Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488
Rev. Colomb. Entomol. vol.40 no.1 Bogotá Jan./June 2014
Sección control
Biological control of Tetranychus urticae (Tetranychidae) on rosebushes using Neoseiulus californicus (Phytoseiidae) and agrochemical selectivity
Control biológico de Tetranychus urticae (Tetranychidae) en rosales con Neoseiulus californicus (Phytoseiidae) y selectividad de plaguicidas
GISELLE C. DE SOUZA-PIMENTEL1,3, PAULO R. REIS1,2, ERIKA C. DA SILVEIRA1,3, PATRÍCIA DE P. MARAFELI1,3, ESTER A. SILVA4 AND HELENA B. DE ANDRADE3
1 Empresa de Pesquisa Agropecuária de Minas Gerais - EPAMIG Sul de Minas/EcoCentro, Caixa Postal 176, CEP 37200-000, Lavras, MG, Brazil.
2 D. Sc., CNPq Researcher. paulo.rebelles@epamig.ufla.br.
3 M. Sc., doctoral students of Postgraduate Program in Entomology - Universidade Federal de Lavras - UFLA, Caixa Postal 3037, CEP 37200-000, Lavras, MG, Brazil. gitostes@yahoo.com.br. Corresponding author. erika.silveira@yahoo.com.br; paduamara@ yahoo.com.br; heleninhaba@yahoo.com.br.
4 D. Sc., Universidade Estadual do Maranhão - UEMA /DFF, Av. Lourenço Vieira da Silva, s/n, Tirirical, São Luis, MA, CEP 65055-310, Brazil. esterazevedo@yahoo.com.br.
Received: 30-Sep-2013 • Accepted: 14-May-2014
Abstract: The two-spotted spider mite, Tetranychus urticae (Tetranychidae), is an important pest in rosebush (Rosa spp.), currently controlled by using agrochemicals. Phytoseiidae mites are used for biological control of pest mites. This study aimed to evaluate the biological control of the T. urticae on rosebushes grown in greenhouse after releases of the predatory mite Neoseiulus californicus (Phytoseiidae). In addition, the selectivity of some agrochemicals for disease and mite control was tested. Three experiments were conducted in a completely randomized design using potted roses. Twenty females per plant of the two-spotted spider mite were placed for infestation. After nine days, 0 to 14 predatory mites per plant were released. After 15 days a new release was made in one of the experiments. Predation was assessed one month after release by randomly collecting rosebush leaves from the apical, medial and basal regions, and the number of living T. urticae was counted. Selectivity was studied by using the laboratory method of residue spraying on a glass surface. Products were sprayed in a Potter tower with the highest doses recommended for the products. The number of live females and eggs laid by the predatory mite were evaluated. It was concluded that N. californicus is efficient in controlling the T. urticae under greenhouse conditions. The mineral oil, acephate, tebuconazole, iprodione, fenpropathrin and abamectin showed to be slightly harmful to the predator and could be used for integrated control. On the other hand, fenpyroximate was highly toxic to the predator mite.
Key words: Agricultural acarology. Rosa alba. Protected cropping. Two-spotted spider mite. Physiological selectivity.
Resumen: La ácaro rojo, Tetranychus urticae es una plaga importante en el rosal (Rosa spp.) siendo el control químico el método más utilizado. Los ácaros Phytoseiidae se utilizan en el control biológico de ácaros plagas. Este estudio tuvo como objetivo evaluar el efecto de liberaciones del fitoseido Neoseiulus californicus sobre T. urticae en rosales en invernadero. Además, se estudió la selectividad de algunos plaguicidas para el control de enfermedades y del ácaro rojo. Se realizaron tres experimentos en un diseño completamente al azar con rosal en macetas. Para la infestación se colocaron 20 hembras del ácaro rojo por planta. A los nueve días, entre 0 y 14 ácaros depredadores fueron liberados por planta. Después de 15 días se repitió la liberación en uno de los experimentos. Para la evaluación de la depredación se colectaron al azar hojas de rosal, de las regiones apical, media y basal, contando el número de ácaros vivos. Para determinar la selectividad, se utilizó el método residual de aspersión en superficie de vidrio en laboratorio, mediante una torre de Potter y las dosis más altas recomendadas por los productos. Se evaluó el número de hembras vivas y huevos puestos por el depredador. En conclusión, N. californicus es eficiente en el control de T. urticae en invernadero. El aceite mineral, acefate, tebuconazol, iprodiono, fenpropatrin y abamectin fueron poco perjudiciales para el depredador y pueden ser utilizados en control integrado. En cambio, fenpyroximato fue altamente tóxico al ácaro depredador.
