Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488
Rev. Colomb. Entomol. vol.40 no.1 Bogotá Jan./June 2014
Sección control
Heterorhabditis amazonensis RSC5 (Rhabditida: Heterorhabditidae) movement and host recognition
Desplazamiento y capacidad de búsqueda del nematodo entomopatógeno nativo Heterorhabditis amazonensis RSC5 (Rhabditida: Heterorhabditida)
VANESSA ANDALÓ1, GRAZIELLE FURTADO MOREIRA2 and ALCIDES MOINO JUNIOR3
1 Ph. D. Entomologia. Universidade Federal de Uberlândia, Campus Monte Carmelo, Av. Goiás, 2000, 38500-000 Monte Carmelo, MG, Brazil. vanessaandalo@gmail.com. Corresponding author.
2 Ph. D. Entomologia. UNESP, FCAV, Campus Jaboticabal, Via Prof. Paulo Donato Castellane, Departamento de Fitossanidade, 14884 900 Jaboticabal, SP, Brazil.
3 Ph. D. Entomologia. Universidade Federal de Lavras, Departamento de Entomologia, CP 3037, 37200- 000, Lavras, MG, Brazil.
Received: 19-Apr-2013 • Accepted: 27-Apr-2014
Abstract: Response of Heterorhabditis amazonensis RSC5 to compounds released by different host insects and its virulence level to several insect hosts like Galleria mellonella, Mycotretus apicalis and Tenebrio molitor were evaluated in this study, and compared with other entomopathogenic nematode species like Steinernema carpocapsae All and Steinernema riobrave 355. Tests were performed in Petri dishes with agar-water 2% to determine nematode movement toward the insect with and without opportunity of choosing different insect hosts. Evaluations were made quantifying the proximity of infective juveniles (IJs) to the insect as a source of allurement. In order to determine the displacement of IJs in a closed soil condition, a test was carried out in an arena with sand. The nematode was virulent to the target insects. When nematode and insect were released on agar-water, IJs moved toward the stimulus, with H. amazonensis howing preference for certain insects. In the arena with sand S. carpocapsae caused lower insect mortality (70% ± 8.9 for G. mellonella) than H. amazonensis and S. riobrave (80% ± 6.5 and 99% ± 0.0). Heterorhabditis amazonensis was able to find and choose its hosts (G. mellonella and T. molitor), similarly to S. riobrave behavior, and located them more effectively than S. carpocapsae. The virulence of H. amazonensis was thus similar to S. riobrave, and this characteristic could be promising to introduce this native species in integrated pest management programs.
Key words: Behavior. Chemical stimulus. Chemoreception. Steinernematidae.
Resumen: La respuesta de Heterorhabditis amazonensis RSC5 en comparación con otras especies de nematodos entomopatógenos como Steinernema carpocapsae All y Steinernema riobrave 355, a los compuestos liberados por diferentes hospederos (Galleria mellonella, Mycotretus apicalis y Tenebrio molitor) y su nivel de virulencia a estos insectos fue evaluada. Las pruebas se realizaron en placas de Petri con agar-agua 2% para determinar el movimiento de los nematodos con y sin posibilidad de escogencia por diferentes hospederos. Se cuantificó la proximidad de juveniles infectivos (JIs) al hospedero como una fuente de atracción. Con el fin de determinar el desplazamiento de JIs en una condición similar al suelo, un ensayo se llevó a cabo en un área con arena. Los nematodos fueron virulentos para los hospederos. Cuando los nematodos e insectos fueron puestos en agar-agua, JIs se movieron hacia el estímulo, con preferencia de H. amazonensis a ciertos insectos. En la arena, S. carpocapsae causó menor mortalidad (70% ± 8,9 para G. mellonella) que H. amazonensis y S. riobrave (80% ± 6,5 y 99% ± 0,0). Heterorhabditis amazonensis fue capaz de encontrar y elegir a sus hospederos (G. mellonella y T. molitor) similar al comportamiento de S. riobrave y localizar sus hospederos con más eficacia que S. carpocapsae. De este modo, la virulencia de H. amazonensis fue similar a S. riobrave y esta característica podría ser promisoria para introducir esta especie nativa en programas de manejo integrado de plagas.
Palabras clave: Comportamiento. Estímulo químico. Quimiorrecepción. Steinernematidae.
Introduction
Nematodes are strongly limited in their use of visual information, but rather use chemical and tactile cues to communicate and behave. Chemoreception is undoubtedly the most important source of stimulus for nematodes (Jones 2002). Nematodes' sensory apparatus allows them to use chemical, electrical, light, mechanical, and temperature stimuli to orientate, move, and locate a sexual partner, as well as energy sources in the soil (Jones 2002; Lee 2002). Some nematodes' sense organs are exposed to the external environment by a pore in the cuticle, primarily functioning as chemoreceptors (Hilliard et al. 2002).
