Cordycipitaceae) on red oak borer, Enaphalodes rufulus (Haldeman) (Coleoptera: Cerambycidae). Biological Control 65: 258-264. [ Links ]
PAINE, T. D.; MILLAR, J. G.; PAINE, E. O.; HANKS, L. M. 2001. Influence of host log age and refuge from natural enemies on colonization and survival of Phoracantha semipunctata. Entomologia Experimentalis et Applicata 98: 157-163. [ Links ]
PARO, C. M.; ARAB, A.; VASCONCELLOS-NETO, J. 2011. The host-plant range of twig-girdling beetles (Coleoptera: Cerambycidae: Lamiinae: Onciderini) of the Atlantic rainforest in southeastern Brazil. Journal of Natural History 45: 1649-1665. [ Links ]
PAULINO NETO, H. F.; ROMERO, G. Q.; VASCONCELLOS NETO, J. 2005. Interactions between Oncideres humeralis Thomson (Coleoptera: Cerambycidae) and Melastomataceae: Host-plant selection and patterns of host use in south-east Brazil. Neotropical Entomology 34: 7-14. [ Links ]
PAULINO NETO, H. F.; VASCONCELLOS NETO, J.; CARMELO GUERREIRO, S. M. 2006. The biology of Oncideres humeralis Thorms (Coleoptera: Cerambycidae: Lamiinae) and new Cerambycidae- Melastomataceae host-plant associations. Studies on Neotropical Fauna and Environment 41: 227-233. [ Links ]
PENG, F.; GARDESCU, S.; HAJEK, A. E. 2011. Transmission of Metarhizium brunneum conidia between male and female of Anoplophora glabripennis adults. BioControl 56: 771-780. [ Links ]
POLK, K. L.; UECKERT, D. N. 1973. Biology and ecology of a mesquite twig girdler, Oncideres rhodosticta, in West Texas. Annals of the Entomological Society of America 66: 411-417. [ Links ]
RAJE, K. R.; ABDEL-MONIEM, H. E. M.; FARLEE, L.; FERRIS, V. R.; HOLLAND, J. D. 2012. Abundance of pest and benign Cerambycidae both increase with decreasing forest productivity. Agricultural and Forest Entomology 14: 165-169. [ Links ]
RAO, M. S.; RAMA RAO, C. A.; SRINIVAS, K.; PRATIBHA, G.; VIDYA SEKHAR, S. M.; SREE VANI, G.; VENKATSWARLU, B. 2012. Intercropping for management of insect pests of castor, Ricinus communis, in the semi–arid tropics of India. Journal of Insect Science 12:14 available online: insectscience.org/12.14. [ Links ]
RICE, M. E. 1989. Branch girdling and oviposition biology of Oncideres pustulatus (Coleoptera: Cerambycidae) on Acacia farnesiana. Annals of the Entomological Society of America 82: 181-186. [ Links ]
RIGHI, C. A.; CAMPOE, O. C.; BERNARDES, M. S.; LUNZ, A M. P.; PIEDADE, S. M. S.; PEREIRA, C. R. 2013. Influence of rubber trees on leaf-miner damage to coffee plants in an agroforestry system. Agroforestry Systems 87: 1351-1362. [ Links ]
RODRÍGUEZ-DEL-BOSQUE, L. A.; GARZA-CEDILLO, R. D. 2008. Survival, emergence, and damage by Oncideres pustulata (Coleoptera: Cerambycidae) on Huisache and Leucaena (Fabaceae) in Mexico. Southwestern Entomologist 33: 209-217. [ Links ]
RODRÍGUEZ-DEL-BOSQUE, L. A. 2013. Feeding and survival of Oncideres pustulata (Coleoptera: Cerambycidae) adults on Acacia farnesiana and Leucaena leucocephala (Fabaceae). Southwestern Entomologist 38: 487-498. [ Links ]
ROGERS, C. E. 1977. Bionomics of Oncideres cingulata (Coleoptera: Cerambycidae) on Mesquite. Journal of Kansas Entomological Society 50: 222-228. [ Links ]