Palabras clave: Acarología Agrícola. Rosa alba. Cultivo protegido. Ácaro rojo. Selectividad fisiológica.
Introduction
Rosebushes (Rosa spp.) are extensively cultivated in Brazil (Martins et al. 2009). The main production centers are the municipalities of Barbacena, Araxá and Munhoz, in Minas Gerais, which stand out not only for the production of roses, but for other conventional cut flowers as well (Landgraf and Paiva 2009).
The consumer market and foreign competition require constant concern with the quality in the production process adopted by producers. One of the challenges in the production of this ornamental is pest control, given that any injury is unacceptable as it depreciates as the final product. In rosebushes cultivation, the two-spotted spider mite, Tetranychus urticae Koch, 1836 (Tetranychidae), stands out as a major pest in greenhouse cultivation (Carvalho et al. 2009). For this reason, a high number of preventive spraying of pesticides is conducted, with high concentrations of active ingredients, which may lead to selection of populations resistant to the compounds, phytotoxicity, resurgence and emergence of secondary pests (Torres et al. 2007).
Pest control in greenhouse cultivated roses, if done through biological methods, provides reduced exposure to natural enemies and the pesticide applicators reduces the risk environmental hazards. In addition, this type of control requires no lag period between application and harvesting.
The use of predatory mites of the family Phytoseiidae, including the phytoseiids Neoseiulus californicus (McGregor, 1954) and Phytoseiulus macropilis (Banks, 1904), has been shown as very promising for the control of the two-spotted spider mite in greenhouses in Brazil (Reis et al. 2005). N. californicus is considered an excellent generalist predator, that can survive for long periods in the absence of prey by consuming pollen (Bambara 1998), and can also be used in combination with other predatory mites such as P. persimilis Athias-Henriot, 1957 and P. macropilis. However, what makes it the best choice among other species is its ability to stand adverse conditions, such as high temperature, low humidity and low prey densities (Fraulo and Liburd 2007; Weintraub and Palevsky 2008). Furthermore, in selectivity studies, N. californicus has been shown to be more tolerant to pesticides than other phytoseiid species (Amano et al. 2004; Silva and Oliveira 2006; Poletti et al. 2008). Under an IPM program, the use of selective products is highly desirable under certain conditions, as when the pest is at excessively highç levels (Silva et al. 2006).
Although N. californicus is already being marketed in Brazil, there are few studies on this mite for the control of T. urticae on roses. Thus, the aim of this work was to evaluate the efficiency releases of the predatory mite N. californicus for the control of the two-spotted spider mite on greenhouse grown rosebushes, as well as to evaluate selectivity of some agrochemicals registered for the control of diseases and of T. urticae on rosebushes.
Material and methods
Field work was conducted in a greenhouse, with top cover of clear plastic with fine mesh screen on the sides and at room temperature, located at the EPAMIG Experimental Farmç (FELA) in Lavras with a total area of 102.4 m2, whereas laboratoryç work was carried out at the Acarology Laboratory of EPAMIG Sul de Minas/EcoCentro, in Lavras, Minas Gerais state, Brazil, at 25 ± 2 °C, RH 70 ± 10% and photoperiod of 14 h.
Rearing of two-spotted spider mite. Specimens of T. urticae were taken from naturally infested plants of FELA and Instituto Federal de Educação, Ciência e Tecnologia do Sul de Minas Gerais, Inconfidentes campus (IFET Sul de Minas) to start a colony that was maintained in the greenhouse and in the laboratory on jack bean (Canavalia ensiformis L., Fabaceae).Specimens of N. californicus were also initially obtained from IFET Sul de Minas, Inconfidentes campus, and raised under the same controlled conditions. The predator mites were placed on a sheet of PVC over Styrofoam® plates floating in water, in a plastic tray (32 x 26.5 x 5.5 cm), surrounded by cotton to prevent the escape of the mites. Jack bean leaves infested with the pest mites were provided as for the predaceous mites, and pollen from castor bean (Ricinus communis L., Euphorbiaceae) pollen was used as supplementary food source.