In the biological control context, predators respond to physical and chemical stimuli that lead them to potential prey (Miller and Strickler 1984). So, the ability of the infective juveniles of entomopathogenic nematodes (EPN) to locate the host and to disperse actively in soil is fundamental to the successful application of many species of these organisms (Cutler and Webster 2003). The nematode's host search behavior and its infective capacity can be affected by many factors, including soil characteristics (Kaya 1990), electrical current (Shapiro-Ilan et al. 2009) and the infective juveniles' (IJ) search strategy ("ambush" and "cruiser"). Thus, the IJs are responsible for finding and penetrating a suitable host, and they use chemoreception during the search for the host, responding to volatile compounds and temperature (Lewis et al. 1992; Grewal et al. 1994). The stimuli obtained by IJs might be associated with the host directly or with their products, like feces or volatiles from the insect's metabolism. Lewis et al. (2006) observed that nematode orientation and aggregation is due to unspecific signaling, such as CO2 emissions. According to Kaplan et al. (2012), infective juveniles (IJs) are also able to recognize an already parasitized host moving very actively away from the point of release on a dish with agar. However, nematodes adopted different strate gies to find their host, following chemical stimuli, but thesestrategies can differ between species or strains. Grewal et al. (1994) concluded that IJs of Heterorhabditis megidis (HO1 strain) cruise to find hosts. However, it is not known if the searching behavior presented by this nematode strain can be the same as in another H. megidis strain isolated.
In this context, the objectives of this research were to evaluate the virulence of EPN species like Heterorhabditis amazonensis RSC5, Steinernema carpocapsae All and Steinernema riobrave 355 to different host insects and the response to compounds released by different bait insects.
Material and methods
Heterorhabditis amazonensis RSC5 were obtained from a nematode collection at the University of Lavras (MG), Brazil, and were maintained in Erlenmeyer flasks in acclimated chambers at a temperature of 16 ± 1 °C, in an aqueous suspension containing 500 to 1,000 IJs/mL. They were identified by morphological and molecular techniques. Steinernema carpocapsae All and Steinernema riobrave 355 were acquired from North Carolina, and Texas, USA, respectively, and were identified by morphological techniques. Steinernema carpocapsae and S. riobrave were chosen because of their known pattern of search behavior on soil, and were used as a control to study H. amazonensis RSC 5 displacement. Nematodes were reared on Galleria mellonella larvae (Linnaeus, 1758) (Lepidoptera: Pyralidae), using an artificial diet modified by Parra (1998). The nematode culture and maintenance were as described by Molina and López (2001). After nematode suspension purification, the suspension was diluted and quantified using a stereo-microscope.
Target insects were G. mellonella, Mycotretus apicalis Lacordaire, 1842 (Coleoptera: Erotylidae) and Tenebrio molitor Linnaeus, 1758 (Coleoptera: Tenebrionidae) obtained from the Entomology Laboratory of Lavras University (Lavras, MG, Brazil). Ascia monuste (Godart, 1818) (Lepidoptera: Pieridae) obtained from kale crop, Cornitermes cumulans (Kollar, 1832) (Isoptera: Termitidae) from pasture, Musca domestica Linnaeus, 1758 (Diptera: Muscidae) from manureç and Astylus variegatus (Germar, 1824) (Coleoptera: Melyridae) from corn crop. Galleria mellonella was raised according to Dutky et al. (1964), using an artificial diet modified by Parra (1998). Tenebrio molitor was reared in the laboratory and larvae and adults were fed on wheat bran and chayote or carrot. Mycotretus apicalis was raised following Moreira et al. (2010) techniques, using Pleurotus sajor-caju (Fr.) Singer as food source.
Virulence evaluation. Virulence of S. carpocapsae, S. riobrave and H. amazonensis RSC5 to G. mellonella larvae, T. molitor larvae, A. monuste larvae, C. cumulans adult, M. domestica larvae, M. apicalis larvae and A. variegatus adult was evaluated. Insects were placed individually in plastic cups of 50 mL volume and covered with 40 g of sterilized sand. A nematode suspension was prepared with water and 0.5 mL (800 IJs/insect) was applied for each replication, with 7.5 mL of water (20% of the weight of sand) being added to each cup. Treatments were designed by using the three nematodes tested against six different insects. As control treatment, water was applied instead of IJ suspension, totalizing seven treatments for each nematode, plus the control (insects with water only). After that, the plastic cups were closed with a plastic film, arranged in a completely randomized design and incubated in a growth chamber at 24 ± 1 °C, RH of 70 ± 10% with 12 h photoperiod. Twelve replications of each treatment (three nematodes versus six insects and the control) were prepared. The mortality evaluation was done after five days of application of IJs and to confirm the mortality caused by nematodes a dissection of dead insects was performed, observing the presence of IJs in stereoscopic microscope.
