Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488
Rev. Colomb. Entomol. vol.40 no.1 Bogotá Jan./June 2014
Sección control
Conidial production, virulence, and stress tolerance of Beauveria bassiana conidia after successive in vitro subculturing
Producción conidial, virulencia y tolerancia al estrés de Beauveria bassiana de subcultivos sucesivos in vitro
PATRICIA H. SANTORO1, JANAINA ZORZETTI2,3, KELLY CONSTANSKI2,3 AND PEDRO M. O. J. NEVES2,4
1 Ph. D. Instituto Agronômico do Paraná, Rod. Celso Garcia Cid, PR 445, km 375, CP: 481, CEP: 86047-902, Londrina, PR, Brazil. patriciasantoro@iapar.br. Corresponding author.
2 Universidade Estadual de Londrina, Centro de Ciências Agrárias Rod. Celso Garcia Cid, PR 445, km 380, CP: 6001, CEP: 86051-990 Londrina, PR, Brazil.
3 Ph. D. Candidate. jzorzetti@hotmail.com; kconstanski@hotmail.com.
4 Ph. D. pedroneves@uel.br
Received: 14-Oct-2013 • Accepted: 23-Apr-2014
Abstract: The effect of successive subculturing of Beauveria bassiana under different in vitro nutritional conditions was evaluated on vegetative growth, conidial production, virulence, tolerance to UV radiation and heat tolerance. Potato dextrose agar (PDA) and a medium based on adult Alphitobius diaperinus (MAD) were used. The fungus was inoculated on the insects, isolated, and subcultured 17 times on the different media. After subculturing, the fungus was again inoculated on the insects, and it was then re-isolated. Successive subculturing and nutritional conditions influenced fungus quality. MAD favored maintenance of vegetative growth and conidial production on culture medium and on rice after a greater number of successive subcultures than PDA. Furthermore, a decrease in conidial production in MAD was less pronounced than in conidia from the PDA medium. Conidia cultured on MAD maintained virulence after 17 successive subcultures. Conidia cultured on PDA retained initial thermotolerance levels. Conidial viability decreased after UV irradiation, but this decrease was unaffected by successive subculturing on PDA and MAD. Virulence, conidial production, and temperature tolerance, which were reduced after successive in vitro subculture, were restored with fungus passage through the host.
Key words: Alphitobius diaperinus. Abiotic factors. Entomopathogenic fungi. UV radiation. Temperature.
Resumen: El efecto de subcultivos sucesivos in vitro de Beauveria bassiana a diferentes condiciones nutricionales fue evaluado mediante el crecimiento vegetativo, la producción conidial, la virulencia, tolerancia a la radiación UV y al calor. Se usaron los medios agar dextrosa papa (PDA) y un medio basado en adultos de Alphitobius diaperinus (MAD) para la producción conidial. El hongo se inoculó en los insectos, se aisló, y subcultivó 17 veces en medios diferentes. Después de subcultivados, los hongos se inocularon en los insectos, y se aislaron nuevamente a partir de ellos. Los subcultivos sucesivos y las condiciones nutricionales influenciaron la calidad de los hongos. El medio MAD mantuvo el crecimiento vegetativo y la producción conidial en cultivo y en arroz, después de un gran número de subcultivos en PDA. Así mismo, la reducción en la producción de conidias en MAD fue poco percibida comparado con la producción en PDA. El cultivo de conidias en MAD mantuvo la virulencia luego de 17 subcultivos sucesivos. Las conidias cultivados en PDA preservaron los niveles iniciales de termotolerancia. La viabilidade de los conídios disminuyó después de recibir radiación UV, pero esta disminución no afectó los subculivos sucesivos en PDA y MAD. La virulencia, la producción conidial y la tolerancia a alta temperatura, se redujeron después de los sucesivos subcultivos in vitro, pero restauradas con el paso de los hongos a través del hospedero.
Palabras clave: Alphitobius diaperinus. Factores Abióticos. Hongos entomopatógenos. Radiación UV. Temperatura.