Biological control experiments. For the greenhouse experiments, potted grafted white roses (Rosa alba L., Rosaceae) (bench graft) were used, planted in pots with a capacity of 22 liters of soil and approximately 60 cm high (excluding the height of the pot). Three experiments were conducted in a completely randomized design. The first experiment consisted of three treatments and seven replicates. Treatments corresponded to: (1) - roses without pest or predator (controlç treatment), (2) - roses with only T. urticae and (3) - roses with T. urticae and N. californicus.
Initially each rosebush was infested with 20 specimens of two-spotted spider mites females; after nine days, the first predator release was conducted with two predators per plant per week up to a total of six per plant.
The second and third experiments consisted of eight treatments with three replicates each. The control treatment (T1) consisted only of plants infested with T. urticae and the other treatments with combinations of pest and predator, in increasing order of two as the number of predators released, i.e., T2 = 2, T3 = 4, T4 = 6, T5 = 8, T6 = 10, T7 = 12 and T8 = 14 predators / plant. The difference between the second and third experiments was the number of releases of the predatory mites. In the second experiment, a single release was done in the first week, whereas in the third xperiment releases were done in the first and the third week. Each test was conducted for four weeks.
Each plant was placed in a wood cage covered with Voile fabric (0.60 x 0.60 x 1.50 m) to prevent dispersion of the phytophagous mites as well as predators and the entrance of unwanted organisms. At each week before the release of predators, one leaflet (first experiment) or two leaflets (second and third experiments) were taken from each plant section (basal, middle and apical). The leaves collected from each plant section were placed separately in a plastic bag, which in turn were placed in refrigerated polystyrene boxes for immediate transport to the laboratory, to count the active forms of mites present under a stereoscopic microscope. The data obtained were subjected to regression analysis (Ferreira 2008).
Selectivity. Seven products commonly used in rose cultivation in southern Minas Gerais state, Brazil were evaluated: two fungicides (tebuconazole and iprodione), four insecticides- acaricides (fenpropathrin, fenpyroximate, abamectin and acephate) and an insecticide (mineral oil) (Table 1). The test was conducted in laboratory using the method of residual spraying on a glass surface. The results obtained using this methodology is equivalent to those when using vegetablesleaves (Bakker et al. 1992). Glass coverslips (20 x 20 mm) (Reis et al. 1998) were sprayed with the recommended doses (Agrofit 2010) of the products being tested, in a Potter tower at a pressure of 15 lb./in2, with the tower spray table at a distance of 1.7 cm from the spray tube; each coverslip received a deposit of approximately 2 mg These procedures were in accordance with those proposed by the IOBC/WPRS (Hassan et al. 1994; Overmeer 1988). After application, the glass coverslips were placed to dry at room temperature for one hour and then placed to float in water in a 5 cm diameter by 2 cm deep Petri dish which was kept open. Soon afterwards, five N. californicus females were transferred with a brush to each glass coverslip, which also received a small amount of castor bean pollen as food for the surviving mites.
Each test remained eight days, with a daily count of the live females and the number of eggs laid that resulted in viable larvae, and dead females were removed. The adverse or total effect (E%) was alculated by taking into account ortality in treatment, corrected in function of the control mortality, and the effect on reproduction, according to Overmeer and van Zon (1982) and according to the IOBC/WPRS (Bakker et al. 1992) by using: E % = 100% - (100% - Mc) x Er, were: Mc = corrected mortality (Abbott, 1925) and Er = effect on reproduction.
The effect on reproduction (Er) was obtained by dividing the average egg production of the females in treatment (R) by the egg production in the control group (Er = RTreatment/RControl). The average egg production per female (R) was obtained by the relationship: R = number of viable eggs/number of live females. Were considered valid only the tests where the mortality in the control plot was ≤ 20% (Bakker et al. 1992).
The total effect values found for each product were classified in Classes 1 to 4 according to the criteria established by the IOBC/WPRS for classifying plant protection products on the asis of the adverse effect caused to beneficial organisms in laboratory tests (Bakker et al. 1992; Hassan et al. 1994; Overmeer 1988; Boller et al. 2005) which are: Class 1 = Eç < 30% (innocuous, not harmful), Class 2 = 30% ≤ E ≤ 79% (slightly harmful), Class 3 = 80% ≤ E ≤ 99% (moderately harmful), and Class 4 = E > 99% (harmful).
