Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488
Rev. Colomb. Entomol. vol.40 no.1 Bogotá Jan./June 2014
Induced resistance to Diatraea saccharalis (Lepidoptera: Crambidae) via silicon application in sugarcane
Resistencia inducida a Diatraea saccharalis (Lepidoptera: Crambidae) vía aplicación de silicio
MICHELLE VILELA1,2, JAIR C. MORAES1,3, EDUARDO ALVES 1,4, TEREZINHA M. SANTOS-CIVIDANES5 AND FABÍOLA A. SANTOS1,6
1 Universidade Federal de Lavras, Caixa Postal 3037, CEP 37200-000 Lavras, MG. Brasil.
2 Doutora. Departamento de Entomologia. michellevilela@live.com Corresponding author.
3 Professor. Departamento de Entomologia. jcmoraes@den.ufla.br.
4 Professor. Departamento de Fitopatologia. ealves@dfp.ufla. br.
5 Doutora, Pesquisador Científico. Agencia Paulista de Tecnologia dos Agronegócios - APTA, Polo Regional Centro Leste, Avenida Bandeirantes, 2419, Vila Virginia, CEP 14030-670 Ribeirao Preto, SP. Brasil. terezinha@apta.sp.gov.br.
6 M. Sc. Departamento de Entomologia. faby_minduri@yahoo.com.br.
Abstract: Integrated pest management (IPM) strategies include factors that induce host plant resistance. Silicon may increase plant resistance to attack by insect pests. In a greenhouse, two sugarcane cultivars RB72454 (moderately resistant) and SP801842 (susceptible), treated and untreated with silicon (Si) were infested with adult Diatraea saccharalis (Lepidoptera: Crambidae) and checked after 60 days. The accumulation of silicon increased in the susceptible cultivar, resulting in a silicon content that was not significantly different than that of the untreated moderately resistant cultivar. The number of holes in the susceptible cultivar grown in silicon-treated soil was similar to that of the moderately resistant cultivar. Silicon application promotes cuticle thickening and the accumulation of crystals on the leaf stomata.
Key words: Insects. Diatraea saccharalis. Resistance. Integrated pest management.
Resumen: Dentro de las estrategias de manejo integrado de plagas (MIP), la inducción de resistencia de la planta hospedera es una opción de control, siendo el silicio un factor que puede aumentar el grado de resistencia al ataque de plagas de insectos. En el desarrollo vegetativo de los cultivares de caña de azúcar RB72454 (moderada resistencia) y SP801842 (susceptible), tratados y no tratados con silicio (Si) fueron infestadas con adultos Diatraea saccharalis (Lepidoptera: Crambidae) y revisados después de 60 días. La acumulación de silicio aumentó en el cultivar susceptible, lo que resulta en un contenido de silicio que no fue significativamente diferente que la del cultivar moderadamente resistente sin tratar. El número de orificios en el cultivar susceptible cultivado en el suelo tratado con silicio fue similar al del cultivar de moderada resistencia. La aplicación de silicio promueve el engrosamiento de la cutícula y la acumulación de cristales en los estomas de las hojas.
Palabras clave: Insectos. Diatraea saccharalis. Resistencia. Manejo integrado de plagas.
Introduction
Silicon (Si) application may improve the ability of crops to withstand biological, climatic and edaphic adversity by affecting the interactions among various factors that promote plant nutrition (Epstein 1994; 1995). The beneficial effects of these changes include increased and quality production and reduced plant susceptibility to diseases and attack by certain pests (Marschner 1995; Lima Filho et al. 1999).
As with most Poaceae species, sugarcane responds well to silicon fertilization because it is a silicon-accumulating crop (Epstein 1999; Ma and Yamaji 2008). The accumulation of the element to high levels in the tissues may increase stalk length and diameter, the number of tillers and, ultimately, productivity (Lima Filho et al. 1999; Korndörfer et al. 2002). The highest response in terms of sugarcane productivity is obtained during the first year due to the increase in silicon absorption resulting from the application of calcium silicates in the form of steelmaking slag or other sources (Anderson 1991; Raid et al. 1992).