Displacement of nematodes toward insects with no choice. Test was performed in Petri dishes (9 cm) by adding 30 mL of agar-water 2% to the dishes, which were sealed with Parafilm®. The insects were placed within a tip (used for micropipette of 100 - 1.000 µL volume), closed with cotton on one side of the dish, and the IJs were released on the opposite side. This procedure was performed to evaluate movement of the nematodes, S. carpocapsae, S. riobrave and H. amazonensis RSC5, toward different insects. Insects tested were G. mellonella, T. molitor, C. cumulans, M. apicalis and A. variegatus, so treatments were compounded by three nematodes tested with five insects, totaling fifteen treatments plus the control, which was prepared using a Petri dish but instead of an insect inside, the tip was empty. Dishes were kept in an environmentally controlled chamber at 24 ± 1 °C, RH of 70 ± 10% with 24 h scotophase. The experiment was conducted in a completely randomized design and seven replications per treatment were used. To perform the evaluation after 12 h the IJs observed in a stereoscopic microscope that were located within a 1 cm diameter around the tip were considered attracted to the insect. Percentage of displacement was calculated based on the number of nematodes that moved related to the number of nematodes applied.
Displacement of nematodes toward insects with choice. In the following test, a procedure similar to that of the previous experiment was executed; however, beyond the tip containing the insect on one side of the dish, another tip, in this case empty, was placed on the opposite edge. An aqueous suspension containing 75 IJs in 0.03 mL was released in the middle of the dish. Test had seven repetitions per treatment. Dishes were kept in an environmentally controlled chamber at 24 ± 1 °C, RH of 70 ± 10% with 24 h scotophase. After 12 h, in order to evaluate the attraction of IJ toward the stimulus, the nematodes observed in a stereoscopic microscope that were disposed 1 cm diameter around the tip were considered attracted to that. Percentages of nematodes that moved toward the tips were calculated.
Displacement of nematodes with choice between two insects. In this experiment the nematodes were placed on one side of the dish; the tip containing one of the tested insect species and other tip with insect inside in opposite positions, in order to evaluate if the IJs chose the host insect. The treatments were G. mellonella x T. molitor, G. mellonella x C. cumulans, G. mellonella x M. apicalis, G. mellonella x A. variegatus, T. molitor x C. cumulans, T. molitor x M. apicalis, T. molitor x A. variegatus, C. cumulans x M. apicalis, C. cumulans
x A. variegatus and M. apicalis x A. variegatus. Seven repetitions were performed for each combination of insects and treatment control. No insects were placed in the tips. At the center of the dish were released 75 IJs in 0.03 mL of aqueous suspension through a hole that was thereafter sealed with Parafilm®. The dishes were kept in an environmentally controlled chamber at 24 ± 1 °C, RH of 70 ± 10% with 24 h scotophase. Evaluations were made after 12 h in stereoscopic microscope, observing and quantifying the percentage of IJs close to the insects used as a source of allurement.
Displacement of nematodes with choice among insects. For this study the IJ were placed in relation to the source of stimulation, in a free choice arena. Six divisions were performed on the Petri dish of 15 cm diameter with agar-water 2%. In each division was placed a tip containing one of the insects used as stimulus for nematode displacement. In this case, we used five insects at the same time, which were G. mellonella, T. molitor, C. cumulans, M. apicalis and A. variegatus. In one division an empty tip was added, as the control treatment. Nematodes tested were S. carpocapsae, S. riobrave and H. amazonensis RSC5. An aliquot of 0.1 mL containing 400 IJs was released through a hole at the center of the dish and after the application the hole was closed with Parafilm®. The area of each division was arranged to be approximately 29.5 cm2. Fifteen replications were performed and the nematode quantification close to insects was performed after 12 hours using a stereoscopic microscope calculating the percentage of nematodes close to the insects. Dishes were kept in an environmentally
controlled chamber at 24 ± 1 °C, RH of 70 ± 10% with 24 h scotophase.
Nematode displacement on sand. In order to determine the displacement of IJs in a closed condition of soil, a similar test to the one above was developed, but in an arena containing sand instead of agar. A plastic plate, measuring 20 cm in diameter and 4 cm in height, was used as a container to set up the test. Eleven hundred grams of autoclaved sand were added to the container and wetted with 330 mL of water (30% of the sand weight). In the center of the arena (sand circle), on the central 5 cm diameter of the dish, were released 5 mL of nematode suspension containing 1,000 IJs. To avoid insect movement in the arena, they were placed in cages (made of high density polyethylene with 0.2 cm diameter) with wire measuring 3.0 x 3.0 cm, and inside them was added a rubber tube 1.0 cm long and 0.5 cm in diameter, to prevent crushing the insect in the cage. Cages were placed on the opposite side to that where the nematode was released. Six divisions were performed, one for each insect-bait (G. mellonella, T. molitor, C. cumulans, M. apicalis and A. variegatus) and one for the treatment control, in which an empty cage was added in one of the fields of the container. Each division had a volume of sand of approximately 209.4 cm3. Containers were maintained in a greenhouse with an average temperature of 20.2 °C and air of 58% relative humidity. Ten repetitions were executed. Insects' mortality was evaluated eight days after the displacement. Insect mortality caused by nematodes a dissection was confirmed under stereoscopic microscope.