Introduction
Previous studies have shown physiological changes of entomopathogenic fungi after successive subculture in vitro. For instance, Metarhizium anisopliae (Metsch.) showed reduced virulence in Tenebrio molitor (Linnaeus) (Coleoptera: Tenebrionidae) and Pr1 enzyme production after successive subculturing on sabouraud dextrose agar (SDA) medium (Shah et al. 2007). Quesada-Moraga and Vey (2003) believe that changes in virulence depend on the nutritional conditions of the subculture. These authors reported that conidia of Beauveria bassiana (Bals.) Vuill showed reduced virulence to Dociostaurus maroccanus (Thunberg, 1815) (Orthoptera: Acrididae) after two subcultures on SDA, but increased virulence after two subcultures on malt agar (MA) medium. Virulence of Paecilomyces fumosoroseus (Wise) in Diuraphis noxia (Mordvilko) (Hemiptera: Aphididae) and Plutella xylostella (Linnaeus, 1758) (Lepidoptera: Plutellidae) was unaffected after 30 successive subcultures on sabouraud dextrose yeast (SDY) extract medium. However, some isolates showed a decrease in conidial production and viability (Vandenberg and Cantone, 2004). Neither morphological changes nor altered virulence to Bemisia tabaci (Gennadius, 1889) (Hemiptera: Aleyrodidae = B. argentifolii Bellows and Perring) were detected in conidia of B. bassiana after 15 subcultures on SDY; thus, the isolate exhibits sufficient stability for mass production (Brownbridge et al. 2001).
Variable outcomes after successive in vitro subculturingmay be associated with inter- and intraspecific variation and the use of monosporic or multisporic cultures. Brownbridge et al. (2001) believe that attenuation of virulence may be random (mutation) or caused by subculture conditions. However, the effects of successive in vitro subculturing on conidia tolerance to abiotic factors limiting the efficiency of fungi, such as heat and UV radiation, are unknown.
We evaluated the effects of successive in vitro subculture of B. bassiana under different nutritional conditions on vegetative growth, conidial production, virulence, and tolerance to heat and UV radiation.
Material and methods
Inoculum growth of B. bassiana and successive subculturing in vitro. Unioeste 4 isolate of B. bassiana, which is part of the entomopathogen collection maintained by the Microbial Control laboratory of Universidade Estadual Londrina, was selected for its virulence in Alphitobius diaperinus (Panzer, 1797) (Coleoptera: Tenebrionidae) (Santoro et al. 2008). Initial conidial multiplication was performed on medium for spore production of Beauveria spp. (MSP) (Alves et al. 1998) in an incubator (25 ± 1 °C and a 12 h photophase) for 10 days. Conidia produced on MSP were inoculated on adult A. diaperinus collected in poultry houses and previously surface sterilized with a solution of 2% sodium hypochlorite. The insects were submerged in a suspension of 1.0 × 107 conidia mL-1 for 15 s. Dead insects were again surface sterilized and placed in a humid chamber (25 ± 1 °C) for five days allowing for conidial multiplication. These conidia were called (A).
Successive subculturing was performed on potato dextrose agar (PDA) medium and the medium of A. diaperinus (MAD). To prepare the MAD, adult insects were frozen, then were surface sterilized and ground in a blender with distilled water at a 10% (w/v) concentration. Media were adjusted to pH 7 with NaOH (1N), solidified with agar (20 g·L-1), sterilized by autoclaving for 30 min, and poured into sterile Petri dishes (diameter 5.2 cm).
Conidia (A), produced on dead insects, were grown onPDA and MAD under the same conditions described for MSP, giving rise to the first conidial subculture, 1st(A). These were grown in their respective media to obtain the second conidial subculture and so on, up to 17 subcultures, when they were again inoculated on A. diaperinus. The fungus produced on these insects was called (B) and grown in the same media, producing conidia of the first subculture 1st(B).
Conidia produced from each in vivo and in vitro subculture were stored at 6 °C. Because of the time required to obtain all subcultures (i.e., approximately 200 days), it was necessary to standardize the age of the conidia to eliminate possible interferences arising from the storage time. Thus, conidia produced and stored in (A), 3rd, 7th, 11th, 16th, and (B) were grown in their respective media (PDA or MAD), giving rise to conidial subcultures 1st (A), 4th, 8th, 12th, 17th, and 1st(B), respectively.
Vegetative growth and conidial production on culture media. Petri dishes (9 cm diameter) with MSP were needleinoculated in the center with the fungus to obtain a single colony. Plates were placed in an incubator for 10 days. Vegetative growth was determined by calculating the area of the colony based on the average measurements of two opposite diameters. The conidial production in the same colonies was assessed. Conidia were removed from the medium with a spatula, suspended in an aqueous solution of 0.005% (v/v) tween 20, and vortexed for 30 s. After dilution, the conidia were quantified in a hemocytometer.