The sugarcane borer Diatraea saccharalis (Fabricius, 1794) (Lepidoptera: Crambidae) is a pest that directly damages the plant by boring into the stalk; the resulting galleries reduce sap flow and make the plants susceptible to toppling by wind and rain as well as indirect damage by pathogenic microorganisms that penetrate the entry holes (Gallo et al. 2002; Bortoli et al. 2005).
Because controlling this lepidopteran pest is difficult once the larvae are inside the stalk, greater emphasis has been placed on alternative control measures, such as biological control and host plant resistance (Lara 1991). Silicon application may induce host resistance by forming a mechanical barrier (Goussain et al. 2002) and/or through physiological responses of the plant (Kvedaras et al. 2010) when under herbivore pressure.
The aim of this study was to evaluate the effects of silicon application to sugarcane cultivars RB72454 (moderately resistant) and SP801842 (susceptible) to D. saccharalis infestation.
Materials and methods
The experiment was conducted in a climate chamber in the Laboratory of Plant Resistance to Insects and in a greenhouse (Department of Entomology, Federal University of Lavras – UFLA, Lavras, Brazil) from March to November 2011.
A D. saccharalis culture was established using eggs provided by Usina Santa Adélia S/A mill (Jaboticabal, São Paulo) and the Biocontrol company (Sertãozinho, São Paulo). After hatching, the larvae were maintained in a environmental chamber at 25 ± 2 °C and 70 ± 10% relative humidity with a 12-hour photoperiod. The larvae were fed a diet consisting mainly of soybean meal, sugarcane yeast and sugar (King and Hartley 1985).
The cuttings of sugarcane stalks used for planting contained one node plus half of the internode lengths above and below the node and were obtained from the middle third of sugarcane cultivars RB72454 (moderately resistant) and SP801842 (susceptible) stalks. The cultivars were provided by the Usina Itaiquara factory (Passos, Minas Gerais) and the Cachaçaria João Mendes cachaça brewery (Perdões, Minas Gerais).
Polyethylene pots (5 kg) were filled with hillside soil (dark red latosol) and fertilized with 3.83 g of 4-14-8 NPK prior to planting. For earth sugarcane cultivar, 20 pots were planted with six cuttings per pot, and the cuttings were covered with a 5 cm layer of soil. Thinning was performed 20 days post-emergence, leaving two plants per pot. The pots were placed on benches in a greenhouse at the UFLA Department of Entomology and irrigated daily with 100 mL of water.
Two 500 mL applications of 1% silicic acid solution (SiO2.XH2O) (Vetec Fine Chemicals [Vetec Química Fina], Duque de Caxias, Brazil) were applied to the soil of each 5 kg pot, 120 and 125 days post-emergence. Each application was equivalent to 4 t SiO2/ha. For the control pots, two 500 ml applications of water were used. Each pot was enclosed within a cage made of voile fabric and was 40 cm in diameter and 100 cm in height. The fabric was supported using three iron rods (0.50 cm diameter) placed in the soil. The base of the fabric was secured to the bottom of the pot with a rubber band.
Twenty D. saccharalis adults at a maximum age of 24 hours were released into each caged pot 185 days after shoot emergence. Infestation intensity was evaluated 60 days after the release of adult D. saccharalis. Plants from each pot were cut, and the stalks dissected to determine the number of borer entry holes, galleries, larvae and pupae. The total number of internodes and the number of internodes per stalk bored were also recorded. Infestation intensity was calculated as follows: (number of internodes bored/number of total internodes per plant) × 100.