Statistical analysis. The experiments were submitted to a completely randomized design. The data were subjected to ANOVA and to Tukey comparison test (P < 0.05).
Results and discussion
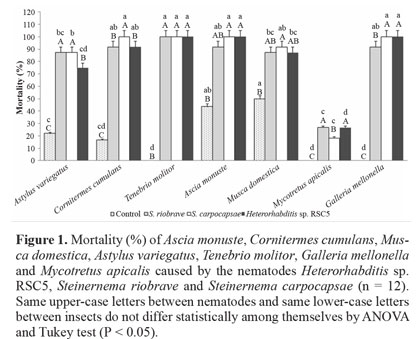
Virulence evaluation. Heterorhabditis amazonensis was the main cause of mortality in all hosts, like other EPN species. Lowest mortality in all species was in M. apicalis. H. amazonensis, S. riobrave and S. carpocapsae were pathogenic to the insects tested in this study and showed differences in virulence level. Heterorhabditis amazonensis caused lower mortality of M. apicalis at 26% ± 13.6. Steinernema carpocapsae and S. riobrave also caused low mortality to M. apicalis, and all other insects had mortality above 80%. There was no difference among the three nematode species as regards causing mortality of T. molitor. However, there was a difference comparing the nematodes for the other insects. For example, H. amazonensis caused higher mortality in G. mellonella (100% ± 0.0) than S. riobrave (91% ± 8.3) (Fig. 1). The low mortality of M. apicalis by the three nematode species, under these conditions, suggests that this insect is not a good host for the EPNs tested. In the control there was no mortality of G. mellonella, T. molitor and M. apicalis, and low mortality (20%) for C. cumulans and A. variegatus. The mortality for M. domestica and A. monuste was 40% and 50%, respectively. The insect mortality in the control was statistically different from the treatments with nematodes (Fig. 1). Since it was found that the EPN are pathogenic to the tested insects, they were used for further studies to evaluate nematode movement to ward host insect. As the mortality of A. monuste and M. domestica in the control was higher than for the other insects, they were not used in subsequent trials.
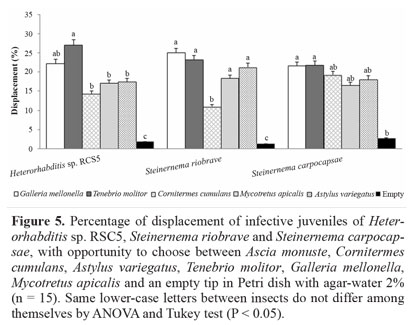
Displacement of nematodes toward insects with no choice. When the infective juveniles were released over agar-water 2% on one side of the Petri dish opposite to an insect trap, the IJs moved toward the stimulation source (insect). Some IJs were seen inside the tips. They were not quantified because of the vagueness of collecting all that were within the tip, and some may have already penetrated the insect. In the control treatment, in which the tip was empty, no uniform orientation of the nematodes toward the tip was observed. Orientation was perceived when the tips had an insect inside them. Heterorhabditis amazonensis was closer to the baits G. mellonella and T. molitor. Seventy-eight percent (78% ± 6.6) and 81% ± 4.6 of the IJs released in the dish were near the tip with these insects. All the other insects also had higher percentage of IJs near the tip (P < 0.05), differing significantly from the control. Steinernema riobrave showed large displacement of the IJs toward the insects, but G. mellonella, T. molitor and M. apicalis, with the highest percentage of IJs near the tips, differed from C. cumulans and A. variegatus. For C. cumulans and A. variegatus, IJs were also observed near the tip, differing from the control treatment. Steinernema carpocapsae approached primarily T. molitor, differing from the other insects tested, G. mellonella, M. apicalis, C. cumulans and A. variegatus, for which the IJs were also observed near to their tips, differing from the control (Fig. 2). There were significant differences between G. mellonella and A. variegatus with regard to the closeness of the nematodes H. amazonensis, S. riobrave and S. carpocapsae. There were no statistical differences among the three nematodes when they were tested against T. molitor. With regard to M. apicalis the nematode that was closest to the tip was S. riobrave (Fig. 2). So, in this case, it was found that the three nematodes tested displaced themselves in the treatments containing insects, which differed from the control, highlighting the presence of the insect as the director of motion. According to Lewis (2002), entomopathogenic nematodes respond differently to chemical volatiles of their hosts. So, although M. apicalis was not evaluated as a good host for H. amazonensis, S. riobrave and S. carpocapsae (Fig. 1), in the present assay these nematodes directed themselves to M. apicalis, so this insect was retained for the later assays.