Conidial production on rice. Parboiled rice (500 g) was added to 1 L boiling distilled water, and cooked in a microwave at full power for 3 min. The unabsorbed water was discarded, and 65 g of rice were placed in glass bottles (500 mL). These were capped with a paper towel and sterilized by autoclaving for 30 min. After cooling, the rice was inoculated with 1.5 mL of a suspension containing 1.0 × 107 conidia mL-1 and placed in an incubator for 15 days. To assess conidial production, 300 mL of an aqueous solution of 0.005% (v/v) tween 20 was added to each bottle and shaken manually for 3 min. After dilution, conidia were quantified in a hemocytometer.
Virulence to A. diaperinus. Adult A. diaperinus (n = 50) were placed on an acrylic plate (6 cm diameter), and 0.5 mL of a suspension containing 8 × 106 conidia mL-1 was sprayed. This corresponds to the LC50 of the isolate used in this experiment (Santoro et al. 2007). Controls were sprayed with an aqueous solution of 0.005% (v/v) tween 20. Insects were fed sterilized corn food and kept in an incubator (25 ± 1 °C). After 10 days, the number of dead insects was assessed, and these insects were placed in a humid chamber (25 ± 1 °C) for another five days to confirm mortality by the pathogen.
Tolerance to UV radiation. The colony-forming units (CFUs) method was used because exposure to UV radiation cause a delay in germination of the surviving conidia (Nascimento et al. 2010). A suspension containing 1 × 103 conidia mL-1 (0.1 mL) was spread on the surface of the MSP using a sterile glass spreader. Uncovered plates were placed in a laminar flow hood under a low-pressure germicidal lamp (253.7 nm; Philips TUV 30W) at a distance of 52 cm for 1 min. The plates were closed and transferred to an incubator. Conidia that were not exposed to UV radiation were used as controls. The CFUs were quantified on fifth day.
Temperature tolerance. Conidia were stored in sterile test tubes and placed in an incubator at 30 °C for 15 days in the dark. Conidia not exposed to 30 °C were used as controls. Viability was assessed by the CFU method.
Experimental design and statistical analysis. The study was a completely randomized factorial design (2 × 6) (conidia media of origin × subcultures), with four replicates for vegetative growth, conidial production in culture medium, and tolerance to UV radiation, and five replicates for conidial production on rice and temperature tolerance. Experimental design for virulence to A. diaperinus was a completely randomized factorial design (2 × 6) + 1 (conidia media of origin × subcultures) + control, with six replicates of 50 insects. Data analysis were performed using ANOVA. Mean values were compared using the Tukey test (P < 0.05); the mean values of factorial analysis of virulence testing were compared using the Dunnett test (P < 0.05).
Results and discussion
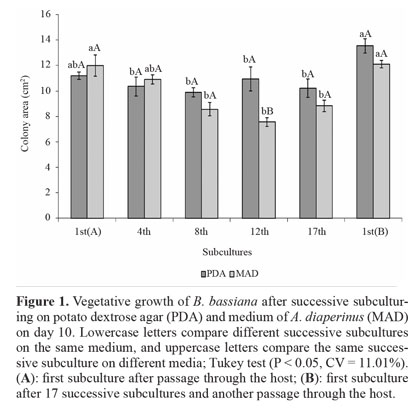
Vegetative growth and conidial production on culture media. Vegetative growth was influenced by a significant interaction between successive subculturing and media. No difference in vegetative growth was observed from the 1st(A) to the 17th successive subculture for the fungus isolated from PDA. The vegetative growth from subculture 1st(B) did not differ from 1st(A) although it was higher than others subcultures (Fig. 1).
For conidia produced on MAD, no change was observed from subculture 1st(A) to the 4th subculture, with a decrease from subculture 8th to 17th, and recovery on subculture 1st(B), similar to 1st(A). Comparison between the two media showed that the only difference was in the 12th successive subculture, wherein more conidia were produced on PDA (Fig. 1). The ef- fect of successive in vitro subculturing on vegetative growth has been reported. Isolates of P. fumosoroseus have shown a decrease in mycelium production in liquid medium after 30 successive subcultures (Vandenberg and Cantone 2004). Morphological changes and reduced growth were observed for Lecanicillium lecanii (Zimmerman) after 98 successive subcultures (Hall 1980).