For the determination of silicon content, the plant leafs were dried at 60 °C and ground to a powder in a Willey-type grinder. These samples were sent to the Fertilizer Laboratory of the Federal University of Uberlândia, Institute of Agricultural Sciences (Laboratório de Fertilizantes da Universidade Federal de Uberlândia, Instituto de Ciências Agrárias – UFU/ LAFER) for analysis. For the determination of lignin content, some of the material was sent to the UFLA Department of Food Sciences Biochemistry Laboratory.
Plant leafs microscopic analysis were performed at the UFLA Department of Plant Pathology Laboratory of Electron Microscopy and Ultrastructural Analysis [Laboratório de Microscopia Eletrônica e Análise Ultraestrutural – LME]. The third complete leaves of each the sugarcane plant were sectioned and immersed in Karnovsky fixative solution in Eppendorf tubes for 24 hours. Immediately after fixation, the sections were transferred to a cryoprotective solution (30% glycerol) for 30 minutes. The samples were then frozen in liquid nitrogen and fragmented with a scalpel on a cooled metal surface. The fragments were subsequently placed in Petri dishes containing distilled water, dried on paper towels and then fixed with double-sided carbon tape onto a stub specimen holder (brass disc 12-13 mm [0.5 in] in diameter) wrapped in aluminum foil. Some fragments were fixed with the abaxial leaf surface facing up, while others were fixed with the abaxial side down. The stubs containing the specimens were placed in a desiccator containing silica gel, and after three days, the stub-mounted samples were placed in a Balzers SCD 050 evaporator for plating (covering of the samples with gold). Sample analysis was performed using a LEO Evo 40XVP scanning electron microscope. Several images at different magnifications were generated and digitally recorded. Photopaint software in the Corel Draw 12 package was used for image preparation.
A completely randomized experimental design with 10 replicates for the four following treatments was used: T1) SP801842 with no silicon application, T2) SP801842 with silicon application, T3) RB72454 with no silicon application and T4) RB72454 with silicon application. Six replicates were used for the determination of silicon and lignin content. Due to cost of analyzes only six replicates were used for the determination of silicon and lignin content. The data were subjected to analysis of variance, and the mean values were compared using Tukey's test (P ≤ 0.05) in the statistical program SAEG (Ribeiro Júnior 2001).
Results
Significant differences were observed for the number of holes in the stalks stalks (P = 0.0489; df = 36); although there was no evidence within cultivar for an increase in resistance of Sitreated cultivars compared with Si-untreated cultivars (Table 1). The highest number of entry holes was found in the untreated susceptible SP801842 cultivar, and the lowest number of holes was found in the silicon-treated resistant RB72454 cultivar. There were no significant differences observed for infestation intensity or the number of galleries among the four treatments (P > 0.05) (Table 2 ).
Because of the reduced occurrence of insect pests, it was not possible to perform statistical analysis on the data. However, the number of caterpillars and pupae was highest in the Si-untreated susceptible cultivar (5 caterpillars and 1 pupa) and lowest in the Si-treated moderately resistant cultivar (2 caterpillars and 0 pupa).
For both cultivars, Si-treatment led to a significant increase (P = 0.0001) in leaf Si-accumulation compared to Si-untreated plants (Table 3). Levels Si-leaf of the Si-treated susceptible cultivar did not reach that of the Si-untreated resistant cultivar. There were no significant differences in lignin content among the different treatments (P > 0.05).
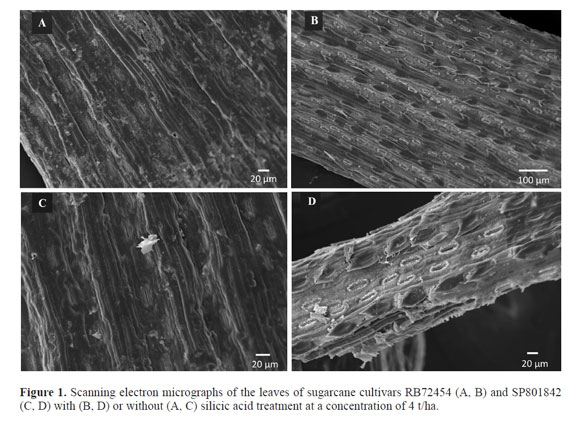
The scanning electron microscopy of the leaf surfaces revealed small crystals partially covering the stomata of leaves from the silicon-treated plants (Fig. 1). Crystals were not observed on the leaf stomata of plants that did not receive silicon application (Fig. 1). The opaque and whitish appearance of the leaves also indicated cuticle thickening.