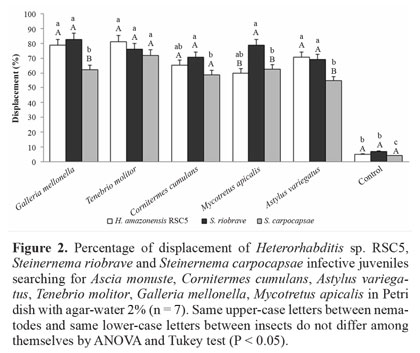
Displacement of nematodes toward insects with choice. In the experiment where the IJs were released in the central part of the Petri dish and they had an opportunity to choose between the tip with the insect or the empty tip, there was a preference for the tip with the bait insect inside. No IJs clustering near the empty tip were observed, suggesting that the IJs are not directing themselves due to the presence of the tip, but to the insect. According to Boff and Smits (2001), IJs move by sinuous movements and in permanent contact with the agar surface. Presence of a host insect stimulates their dispersion, and nematodes move randomly over the agar surface for more than one hour before a response to the host can be observed. After 90 min, nearly 40% of the IJs aggregate around G. mellonella. A higher percentage of H.amazonensis IJs was seen near the tips with G. mellonella, T. molitor and A. variegatus than with the other tested insects, whereas a higher percentage of S. riobrave was found near the tips with G. mellonella and T. molitor than with the other insects. A regards S. carpocapsae, more IJs clustered around T. molitor. Steinernema riobrave and H. amazonensis were the nematodes that moved closer to G. mellonella and A. variegatus. There was no statistical difference among the three nematodes tested and T. molitor. In general, the majority of nematodes also moved toward C. cumulans, M. apicalis and A. variegatus (Fig. 3). Steinernema riobrave showed great ability to orient itself toward all the sources of stimulus. More than 70% of nematodes released into the plate moved toward the insects. For the other nematodes, more than 60% also moved toward the insects, showing the displacement to the side of the dish with the tip containing the insect rather than the empty ones (Fig. 3). These results indicate that IJs were attracted by compounds released by insects. Some nematodes were unaccounted for because they were positioned at points distant from the area near the tip that was used as the counting area. Furthermore, some IJs were seen within the tips with insect, and were not quantified because of the imprecision of collecting all juveniles remaining within the tips, since some of them may have already penetrated the insect. It is possible the IJs were attracted to the insects due to the release of volatile compounds regardless of the specific search behavior used by nematodes.
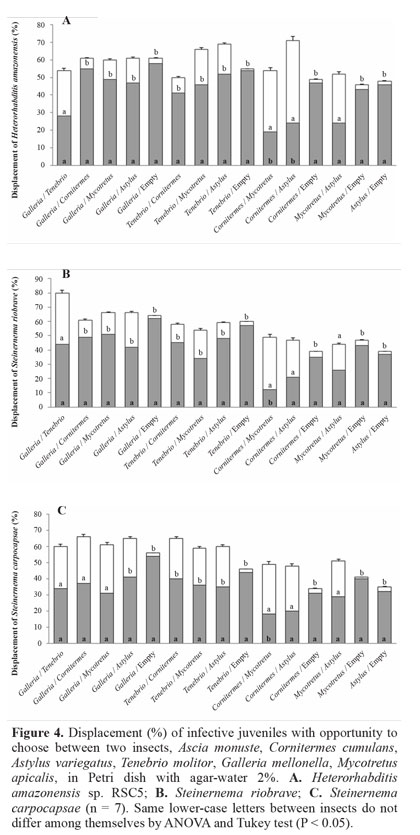
Displacement of nematodes with choice between two insects. Heterorhabditis amazonensis had preference for G.mellonella and T. molitor compared to the other hosts tested (C. cumulans, A. variegatus and M. apicalis). Cornitermes cumulans was preferred by this nematode in relation to M. apicalis and A. variegatus and there was no preference between these two insects (Fig. 4A). The same result was found with S. riobrave, with no statistical difference between G. mellonella and T. molitor. Cornitermes cumulans was chosen over M. apicalis and there was no difference when tested with A. variegatus. There was no significant difference between M. apicalis and A. variegatus (Fig. 4B). Steinernema carpocapsae was the nematode with the lowest difference regarding preference for hosts. Galleria mellonella was preferred compared to A. variegatus, but there was no preference when placed with choice among other insects. Tenebrio molitor was chosen over the other insects, except to G. mellonella where no difference was observed (Fig. 4C). In the control, where one side had an insect and the other side did not, the nematode sought the side with the insect (Figs. 4 A-C). When two insects were placed in the same area H. amazonensis showed greater preference for presenting some hosts than S. carpocapsae. Based on these results we can infer that the search behavior of H. amazonensis is in direct pursuit of its host, mainly by the chemical compounds released by the insects. This characterizes the behavior of a cruiser nematode, which searches for the host in the soil. Steinernema carpocapsae presents ambusher behavior, waiting for the host insect to pass by. It is possible that this nematode does not use many chemical cues to find its host.