Results and discussion
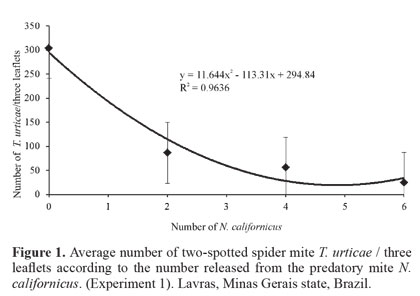
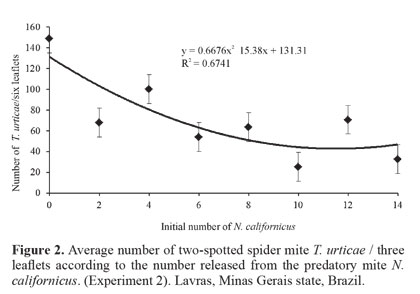
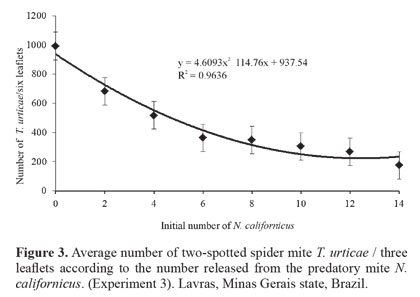
Biological control. The results of the three biological control experiments showed that there is a negative and highly significant relationship between the increase in the number of the predatory mite (F = 9.82 and P = 0.0003, F = 20.24 and P = 0.0000, F = 5.82 and P = 0.0002, respectively) and the average number of T. urticae/leaflet. For all experiments, the quadratic regression (P = 0.0321, P = 0.0004 and P = 0.0412, respectively) fit the infestation data of T. urticae/leaflet better (Figs. 1-Fig 3). Thus, there was a reduction in the number of T. urticae mites with the increase of the predatory mite N. californicus on rosebush leaves.
Findings of this study are similar to those reported by Greco et al. (2005) who performed a study with different ratios between the number of T. urticae and N. californicus on strawberry (Fragaria spp., Rosaceae). They concluded that at ratios of up to 1:10 (predator: pest) N. californicus prevented the pest from reaching the economic damage level (i.e. 50 T. urticae/leaflet). Fraulo and Liburd (2007) also observed that N. californicus, when released at ratios of up to 1:10 (predator: prey) was effective in controlling T. urticae on strawberry in the field and greenhouse, maintaining the pest population at low levels for long periods.
Results obtained in the present study are also similar to those of Bellini (2008) in studies of the potential of N. californicus to control T. urticae on rosebushes. According to that author, initial density of 10 predators/m2 was not enough to control the pest, when the latter was above the level of the control (mentioned as 10 T. urticae/leaf), whereas initial density of 20 predators/m2 provided quick and efficient control, even with a higher infestation of T. urticae. Still according to that author, the number of predators initially released seems related to the speed of reduction of the pest population, also observed in the present work.
Selectivity. Given that no mortality was observed in the control treatment, the correction of the mortality were not necessary (Table 1). Six of the tested products were shown as selective to N. californicus; mineral oil (insecticide) was classified as harmless (Class 1), acephate (insecticideacaricide), tebuconazole (fungicide), iprodione (fungicide), fenpropathrin (insecticide-acaricide) and abamectin (insecticide acaricide) caused minor effects on the predator being classified as slightly harmful (Class 2). Fenpyroximate (acaricide) was rated as moderately harmful (Class 3). The products that were classified in Class 2 (slightly harmful) caused a reduction in mite reproduction (Er < 1), but mineral oil (Class 1) stimulated their reproduction, Er > 1 (r = 1.3) value (Table 1).
Abamectin (insecticide-acaricide) was slightly harmful (Class 2) to the N. californicus mite. Sato et al. (2002) reported that abamectin was significantly detrimental to N. californicus soon after application of the product. However, this effect was short and one day after the application is not showed any more toxic effect to the predatory mite. Nadimi et al. (2011) also reported that for P. persimilis after 10 days of application, there is no effect of the product to the predaceous mite. Ibrahim and Yee (2000) reported that abamectin showed relative selectivity to Neoseiulus longispinosus (Evans, 1952) and reduced its longevity by 40%. However, Silva et al. (2006), reported high toxicity of abamectin to Euseius alatus DeLeon, 1966 causing 100% mortality of those mites within 72 h. Also, this product was considered harmful to P.ç macropilis on strawberry by Lorenzato (1998).