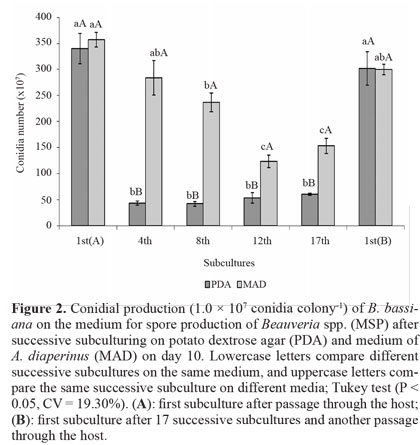
Most studies of successive subculturing in vitro have only assessed virulence (Barbosa et al. 1985; Brownbridge et al. 2001; Shah et al. 2007). However, the success of biological control by fungi depends, among other factors, on the ability to produce high conidial concentrations in vitro. Although we did not find any difference in the vegetative growth of conidia produced on PDA from 1st(A) to the 17th successive subculture, conidial production suffered a marked decrease on the 4th subculture, remaining low up to the 17th subculture (Fig. 1). After fungus passage through the host on 1st(B) subculture, conidial production was recovered to the level of 1st(A). For conidia produced on MAD, there was a decrease on the 8th subculture that was more pronounced on the 12th and 17th, which did not differ from each other. Again, productive capacity was recovered on 1st(B), similar to 1st(A). Comparison of the two media showed that PDA did not differ from MAD on 1st(A) and 1st(B), but on all remaining subcultures, the conidial production was higher on MAD (Fig. 2).
MAD, which consists of insects, favored maintenance of the conidial production of B. bassiana after a greater number of subcultures, which may be a consequence of fungal adjustment to medium containing insects. Regardless of the medium for successive subculturing, fungus passage through the host restored vegetative growth and conidial production, which had been reduced. This information is important for mass production; if conidia that give rise to matrices are not produced in media that preserve these characteristics, a periodic passage of the fungus through the host is essential.
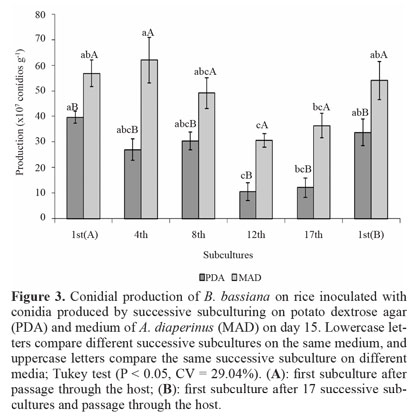
Conidial production on rice. Conidia produced on PDA did not decrease in the production from 1st(A) to the 8th subculture, but that in the 12th and 17th subcultures was reduced compared to the 1st(A) subculture. Initial production was restored after fungus passage through the host on the 1st(B) subculture (Fig. 3). For conidia produced on MAD, the production did not differ between 1st(A), 4th, and 8th subcultures. There was a decrease on the 12th passage relative to subcultures 1st(A) and 4th, and on the 17th passage relative to the 4th subculture. Furthermore, the 1st(B) subculture showed an increase in the production compared to the 12th subculture. Comparison of the two media showed that conidial production was always higher in cultures produced on MAD, reaching a 3-fold maximum on the 12th and 17th subcultures. Although conidial production on MSP and rice showed the same trend, with the largest reductions produced on PDA and maintenance of conidia production through a greater number of subcultures on MAD, the decrease in the production on rice were less pronounced.
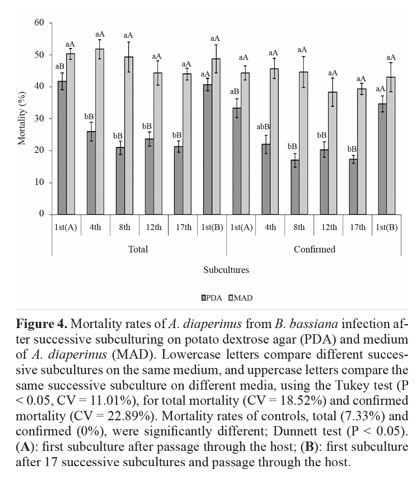
Virulence to A. diaperinus. Nutritional conditions and the number of successive subcultures significantly affected the virulence of B. bassiana in A. diaperinus. Total and confirmed mortality of insects treated with the fungus were higher than in controls. On PDA, reduced virulence was observed when using conidia from the 4th subculture (total mortality) and from the 8th subculture (confirmed mortality). The virulence was reduced until the 17th subculture, with a reduction of up to 50%. However, after fungus passage through the host on 1st(B) subculture, virulence was restored to the level of 1st(A) (Fig. 4).