Grewal et al. (1994) state that some entomopathogenic nematodes, such as S. carpocapsae and Steinernema scapterisci, wait for passing hosts at or near the soil surface, whereas other species such as Heterorhabditis bacteriophora, H. megidis, Steinernema glaseri and Steinernema anomali continuously move through the soil in search of hosts. Also, active dispersal movement has advantages for IJs because it increases the chances for encountering a susceptible host as well as for survival (Ishibashi and Kondo 1990; Kaya and Gaugler 1993). For example, Lewis et al. (1993) found thatç S. glaseri responded positively to volatile cues from an insect host, and that this response was eliminated if CO2 was removed. A similar response was found by Grewal et al. (1994) for other cruiser Steinernema spp. and for two species of Heterorhabditis.
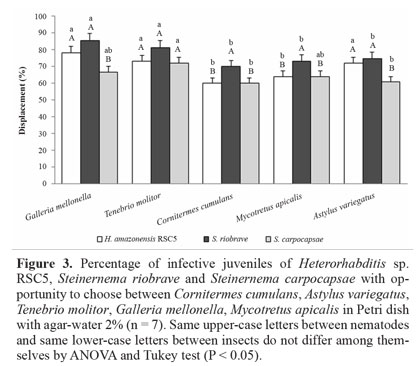
Displacement of nematodes with choice among insects. In the test where the insects were placed in the arena and the nematode was released in the center, the preference of the nematodes for certain hosts was observed. When G. mellonella, T. molitor, C. cumulans, M. apicalis and A. variegatus were arranged equidistantly and the IJs of H. amazonensis were released in the center of the arena T. molitor were preferred in relation to other insects, followed by G. mellonella. A second group of insects was formed differing for the treatment without insect (control) (Fig. 5). There were no statistical differences in preference among C. cumulans, M. apicalis and A. variegatus for H. amazonensis. The presence of IJs was observed near these insects, differing statistically from the control without insect. Steinernema carpocapsae showed no preference for any of the insects tested, and there was no statistical difference among the treatments. The group of bait insects differed only for the treatment control, where no insect was placed in the delimited region (Fig. 5). The result obtained with S. carpocapsae was similar to the previous ones, where S. carpocapsae showed no preference for specific insect (Fig. 4). Both H. amazonensis and S. riobrave showed a preference for certain groups of insects, G. mellonella and T. molitor, which reinforces the idea that these nematodes tracked their hosts preferably by volatile compounds. Lewis (2002) describe S. carpocapsae as an ambusher nematode, while S. riobrave presents an intermediate behavior and H. bacteriophora uses a considerable displacement deep in the soil, as well as cruiser nematode behavior in the soil to find their host. Gaugler et al. (1991) found that the last instar of G. mellonella larva produced more CO2 per hour than last instar larvae of Coleopteran species, Leptinotarsa decemlineata and Popillia japonica, and consequently attracted more IJs. Thurston et al. (1994) observed that IJs of S. carpocapsae were attracted to CO2 and feces produced by G. mellonella larvae, but were repelled by L. decemlineata feces.
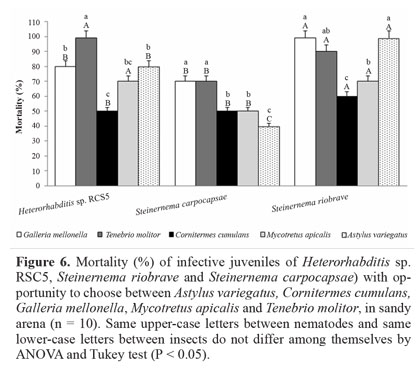
Nematode displacement in sand. When sand was used as substrate, H. amazonensis showed a preference for T. molitor, followed by G. mellonella, M. apicalis and A. variegatus. The preferred insects for S. carpocapsae were G. mellonella and T. molitor, followed by the other three insect tested, A. variegatus, M. apicalis and C. cumulansbut there was a statistical difference for A. variegatus. Steinernema riobrave demonstrated preference for G. mellonella and A. variegatus, differing statistically from the other two insects, M. apicalis and C. cumulans, with a lower percentage of mortality (Fig. 6). Nermut' et al. (2012) observed nematode displacement in agar and sand, and tested the cues of G. mellonella and a slug (Deroceras reticulatum Muller, 1774) to verify the displacement of Phasmarhabditis hermaphrodita (Nematoda: Rhabditida) and Steinernema feltiae. Both nematodes were able to detect host volatile cues and move toward them in the substrates. In this study it was possible to determine IJ movement toward the host in a condition close to the one that occurs in the soil; since the soil matrix is composed of three phases (gas, liquid, and solid) it can impact mobility, behavior, signaling, and interaction between organisms belowground in a different manner to aboveground (Hedlund et al. 2004); and it was possible to verify that the nematodes S. carpocapsae, S. riobrave and H. amazonensis were able to find and cause the mortality of their hosts in a sandy arena.