The insecticide-acaricide fenpropathrin was classified as slightly harmful to N. californicus in this work. However, inç tests conducted by Reis et al. (1998) it was shown as nonselective to the phytoseiid Iphiseiodes zuluagai Denmark and Muma, 1972 (Class 4). Poletti et al. (2008) reported less than 20% mortality of N. californicus by applications of this product, a result similar to that found in the present work.
The acaricide-insecticide acephate, was slightly harmful to N. californicus in this study, similar to that reported by Silva et al. (2009) for the predatory mite Agistemus brasiliensis Matioli, Ueckermann and Oliveira, 2002 (Acari: Stigmaeidae). However, the results differ from those obtained by Ferla and Moraes (2006) with the phytoseiids Euseius concordis (Chant, 1959) and Neoseiulus anonymus (Chant and Baker, 1965) placed by the authors in Class 4 (harmful). The insecticide-acaricide mineral oil was considered in this study as harmless to the predatory mite N. californicus, a result similar to that found by Yamamoto and Bassanezi (2003) for E. concordis and Euseius citrifoliusDenmark and Muma, 1970. However, for these authors mineral oil was considered harmful to the predator I. zuluagai, as also found by Reis et al. (1998) for the same predator.
The fungicides iprodione and tebuconazole were classified as slightly harmful, with predator mortality of 26.7 and 56.7% in this study, respectively. Poletti et al. (2008) also studied the same fungicides for N. californicus and found mortality rates of around 5% for the two fungicides; much lower than the rates in the present work. This difference may be explained to the different origins of the N. californicus populations.
Fenpyroximate (acaricide) was considered moderately harmful to N. californicus in this work, a similar result to those obtained by Nadimi et al. (2011) for P. persimilis and Yamamoto and Bassanezi (2003) for E. concordis and E. citrifolius in selectivity tests performed and was considered harmful to I. zuluagai. However, in a study by Sato et al. (2002) fenpyroximate not showed harmful effect to N. californicus. Poletti et al. (2008) observed that a population of N. californicus showed less susceptibility than a population of P. macropilis to the action of various pesticides used in protected cultivation, concluding that N. californicus can be released in commercial areas where chemical control is often used. The same was reported by Silva et al. (2011). Results of the present study allow similar conclusions.
Conclusion
Releases of the predatory mite N. californicus efficiently reduced T. urticae populations on greenhouse-grown rosebushes. If the use of pesticides for the control of other pests and diseases is necessary, mineral oil, acephate, tebuconazole, iprodione, fenpropathrin and abamectin are safer to use than other products, for having relatively small impact on N. californicus. However, fenpyroximate should not be associated with biological control as it is moderately harmful to the predatory mite N. californicus.
Acknowledgements
To Fundação de Amparo à Pesquisa do Estado de Minas Gerais (Fapemig) for financial support and Technical Support Grant (BAT); to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for granting the research productivity scholarship, and to Prof. Dr. Luiz Carlos Dias da Rocha, IFET Sul de Minas, Inconfidentes campus, for the initial supply of T. urticae and N. californicus.