Attenuation of virulence may be related to the germination and adhesion of conidia to the insect cuticle (Adames et al. 2010), which are indicative of virulence (Inglis et al. 2001). In fact, Shah et al. (2005) showed that conidia with a higher C:N ratio germinated slowly and were less virulent. Thus, the PDA medium, which is rich in carbon and poor in nitrogen, may have favored the marked reduction of virulence after successive subculturing.
Virulence was unaffected by successive subculturing on MAD, and fungus passage through the host did not favor an increase in virulence. Comparison of the two media showed that both total and confirmed mortality were higher for conidia produced on MAD, except for the 1st(B) subculture, which was similar to the results observed for the PDA medium. Maintenance of virulence after several successive cultures on MAD is probably related to nutritional aspects of the medium, which consists of insects. Hussain et al. (2010) believe that the host provides the fungus an adequate nutrition to produce conidia that are more virulent. Research has shown that media containing insect cuticle can induce the production of Pr1 enzyme (Campos et al. 2005; Tiago et al. 2002), which is considered a virulence determinant due to its ability to degrade the insect cuticle (Shah et al. 2005).
Fungus passage through the host is commonly reported as a way of recovering attenuated virulence after subculturing in vitro (Brownbridge et al. 2001; Vandenberg and Cantone 2004; Hussain et al. 2010). Culture medium is considered a less restrictive reproduction environment than the host, allowing for the development of more genetic variants of the fungus, including less virulent or avirulent derivatives. On the other hand, fungus passage through the insect can act as a filter and reduce this diversity (Scully and Bidochka 2006).
Feng et al. (1994) believe that changes in virulence of B. bassiana arising from successive subculturing are a consequence of genetic changes due to the parasexual cycle, which was reported by Paccola-Meirelles and Azevedo (1991), and that monosporic isolates can be used in addition to passage through the host.
In this study, fungus passage through the host may have eliminated the nonpathogenic variants that developed during successive subculturing on PDA. However, our results show that the relationship between successive subculturing and fungus nutriti on is complex, possibly because fungal pathogenicity is not determined by a single factor but depends on coordinated interaction between several factors (Shah et al. 2005). Thus, after selection of a fungal isolate to control a given pest, it is necessary to routinely check if virulence characteristic and sporulation have been preserved after subculturingin vitro.
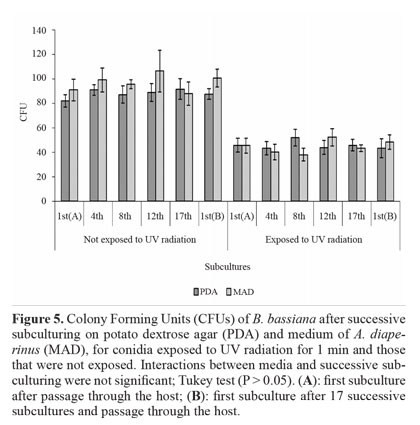
Tolerance to UV radiation. The effect of media and successive subculturing, as well as the interaction between these factors, was not significant for conidia that were exposed to UV radiation for 1 min and for those that were unexposed. Viability reduction (expressed in CFUs) after exposure was approximately 50% (Fig. 5). These results show the sensitivity of this fungus to radiation, which may compromise its effectiveness in the field. Based on the literature, this seems to be the first study on the effect of successive subculturing in vitro under different nutritional conditions on the tolerance of B. bassiana to UV radiation.