Steinernema carpocapsae caused lower mortality than H. amazonensis and S. riobrave. This may have happened because the nematode did not find the hosts through chemical cues. It is also possible that they recognized the volatile compounds released by insects, and may have awaited the passage of the host, a condition that matches with their behavior of searching. According to Grewal et al. (1994) S. carpocapsae is classified as an ambush strategist, for whom volatiles are unimportant in host-finding at a distance; most IJs remain near the soil surface where they lift their body into the air in order to attach to a passing host (Campbell and Gaugler 1997). Ambush strategists are considered to be more successful at infecting mobile, surface dwelling hosts, while cruisers are expected to infect less mobile underground hosts (Gaugler et al. 1997). Nevertheless, S. carpocapsae may sometimes perform better than its described foraging strategy might predict in parasitizing subterranean root-dwelling insects (Ennis et al. 2010).
Steinernema riobrave and H. amazonensis caused high mortality of different insects such as G. mellonella, T. molitor and A. variegatus (Fig. 6), which showed the choice for the different available hosts, performing their search behavior toward the volatile compounds released by insects. These results corroborate the results obtained by Grewal et al. (1994) which observed a cruising search in which IJs of H. bacteriophora and H. megidis move continuously through the environment, searching for prey and cruising to find hosts. Therefore, H. amazonensis showed behavior similar to that of cruiser nematodes, in which it was possible to observe, in agar and in sand, the displacement of their IJs toward the bait and the preference for determined hosts, emphasizing the search using chemical volatiles of the insect.
Conclusion
Heterorhabditis amazonensis had behavior similar to that of S. riobrave, typified by their cruiser behavior, and moved through the substrates to locate its hosts more effectively than S. carpocapsae, a recognized ambusher nematode. In the same way, the virulence of H. amazonensis was similar to S. riobrave and these characteristics could justify introducing this native species in programs of integrated pest management. Furthermore, a preference by specific hosts could be verified, observing the presence of this nematode in a larger number close to G. mellonella and T. molitor, used as insect baits. Beyond that, it is possible to verify that H. amazonensis IJs were targeted by insect volatiles and, based on that statement, the importance of using adequate species of EPNs in programs of biological control is made clear, since the nematode can find its preferred host and cause a better result in controlling the target pest. Understanding mechanisms that drive interactions between EPNs and insects can offer alternatives in pest management in agricultural systems.
Acknowledgements
The authors thank Maria de Lourdes Mendes for providing helpful comments and for giving advice about this paper. We also thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) for financial support.
Literature cited
BOFF, M. I. C.; SMITS, P.H. 2001. Effects of density, age and host cues on the dispersal of Heterorhabditis megidis. Biocontrol Science and Technology 11 (4): 505-514. [ Links ]
CAMPBELL, J. F.; GAUGLER, R. 1997. Inter-specific variation in entomopathogenic nematode foraging strategy: dichotomy or variation along a continuum? Fundamental and Applied Nematology 20: 393-398. [ Links ]
CUTLER, G. C.; WEBSTER, J. 2003. Host-finding ability of three entomopathogenic nematode isolates in the presence of plant roots. Nematology 5 (4): 601-608. [ Links ]
DUTKY, S. R.; THOMPSON, J. V.; CANTWELL, G. E. 1964. A technique for the mass propagation of the DD-136 nematode. Journal of Insect Pathology 6 (4): 417-422. [ Links ]
ENNIS, D. E.; DILLON, A. B.; GRIFFIN, C. T. 2010. Simulated roots and host feeding enhance infection of subterranean insects by the entomopathogenic nematode Steinernema carpocapsae. Journal of Invertebrate Pathology 103 (2): 140-143. [ Links ]
GAUGLER, R.; CAMPBELL, J. F.; GUPTA, P. 1991. Characterization and basis of enhanced host finding in a genetically improvedç strain of Steinernema carpocapsae. Journal of Invertebrate Pathology 57 (2): 234- 241. [ Links ]