Discussion
The main effect of Si in sugarcane was to reduce the number of holes caused by D. saccharalis, especially in cultivar with moderate resistance (RB72454). The reduced number of borer entry holes in the stalks of Si-treated plants as opposed to Si-treated sugarcane may be attributable to silicon accumulation, which forms a mechanical barrier against penetration by D. saccharalis larvae at the initial instars stages. A similar result was reported by Dinardo-Miranda et al. (2012) for the sugarcane cultivar IACSP94-2094, which had less than half the number of holes found in the susceptible cultivar. In maize, D. saccharalis holes are similarly noticeable in the stalks, and the low number of holes in Bt hybrids reflects their high level of resistance against D. saccharalis (Marques et al. 1999; Loche et al. 2009).
Contrary to the results of the present study, field research in South Africa provides evidence of the beneficial effects of silicon application. The incidence of the sugarcane borer Eldana saccharina decreased by more than 30% in Si-treated sugarcane, most notably in susceptible cultivars (Keeping and Meyer 2002, 2006; Kvedaras et al. 2005). These findings differ from the results of the present study, although low D. saccharalis attack was observed in the experiment. One possible explanation for the observed difference is that under water stress conditions, silicon may offer greater protection against borer attack and yield a greater benefit in susceptible cultivars (Kvedaras and Keeping 2007).
The present findings for the silicon content of sugarcane leaves support the hypothesis that sugarcane cultivars vary in their ability to accumulate silicon (Rossetto et al. 2005). However, silicon accumulation could not be correlated with the number of insect pests due to the small number of caterpillars observed. Nevertheless, the findings suggest that silicon prevents D. saccharalis attack on sugarcane plants through increased resistance to penetration of the stalk by caterpillars.
There were no significant differences in the lignin values among the four treatments (Table 3), and these findings are consistent with those of Ferreira et al. (2011) in soybean. However, the results differ from those of Moraes et al. (2009) and Gomes et al. (2008), who observed increases in lignin content following silicon application in soybean and potato, respectively.
The electron microscopy analysis revealed similarities to the thicker cuticles observed in coffee seedlings fertilized with silicic acid (Botelho et al. 2009). The present observations are also consistent with the thicker cuticles reported in other plants treated with silicon (Amaral 2005; Lima 2006).
Sugarcane exhibited a positive response to plant Si uptake with accumulation evident in the stems and leaves.
Acknowledgements
The authors thank the National Council for Scientiï¬c and Technological Development (Conselho Nacional de Desenvolvimento Cientíï¬co e Tecnológico – CNPq) for the fellowship granted and financial support and the Minas Gerais State Research Foundation (Fundação de Amparo à Pesquisa de Minas Gerais – FAPEMIG) for ï¬nancial support.