Among the abiotic factors, solar radiation is considered the most detrimental to entomopathogenic fungi (Braga etal. 2001), given it can damage cell molecules such as DNA, biomembranes, RNA, and ribosomes (Engelberg et al. 1994;Griffiths et al. 1998). Radiation tolerance is a quantitative and complex trait, involving defense mechanisms that prevent or reduce damage, and damage repair mechanisms, which come into play during cell recovery (Chelico et al. 2006). Nevertheless, Yao et al. (2010) believe that even the most tolerant isolates of B. bassiana and M. anisopliae would not survive a day of sunlight exposure. Therefore, protective measures, such as the use of photoprotective agents in formulations, are necessary (Edgington et al. 2000; Reddy et al. 2008).
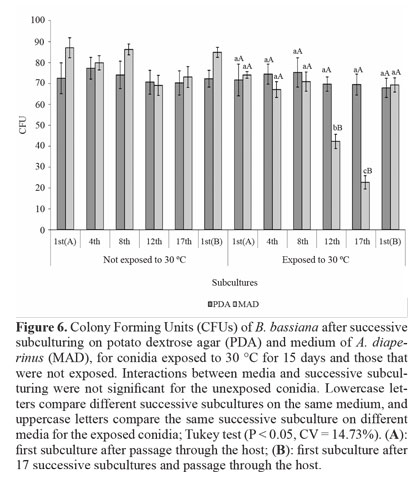
Temperature tolerance. The interaction between media and successive subculturing, and the comparison between subcultures was not significant for conidia not exposed to 30 °C. Comparison of the two media showed that MAD favored a greater number of CFUs. Upon exposure to 30 °C for 15 days, conidia produced on PDA were not affected by temperature, with the average number of CFUs similar to the unexposed conidia. Furthermore, no difference was observed between successive subcultures (Fig. 6).
Conidia produced on MAD maintained their initial toleranceup to the 8th subculture, with reduction on the 12th and 17th subcultures. After passage through the host on subculture 1st(B), the thermotolerance was recovered to that of 1st(A). Comparison of the two media showed that conidia produced on MAD were less tolerant on the 12th and 17th subcultures and did not differ from PDA in the remaining subcultures. As suggested for UV radiation, this may be the first study to assess the effect of successive subculturing in vitro, under different nutritional conditions, on temperature tolerance in this fungus.
Outside the ideal range, temperature can limit the effectiveness of fungi because it directly alters production of enzymes and toxins and indirectly influences germination, penetration, colonization, and reproduction (Alves and Lecuona 1998). One factor that confers high thermotolerance is the accumulation of trehalose in conidia (Hallsworth and Magan 1995; Singer and Lindquist 1998), a process favored by the addition of carbohydrates to the culture medium (Kim et al. 2010). This may explain the greater tolerance of conidia produced on PDA, which is rich in carbohydrates because of the presence of dextrose and potato; MAD consists primarily of insects and is thus richer in protein (Verkerk et al. 2007). In addition to being more tolerant to temperature, conidia produced on PDA maintained this characteristic after several successive subcultures in vitro. Whenever nutritional conditions of the subculture do not favor this maintenance, as was the case with subculture on MAD, the initial level of thermotolerance can be restored after passage through the host.
Conclusions
Successive subculturing in vitro and nutritional conditions affect conidial production, virulence and stress tolerance. MAD, which consists of insects, favors maintenance of vegetative growth and conidial production after a greater number of successive subcultures than on PDA. Even when there is a decrease in conidial production, it is less marked than the
conidia produced on PDA. Virulence is not affected by successive subculturing on MAD but is reduced on PDA, which is the opposite of the effect on temperature tolerance. Conidia of B. bassiana are sensitive to UV radiation, although this feature is not affected by successive subculturing in different media. Successive fungal subculturing attenuates virulence characteristics, conidial production, and temperature tolerance, which can be restored by passage through the host.