Literature cited
ABBOTT, W.S. 1925. A method of computing the effectiveness of an insecticide. Journal of Economy Entomology 18 (2): 265- 267. [ Links ]
AGROFIT- Sistema de agrotóxicos fitossanitários. 2010. Available in: http://extranet.agricultura.gov.br/agrofit_cons/principal_ agrofit_ cons. (Review date: 01 August 2010). [ Links ]
AMANO , H.; ISHII, Y.; KO BORI, Y. 2004. Pesticide susceptibilityof two dominant phytoseiid mites, Neoseiulus californicus and N. womersleyi, in conventional Japanese fruit orchards (Gamasina: Phytoseiidae). Journal of the Acarological Society of Japan 13 (1): 65-70. [ Links ]
BAKK ER, F. M.; GROVE, A.; BLÜMEL, S.; CALIS, J.; OO MEN, P. 1992. Side-effect test for phytoseiids and their rearing methods. IOBC/WPRS Bulletin, Montfavet 15 (3): 61-81. [ Links ]
BAMBARA, S. B. 1998. Predator mites of spider mites greenhouses ornamentals and turf. North Carolina Pest News 13 (6): 1-4. [ Links ]
BARBOSA, J. G. 2003. Produção comercial de rosas. Viçosa: Aprenda Fácil, 200 p. [ Links ]
BELLINI, M. R. 2008. Manejo de Tetranychus urticae (Acari: Tetranychidae) em plantas ornamentais. Piracicaba, 2008. 138 p.ç Tese (Doutorado em Ecologia Aplicada). Escola Superior de Agricultura Luiz de Queiroz. [ Links ]
BOLLER, E. F.; VOGT, H.; TERNES, P.; MALAVOLTA, C. 2005. Working document on selectivity of pesticides (2005). International Organization for Biological and Integrated Control of Noxious Animals and Plants: West Palearctic Regional Section - IOBC/WPRS. Available in: http://www.iobc-wprs.org/ip_ipm/03021_IOBC_WorkingDocumentPesticides_Explanations.pdf. (Review date: 20 May 2013). [ Links ]
CARVALHO, L. M.; BUENO , V. H. P.; SANTA-CECÍLIA, L. V. C; SILVA, R. A.; REIS, P. R. 2009. Pragas na floricultura: identificação e controle. Informe Agropecuário 30 (249): 36-46. [ Links ]
FERLA, N. J.; MORAES, G. J. 2006. Seletividade de acaricidas e inseticidas a ácaros predadores (Acari: Phytoseiidae) encontrados em seringueira no centro-oeste do Brasil. Ciência Rural 36 (2): 357-362. [ Links ]
FERREIRA, D. F. 2008. SISVAR: um programa para análises e ensino de estatística. Revista Científica Symposium 6 (2): 36-41. [ Links ]
FRAULO, A. B.; LIBURD, O. E. 2007. Biological control of twospotted spider mite, Tetranychus urticae, with predatory mite, Neoseiulus californicus, in strawberries. Experimental & Applied Acarology 43: 109-119. [ Links ]
GRECO, N. M.; SÁNCHEZ, N. E.; LILJESTHRÖM, G. G. 2005. Neoseiulus californicus (Acari: Phytoseiidae) as a potential control agent of Tetranychus urticae (Acari: Tetranychidae): effect of pest/predator ratio on pest abundance on strawberry. Experimental & Applied Acarology 37: 57-66. [ Links ]
HASSAN, S. A.; BIGLER, F.; BOGENSCHÜTZ, H.; BOLLER, E.; BRUN, J.; CALIS, J. N. M.; COREMANS-PELSENEER, J.; DUSO, C.; GROVE, A.; HEIMBCH, U.; HELYER, N.; HOK - KANEN, H.; LEWIS, G.B.; MANSOUR, F.; MORETH, L.; POLGAR, L.; SAMSØE - PETERSEN, L.; SAUPHANO R, B.; STÄUBLI, A.; STERK, G.; VAINIO, A.: VAN DE VEIRE, M.; VIGGIANI, G.; VOGT, H. 1994. Results of the sixth joint pesticide testing programme of the IOBC/WPRS - working group "Pesticides and Beneficial Organisms". Entomophaga 39 (1): 107-119. [ Links ]
IBRAHIM, Y. B.; YEE, T. S. 2000. Influence of sublethal exposure to abamectin on the biological performance of Neoseiulus longispinosus (Acari: Phytoseiidae). Journal Economy Entomology 93 (4): 1085-1089. [ Links ]
LANDGRAF, P. R. C.; PAIVA, P. D. D. 2009. Production of cut flowers in the state of Minas Gerais. Ciência e Agrotecnologia 33 (1): 120-126. [ Links ]