Literature cited
AMARAL, D. R. 2005. Indução de resistência em cafeeiro contra Cercospora coffeicola por eliciadores abióticos e extratos vegetais Induction of resistance in coffee plants against Cercospora coffeicola using abiotic elicitors and plant extracts. 96 p. Thesis (Master's in Phytopathology), Universidade Federal de Lavras Federal University of Lavras, Lavras. [ Links ]
ANDERSON, D. L. 1991. Soil and leaf nutrient interaction following application of calcium silicate slag to sugarcane. Fertilizer Research 30: 9-18. [ Links ]
BORTOLI, S. A.; DÓRIA, H. O. S.; ALBERGARIA, N. M. M. S.; BOTTI, M. V. 2005. Aspectos biológicos e dano de Diatraea saccharalis (Lepidoptera: Pyralidae) em sorgo cultivado sob diferentes doses de nitrogênio e potássio. Ciência e Agrotecnologia 29: 267-273. [ Links ]
BOTELHO, D. M. S.; POZZA, E. A.; ALVES, E.; FURTINI NETO, A. E.; BARBOSA, J. P. R. A. D.; CASTRO, D. M. 2009. Aspectos anatômicos e fisiológicos de mudas de cafeeiro (Coffea arabica L.) com cercosporiose (Cercospora coffeicola Berk. & Cook.) adubadas com ácido silícico. Coffee Science 4: 93-99. [ Links ]
DINARDO-MIRANDA, L. L. ; ANJOS, I. A.; COSTA, V. P.; FRACASSO, J. V. 2012. Resistance of sugarcane cultivars to Diatraea saccharalis. Pesquisa Agropecuária Brasileira 47: 1-7. [ Links ]
EPSTEIN, E. 1994. The anomaly of silicon in plant biology. Proceedings of National Academy of Sciences of the United States of America 91:11-17. [ Links ]
EPSTEIN, E. 1995. Photosynthesis, inorganic plant nutrition, solutions, and problems. Photosynthesis Research 46:37-39. [ Links ]
EPSTEIN, E. 1999. Silicon. Annual Review of Plant Physiology and Plant Molecular Biology 50:641-664. [ Links ]
FERREIRA, R. S.; MORAES, J. C.; ANTUNES, C. S. 2011. Silicon influence on resistance induction against Bemisia tabaci biotype B (Genn.) (Hemiptera: Aleyrodidae) and on vegetative development in two soybean cultivars. Neotropical Entomology 40: 495-500 [ Links ]
Gallo, D., Nakano, O., Silve ira Neto, S., Carval ho, R. P. L., Baptista , G. C., Bert i Filho, E., Parra, J. R. P., Zucchi, R. A., Alves , S. B., Vendramim, J. D., Marchini, L. C., Lopes, J. R. S., Omoto, C. 2002. Entomologia Agrícola. FEALQ, Piracicaba, Brasil. 920 p. [ Links ]
GOMES, F. B.; MORAES, J. C.; SANTOS, C. D.; ANTUNES, C. S. 2008. Uso de silício como indutor de resistência em batata a Myzus persicae (Sulzer) (Hemiptera: Aphididae). Neotropical Entomology 37: 185-190. [ Links ]
GOUSSAIN, M. M.; MORAES, J. C.; CARVALHO, J. G.; NOGUEIRA, N. L.; ROSSI, M. L. 2002. Efeito da aplicação de silício em plantas de milho no desenvolvimento biológico da lagartado-cartucho Spodoptera frugiperda (J.E.Smith) (Lepidoptera: Noctuidae). Neotropical Entomology 31: 305-310. [ Links ]
KEEPING, M. G.; MEYER, J. H. 2002. Calcium silicate enhances resistance of sugarcane to the African stalk borer Eldana saccharina Walker (Lepidoptera: Pyralidae). Agricultural and Forest Entomology 4: 265-274. [ Links ]
KEEPING, M. G.; MEYER, J. H. 2006. Silicon-mediated resistance of sugarcane to Eldana saccharina Walker (Lepidoptera: Pyralidae): effects of silicon source and cultivar. Journal of Applied Entomology 130: 410-420. [ Links ]
KING, E. G.; HARTLEY, G. G. 1985. Diatraea saccharalis. In: Singh, P.; Moore, R. F. (Ed.). Handbook of insect rearing. p. 265-270. [ Links ]