Literature cited
ADAMES, M.; FERNÁNDEZ-RUVALCABA, M.; PENÃ-CHORA, G.; HERNÁNDEZ-VELÁSQUEZ, V. M. 2010. Effects of passages through a suitable host of the fungus, Metarhizium anisopliae, on the virulence of acaricide-susceptible and resistant strains of the tick, Rhipicephalus microplus. Journal of Insect Science 11: 1-13. [ Links ]
ALVES, S. B.; LECUONA, R. E. 1998. Epizootiologia aplicada ao controle microbiano de insetos. pp. 97-170. En: Alves, S. B. (Ed.). Controle Microbiano de Insetos. FEALQ Piracicaba. Brazil. 940 p. [ Links ]
ALVES, S. B.; ALMEIDA, J. E. M.; MOINO JR., A.; ALVES, L. F. A. 1998. Técnicas de laboratório. pp. 637-712. En: Alves, S. B. (Ed.). Controle Microbiano de Insetos. FEALQ Piracicaba. Brazil. 940 p. [ Links ]
BARBOSA, F. R.; MOREIRA, W.A; SANTOS, G. 1985. Efeito de sucessivas repicagens em arroz na virulência de Metarhizium anisopliae (Metsch) Sorokin para Deois flavopicta. Pesquisa Agropecuária Brasileira 20: 1115-1118. [ Links ]
BRAGA, G. U. L.; FLINT, S. D.; MILLER, C. D.; ANDERSON, A. J.; ROBERTS, D. W. 2001. Variability in response to UV-B among species and strains of Metarhizium isolated from sites at latitudes from 61°N to 54°S. Journal of Invertebrate Pathology 78: 98-108. [ Links ]
BROWNBRIDGE, M.; COSTA, S.; JARONSKI, S. T. 2001. Effects of in vitro passage of Beauveria bassiana on virulence to Bemisia argentifolii. Journal of Invertebrate Pathology 77: 280-283. [ Links ]
CAMPOS, R. A.; ARRUDA, W.; BOLDO, J. T.; SILVA, M. V.; BARROS, N. M.; AZEVEDO, J. L.; SCHRANK, A.; VAINSTEIN, M. H. 2005. Boophilus microplus infection by Beauveria amorpha and Beauveria bassiana: SEM analysis and regulation of subtilisin-like proteases and chitinases. Current Microbiology 50: 257-261. [ Links ]
CHELICO, L.; HAUGHIAN, J. L.; KHACHATOURIANS, G. G. 2006. Nucleotide excision repair and photoreactivation in the entomopathogenic fungi Beauveria bassiana, Beauveria brongniartii, Beauveria nivea, Metarhizium anisopliae, Paecilomyces farinosus and Verticillium lecanii. Journal of Applied Microbiology 100: 964-972. [ Links ]
EDGINGTON, S.; SEGURA, H; LA ROSA, W.; WILLIAMS, T. 2000. Photoprotection of Beauveria bassiana: Testing simple formulations for control of the coffee berry borer. International Journal of Pest Management 46: 169-176. [ Links ]
ENGELBERG, D.; KLEIN, C.; MARTINETTO, H.; STRUHL, K.; KARIN, M. 1994. The UV response involving the Ras signaling pathway and AP-1 transcription factors is conserved between yeast and mammals. Cell 77: 381-390. [ Links ]
FENG, M. G.; PROPAWSKI, T. J.; KHACHATOURIANS, G. G. 1994. Production, formulation and application of the entomopathogenic fungus Beauveria bassiana for insect control: current status. Biocontrol Science and Technology 1: 3-34. [ Links ]
GRIFFITHS, H. R.; MISTRY, P.; HERBERT, K. E.; LUNEC, J. 1998. Molecular and cellular effects of ultraviolet light-induced genotoxicity. Critical Reviews in Clinical Laboratory Sciences 35: 189-237. [ Links ]
HALL, R. A. 1980. Effect of repeated subculturing on agar and passaging through an insect host on pathogenicity, morphology, and growth rate of Verticillium lecanii. Journal Invertebrate Pathology 36: 216-222. [ Links ]
HALLSWORTH, J. E.; MAGAN, N. 1995. Manipulation of intracellular glycerol and erythritol enhances germination of conidia at low water availability. Microbiology 141: 1109-1115. [ Links ]
HUSSAIN, A.; TIAN, M.; HE, Y.; LEI, Y. 2010. Differential fluctuation in virulence and VOC profiles among different cultures of entomopathogenic fungi. Journal of Invertebrate Pathology 104: 166-171. [ Links ]