ROMERO, G. Q.; VASCONCELLOS-NETO, J.; PAULINO NETO, H. F. 2005. The effects of the wood-boring Oncideres humeralis (Coleoptera, Cerambycidae) on the number and size structure of its host-plants in south-east Brazil. Journal of Tropical Ecology 21: 233-236. [ Links ]
SABATIER, R.; WIEGAND, K.; MEYER, K. 2013. Production and robustness of a cacao agroecosystem: effects of two contrasting types of management strategies. PLoS ONE 8: e80352. [ Links ]
SAEED, Q.; ZAKA, M.; SAEED, S.; BAKHTAWAR, M. 2013. Lucerne as trap crop in wheat for development of predators population against wheat aphids (Aphididae: Homoptera). Pakistan Journal of Zoology 45: 193-196. [ Links ]
SMITH, H. A.; MCSORLEY, R. 2000. Intercropping and pest management: a review of major concepts. American Entomologist 46: 153- 161. [ Links ]
STATSOFT INC. 2009. STATISTICA (data analysis software system), version 9.0. www.statsoft.com. [ Links ]
TANG, G. B.; SONG, B. Z.; ZHAO, L. L.; SANG, X. S.; WAN, H. H.; ZHANG, J.; YAO, Y. C. 2013. Repellent and attractive effects of herbs on insects in pear orchards intercropped with aromatic plants. Agroforestry Systems 87: 273-285. [ Links ]
TEY, Y. S.; LI, E.; BRUWER, J.; ABDULLAH, A. M.; BRINDAL, M.; RADAM, A.; ISMAIL, M. M.; DARHAM, S. 2014. The relative importance of factors influencing the adoption of sustainable agricultural practices: a factor approach for Malaysian vegetable farmers. Sustainability Science 9: 17-29. [ Links ]
UGINE, T. A.; JENKINS, N. E.; GARDESCU, S.; HAJEK, A. E. 2013. Comparing fungal band formulations for Asian longhorned beetle biological control. Journal of Invertebrate Pathology 113: 240-246. [ Links ]
URIBE-MÚ, C. A.; QUESADA, M. 2006. Preferences, patterns and consequences of branch removal on the dioecious tropical tree Spondias purpurea (Anacardiaceae) by the insect borer Oncideres albomarginata chamela (Cerambycidae). Oikos 112: 691-697. [ Links ]
VEHVILÄINEN, H.; KORICHEVA, J.; RUOHOMÄKI, K. 2008. Effects of stand tree species composition and diversity on abundance of predatory arthropods. Oikos 117: 935-943. [ Links ]
VULCANO, M. A.; PEREIRA, F. S. 1978. O gênero Oncideres Serville 1835 (Coleoptera, Lamiidae) do Sul do Brasil e países limítrofes, séria praga dos pomares e da silvicultura. Studia Entomologica 20: 177-220. [ Links ]
XU, Q.; FUJIYAMA, S.; XU, H. 2011. Biological pest control by enhancing population of natural enemies in organic farming systems. Journal of Food, Agriculture and Environment 9: 455-463. [ Links ]
ZHOU, H.; CHEN, L.; CHEN, J; FRANCIS, F.; HAUBRUGE, E.; LIU, Y.; BRAGARD, C.; CHENG, D. 2013.Adaptation of wheat-pea intercropping pattern in China to reduce Sitobion avenae (Hemiptera: Aphididae) occurrence by promoting natural enemies. Agroecology and Suistanable Food Systems 37: 1001-1016. [ Links ]
Citación sugerida:
Lemes, G. P.; dos Anjos, N.; Souza, M. R.; Jorge, R. I. 2014. Effect of intercropping on predation of Oncideres ocularis (Coleoptera: Cerambycidae) in Brazilian Acacia mangium plantations. Revista Colombiana de Entomología 40 (1): 34-39. Enero-julio 2014. ISSN 0120-0488.