LORENZ ATO, D. 1998. Ensaios laboratoriais de controle químico e biológico do ácaro-rajado em mudas de morangueiro. Pesquisa Agropecuária Gaúcha 4 (2): 95-99. [ Links ]
MARTINS, M. V. M.; ADRIGUETO, J. R.; ARTIMON TE, A. P.; MOSCA, J. L. 2009. Produção integrada de flores no Brasil. Informe Agropecuário 30 (249): 64-66. [ Links ]
NADIMI, A.; KAMALI, K.; ARBABI, M.; ABDOLI, F. 2011. Study on persistence tests of miticides abamectin and fenpyroximate to predatory mite Phytoseiulus persimilis (Acarina: Phytoseiidae). African Journal of Agricultural Research 6 (2): 338-342. [ Links ]
OVERMEER, W. P. J. 1988. Laboratory method for testing sideeffects of pesticides on the predaceous mites Typhlodromalus pyri and Amblyseius potentillae (Acari: Phytoseiidae). IOBC/ WPRS Bulletin, Montfavet 11 (4): 65-69. [ Links ]
OVERMEER, W. P. J; van Zon, A. Q. 1982. A standardized method for testing the side-effects of pesticides on the predacious mite, Amblyseius potentillae (Acari: Phytoseiidae). Entomophaga 27 (4): 357-364. [ Links ]
POLETTI, M.; COLLETE, L.de P.; OMOTO, C. 2008. Compatibilidade de agrotóxicos com ácaros predadores Neoseiulus californicu (McGregor) e Phytoseiulus macropilis (Banks) (Acari: Phytoseiidae). BioAssay 3 (3): 1-14. [ Links ]
REIS, P. R.; SILVA, E. A. da; ZACARRIAS, M. S. 2005. Controle biológico de ácaros em cultivos protegidos. Informe Agropecuário 26 (225): 58-67. [ Links ]
SATO, M. E.; SILVA, M.; GON ÇALVES, L. R.; SOUZA FILHO, M. F.; RAGA, A. 2002. Toxicidade diferencial de agroquímicos Neoseiulus californicus (McGregor) (Acari: Phytoseiidae) e Tetranychus urticae Koch (Acari: Tetranychidae) em morangueiro. Neotropical Entomology 31 (3): 449-456. [ Links ]
SILVA, F. R.; VASCON CELOS, G. J. N.; GON DIM JÚNIOR, M. G. C.; OLIVEIRA, J. V. 2006. Toxicidade de acaricidas para ovoe fêmeas adultas de Euseius alatus DeLeon (Acari: Phytoseiidae). Revista Caatinga 19 (3): 294-303. [ Links ]
SILVA, M. Z.; OLIVEIRA, C. A. L. de. 2006. Seletividade de alguns agrotóxicos em uso na citricultura ao ácaro predador Neoseiulu californicus (McGregor) (Acari: Phytoseiidae). Revista Brasileira de Fruticultura 28 (2): 205-208. [ Links ]
SILVA, M. Z.; OLIVEIRA, C. A. L.; SATO, M. E. 2009. Seletividade de produtos fitossanitários sobre o ácaro predador Agistemus brasiliensis Matioli, Ueckermann & Oliveira (Acari: Stigmaeidae). Revista Brasileira de Fruticultura 31 (2): 388-396. [ Links ]
SILVA, M. Z.; SATO, M. E.; OLIVEIRA, C. A. L.; RAIS, D. S. 2011. Toxicidade diferencial de agrotóxicos utilizados in citrosç para Neoseiulus californicus, Euseius concordis e Brevipalpus phoenicis. Bragantia 70 (1): 87-95. [ Links ]
TORRES, F. Z. V.; CARVALHO, G. A.; SOUZA, J. R.; ROCHA, L. C. D. 2007. Seletividade de inseticidas a Orius insidiosus Bragantia 66 (3): 433-439. [ Links ]
YAMAMOTO, P. T.; BASSANEZI, R. B. 2003. Seletividade deprodutos fitossanitários aos inimigos naturais de pragas dos citros. Laranja 24 (2): 353-382. [ Links ]
WEINTRAUB, P.; PALEVSKY, E. 2008. Evaluation of the predatory mite, Neoseiulus californicus, for spider mite control on greenhouse sweet pepper under hot arid field conditions. Experimental & Applied Acarology 45: 29-37. [ Links ]
Suggested citation:
SOUZA-PIMENTEL, G. C. de; REIS, P. R.; DA SILVEIRA, E. C.; MARAFELI, P. de P. SILVA, E. A.; DE ANDRADE, H. B. 2014. Biological control of Tetranychus urticae (Tetranychidae) on rosebushes using Neoseiulus californicus (Phytoseiidae) and agrochemical selectivity. Revista Colombiana de Entomología 40 (1): 80-84. Enero-julio 2014. ISSN 0120-0488.