INGLIS, D. G.; GOETTEL, M. S.; BUTT, T. M.; STRASSER, H.2001. Use of hyphomycetous fungi for managing insect pests. pp. 23-69. En: BUTT, T. M.; JACKSON, C. W.; MAGAN, N. (Eds.). Fungi as biocontrol agents: Progress, problems and potential, CAB International, Wallingford, UK. 390 p. [ Links ]
KIM, J. S.; JE, Y. H; ROH, J. Y. 2010. Production of thermotolerant entomopathogenic Isaria fumosorosea SFP-198 conidia in corn-corn oil mixture. Journal of Industrial Microbiology and Biotechnology 37: 419-423. [ Links ]
NASCIMENTO, E.; SILVA, S. H da, MARQUES, E. dos R.; ROBERTS, D. W.; BRAGA, G. U. L. 2010. Quantification of cyclobutane pyrimidine dimers induced by UVB radiation in conidia of the fungi Aspergillus fumigatus, Aspergillus nidulans,Metarhizium acridum and Metarhizium robertsii. Photochemistry and Photobiology 20: 1-8. [ Links ]
PACCOLA-MEIRELES, L. D.; AZEVEDO, J. L. 1991. Parasexuality in Beauveria bassiana. Journal of Invertebrate Pathology 57: 172-176. [ Links ]
QUESADA-MORAGA, E.; VEY, A. 2003. Intra-specific variation in virulence and in vitro production macromolecular toxins active against locust among Beauveria bassiana strains and effectsof in vivo and in vitro passage on these factors. BioControl Science and Technology 13: 323-340. [ Links ]
REDDY, N. P.; KHAN, D. K. U.; VICTOR, J. S.; SHARMA, H. C. 2008. Assessment of the suitability of Tinopal as an enhancing adjuvant in formulations of the insect pathogenic fungus Beauveria bassiana (Bals.) Vuillemin. Pest Management Science 64: 909-915. [ Links ]
SANTORO, P. H.; NEVES, P. M. O. J.; ALEXANDRE, T. M.; ALVES, L. F. A. 2007. Interferência da metodologia nos resultados de bioensaios de seleção de fungos entomopatogênicos para o controle de insetos. Pesquisa Agropecuária. Brasileira 47: 483-489. [ Links ]
SANTORO, P. H.; NEVES, P. M. O. J.; ALEXANDRE, T. M.; SARTORI, D.; ALVES, L. F. A; FUNGARO, M. H. 2008. Selection of Beauveria bassiana isolates to control Alphitobius diaperinus. Journal of Invertebrate Pathology 97: 83-90. [ Links ]
SCULLY, L. R.; BIDOCHKA, M. J. 2006. The host acts as a genetic bottleneck during serial infections: an insect-fungal model system. Current Genetics 50: 335-345. [ Links ]
SHAH, F. A.; WANG, C. S.; BUTT, T. M. 2005. Nutrition influences growth and virulence of the insectpathogenic fungus Metarhizium anisopliae. FEMS Microbiology Letters 251: 259-266. [ Links ]
SHAH, F. A.; ALLEN, N.; WRIGHT, C. J.; BUTT, T. M. 2007. Repeated in vitro subculturing alters spore surface properties and virulence of Metarhizium anisopliae. FEMS Microbiology Letters 276: 60-66. [ Links ]
SINGER, M. A.; LINDQUIST, S. 1998. Multiple effects of trehalose on protein folding in vitro and in vivo. Molecular Cell 1: 639-648. [ Links ]
TIAGO, P. V.; FUNGARO, M. H. P.; FURLANETO, M. C. 2002. Cuticledegrading proteases from the entomopathogen Metarhizium flavoviride and their distribution in secreted and intracellular fractions. Letters in Applied Microbiology 34: 91-94. [ Links ]
VANDENBERG, J. D.; CANTONE, F. A. 2004. Effect of serial transfer of three strains of Paecilomyces fumosoroseus on growth in vitro, virulence, and host specificty. Journal of Invertebrate Pathology 85: 40-45. [ Links ]
VERKERK, M. C.; TRAMPER, J.; TRIJP, J. C. M. van; MARTENS, D. E. 2007. Insect cells for human food. Biotechnology Advances 25: 198-202. [ Links ]
YAO, S.; YING, S.; FENG, M.; HATTING, J. L. 2010. In vitro and in vivo responses of fungal biocontrol agents to gradient doses of UV-B and UV-A irradiation. BioControl 55: 413-422. [ Links ]
Suggested citation:
SANTORO, P. H.; ZORZETTI, J.; CONSTANSKI, K.; NEVES, P. M. O. J. 2014. Conidial production, virulence, and stress tolerance of Beauveria bassiana conidia after successive in vitro subculturing. Revista Colombiana de Entomología 40 (1): 85-90. Enero-julio 2014. ISSN 0120-0488.