KORNDÖRFER, G. H.; PEREIRA, H. S.; CAMARGO, M. S. 2002. Papel do silício na produção de cana-de-açúcar. Revista STAB 21 (2): 6-9. [ Links ]
KVEDARAS, O. L.; AN, M.; CHOI, Y. S.; GURR, G. M. 2010. Silicon enhances natural enemy attraction and biological control through induced plant defenses. Bulletin of Entomological Research, Farnham Royal, Vol 100 (3): 367-371. [ Links ]
KVEDARAS, O. L.; KEEPING, M. G.; GEOBEL, R.; BYRNE, M. 2005. Effects of silicon on the African stalk borer Eldana saccharina (Lepidoptera: Pyralidae) in sugarcane. pp. 359-362. In: Annual Congress of the South African Sugar Technologists' Association, 79, 2005, Durban. Proceedings. Durban: South African Sugar Technologists Association. [ Links ]
KVEDARAS, O. L.; KEEPING, M. G. 2007. Silicon impedes stalk penetration by the borer Eldana saccharina in sugarcane. Entomologia Experimentalis et Applicata 125: 103-110. [ Links ]
LARA, F. M. 1991. Princípios de resistência de plantas a insetos. 336 p. [ Links ]
LIMA, L. M. 2006. Manejo da ferrugem da soja (Phakopsora pachyrhizi Sydow & P. Sydow) com fungicidas e silício. Thesis (Master's in Phytopathology), Universidade Federal de Lavras, Lavras. 81 p. [ Links ]
LIMA FILHO, O. F.; LIMA, M. T. G.; TSAI, S. M. 1999. O silício na agricultura. Informações Agronômicas 87: 1-7. [ Links ]
LOCHE, E. T.; NOVAKOWISKI, J. H.; BREN, L.; SANDINI, I. E. 2009. Severidade de Diatraea saccharalis, produtividade e massa de grãos em milho Bt e seu similar convencional. In: Semana de integração ensino, pesquisa e extensão, 1., Anais Guarapuava: SIEPE, 2009. Available at: <http://anais.unicentro.br/siepe/2009/pdf/resumo_618.pdf>. (Accessed on December 10th, 2012). [ Links ]
MA, J. F. ; YAMAJI, N. 2008. Functions and transport of silicon in plants. Cellular and Molecular Life Sciences 5:3049-3057. [ Links ]
MARQUES, G. B. C.; ÁVILA, C. J.; PARRA, J. R. P. 1999. Danos causados por larvas e adultos de Diabrotica speciosa (Coleoptera: Chrysomelidae) em milho. Pesquisa Agropecuária Brasileira 34: 1983-1986. [ Links ]
MARSCHNER, H. 1995. Mineral nutrition of higher plants. 2.ed. San Diego: Academic Press. 889 p. [ Links ]
MORAES, J. C.; FERREIRA, R. S.; COSTA, R. R. 2009. Indutores de resistência à mosca-branca Bemisia tabaci Biótipo B (GENN., 1889) (Hemiptera: Aleyrodidae) em soja. Ciência e Agrotecnologia 33: 1260-1264. [ Links ]
RAID, R. N.; ANDERSON, D. L.; ULLOA, M. F. 1992. Influence of cultivar and amendment of soil with calcium silicate slag on foliar disease development and yield of sugar-cane. Crop Protection 11: 84-88. [ Links ]
RIBEIRO JÚNIOR, J. I. 2001. Análises estatisticas no SAEG. Viçosa, MG: UFV 301 p. [ Links ]
ROSSETO, R.; LIMA FILHO, O. F.; AMORIN, H. V.; TSAI, S. M.; CAMARGO, M. S.; MELONIA, A. B. 2005. Silicon content in different sugarcane varieties. pp. 134. In: Silicon in agriculture conference, 3, Uberlândia. Proceedings. Uberlândia: UFU. [ Links ]
Suggested citation:
VILELA, M.; MORAES, J. C.; ALVES, E.; SANTOS-CIVIDANES, T. M.; SANTOS, F. A. 2014. Induced resistance to Diatraea saccharalis (Lepidoptera: Crambidae) via silicon application in sugarcane. Revista Colombiana de Entomología 40 (1): 44-48. Enero-julio 2014. ISSN 0120-0488.