GAUGLER, R.; LEWIS, E.; STUART, R. J. 1997. Ecology in the service of biological control: the case of entomopathogenic nematodes. Oecologia 109 (4): 483-489. [ Links ]
GREWAL, P. S.; LEWIS, E. E.; GAUGLER, R.; CAMPBELL, J. F. 1994. Host finding behaviour as a predictor of foraging strategy in entomopathogenic nematodes. Parasitology 108 (2): 207–215. [ Links ]
HEDLUND, K.; GRIFFITHS, B.; CHRISTENSEN, S.; SCHEU, S.; SETÄLÄ, H.; TSCHARNTKE, T.; VERHOEF, H. 2004. Trophic interactions in changing landscapes: responses of soil food webs. Basic Applied Ecology 5 (6): 495-503. [ Links ]
HILLIARD, M. A.; BARGMANN, C. I.; BAZZICALUPO, P. 2002. Caenorhabditis elegans responds to chemical repellents by integrating sensory inputs from the head and the tail. Current Biology 12 (21): 730-734. [ Links ]
ISHIBASHI, N.; KONDO, E. 1990. Behaviour of infective juveniles. pp. 139-150. En: Gaugler, R.; Kaya, H. K. (Eds.). Entomopathogenic nematodes in biological control. CRC Press, Boca Raton, FL, USA. 365 p. [ Links ]
JONES, J. 2002. Nematode sense organs. pp. 353-368. En: Lee, D.L. (Ed.). The biology of nematodes. Taylor & Francis Inc., London, UK. 635 p. [ Links ]
KAPLAN, F.; ALBORN, H. T.; VON REUSS, S. H.; AJREDINI R.; ALI, J. G.; AKYAZI, F.; STELINSKI, L. L.; EDISON, A. S.; SCHROEDER, F. C.; TEAL, P. E. 2012. Interspecific nematode signals regulate dispersal behavior. PLoS One 7 (6): 1-8. [ Links ]
KAYA, H. K. 1990. Soil ecology. p. 93-115. En: Gaugler, R.; Kaya, H. K. (Eds.). Entomopathogenic nematodes in biological control. CRC Press, Boca Raton, FL, USA. 365 p. [ Links ]
KAYA, H. K.; GAUGLER, R. 1993. Entomopathogenic nematodes. Annual Review of Entomology 38: 181-206. [ Links ]
LEE, D. L. 2002. Behaviour. pp. 369-387. En: Lee, D. L. (Ed.). The biology of nematodes. Taylor & Francis Inc., London, UK. 635 p. [ Links ]
LEWIS, E. E. 2002. Behavioural Ecology. p.p 205-224. En: Gaugler, R. (Ed.). Entomopathogenic nematology. CABI Publishing, Wallingford, UK, 388 p. [ Links ]
LEWIS, E. E.; GAUGLER, R.; HARRISON, R. 1992. Entomopathogenic nematode host finding: response to host contact cues by cruise and ambush foragers. Parasitology 105 (2): 309-319. [ Links ]
LEWIS, E. E.; GAUGLER, R.; HARRISON, R. 1993. Response of cruiser and ambusher entomopathogenic nematodes (Steinernematidae) to host volatile cues. Canadian Journal of Zoology 71 (4): 765-769. [ Links ]
LEWIS, E. E.; CAMPBELL, J. F.; GRIYN, C.; KAYA, H.; PETERS, A. 2006. Behavioral ecology of entomopathogenic nematodes. Biological Control 38: 66-79. [ Links ]
MILLER, J. R.; STRICKLER, K. L. 1984. Finding and accepting host plants. pp. 127-157. En: Bell, W. J.; Carde, R. T. (Eds.). Chemical ecology of insects. Chapman & Hall, London, UK. 524 p. [ Links ]
MOLINA, J. P.; LÓPEZ, N. J. C. 2001. Producción in vivo de tres entomonematodos con dos sistemas de infección en dos hospedantes. Revista Colombiana de Entomología 27 (1-2): 73- 78. [ Links ]
MOREIRA, G. F.; MOREIRA, C. C.; ANDALÓ, V.; MOINO JUNIOR, A.; CARDOSO-FREIRE, M. M.; DIAS, E. S. 2010. Laboratory rearing technique and biology of Mycotretus apicalis (Coleoptera: Erotylidae) on dried Pleurotus sajor-caju mushrooms. Revista Colombiana de Entomología 36 (2): 342-345. [ Links ]
NERMUT', J.; PŮŽA, V.; MRÁÄEK, Z. 2012. The response of Phasmarhabditis hermaphrodita (Nematoda: Rhabditidae) and Steinernema feltiae (Nematoda: Steinernematidae) to different host-associated cues. Biological Control 61: 201-206. [ Links ]
PARRA, J. R. P. 1998. Criação de insetos para estudos com patógenos. pp. 1015-1037. En: Alves, S. B. (Ed.). Controle microbiano de insetos. Editora FEALQ. Piracicaba. 1163 p. [ Links ]
SHAPIRO-ILAN, D.; CAMPBELL, J. F.; LEWIS, E. E.; ELKON, J. M.; KIM-SHAPIRO, D. B. 2009. Directional movement of steinernematid nematodes in response to electrical current. Journal of Invertebrate Pathology 100 (2): 134-137. [ Links ]
THURSTON, G. S.; YULE, W. N.; DUNPHY, G. B. 1994. Explanations for the low susceptibility of Leptinotarsa decemlineata to Steinernema carpocapsae. Biological Control 4 (1): 53-58. [ Links ]
Suggested citation:
ANDALÓ, V.; MOREIRA, G. F.; MOINO JUNIOR, A. 2014. Heterorhabditis amazonensis RSC5 (Rhabditida: Heterorhabditidae) movement and host recognition. Revista Colombiana de Entomología 40 (1): 91-97. Enero-julio 2014. ISSN 0120-0488.