Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488
Rev. Colomb. Entomol. vol.40 no.2 Bogotá July/Dec. 2014
SECCIÓN AGRÍCOLA / AGRICULTURE
ARTÍCULOS DE INVESTIGACIÓN / RESEARCH PAPER
Impact of organic crops on the diversity of insects: a review of recent research
Impacto de los cultivos orgánicos en la diversidad de insectos: una revisión de investigaciones recientes
María N. MontañezI; Ángela Amarillo-SuárezII
IM. Sc. Pontificia Universidad Javeriana, Maestría en Conservación y uso de Biodiversidad, Transversal 4 No. 42-00, piso 8, Bogotá, Colombia. manamove@gmail.com. Corresponding author
IIPh. D. Pontificia Universidad Javeriana, Departamento de Ecología y Territorio, Transversal 4 No. 42-00, piso 8, Bogotá, Colombia. aamarillo@javeriana.edu.co
ABSTRACT
The conversion of forests to conventional agroecosystems is one of the causes of biodiversity loss. In contrast, organic farming practices that promote caring for the environment are seen as an alternative that promotes increased biodiversity. Although insects have one of the largest impacts on crops, to date there have been no published studies that specifically synthetize information on the impacts of organic farming practices on insects. The results of 35 studies that compare the diversity of insects on organic and conventional crops were analyzed by combining a classic review with meta-analysis tools. The purpose was to determine whether organic crops promote better conservation of insects. Species richness and abundance were significantly higher in organic crops, though the reviewed studies indicated a high heterogeneity for species richness and abundance. Likewise, organic farming was associated with higher trophic guild diversity. Insects were 34% more abundant on organic crops. Comparing studies at different landscape scales (plot, farm, landscape matrix), organic crops have a positive effect, with the greatest effect at the plot level. This review also indicates the great need for studies of this nature in the Neotropics and the importance of developing research on the complexity of ecological networks to understand the dynamics of interactions in these agroecosystems in addition to their taxonomic and functional richness.
Key words: Species richness; Abundance; Agricultural systems; Review.
RESUMEN
El uso de la tierra y su conversión a agroecosistemas convencionales es una de las causas de pérdida de la biodiversidad. En contraste, la agricultura orgánica debido a prácticas que favorecen el cuidado del ambiente, es percibida como una forma alternativa que promueve un aumento de la biodiversidad. Aunque los insectos son uno de los grupos que mayor impacto genera en cultivos, a la fecha no existen trabajos publicados que sintetizen información a este respecto y exclusivamente para ellos. Se analizaron los resultados de 35 estudios que comparan la diversidad de insectos en cultivos orgánicos y convencionales combinando herramientas de la revisión clásica y del meta-análisis. El propósito fue determinar si los cultivos orgánicos posibilitan un mejor espacio para la conservación de insectos. Se encontró que la riqueza de especies y su abundancia son significativamente mayores en cultivos orgánicos. Los estudios registraron una alta heterogeneidad tanto para riqueza de especies como para abundancia. Asimismo, los cultivos orgánicos registraron una mayor riqueza por gremios tróficos. Los insectos fueron 34% más abundantes en cultivos orgánicos. Al comparar los estudios en relación con categorías de paisaje (parcela, granja, estudios con matriz de paisaje) los cultivos orgánicos tienen efecto positivo, siendo mayor éste en la categoría de parcela. Esta revisión sugiere que hay una gran necesidad de estudios de esta naturaleza en el neotrópico y que es importante desarrollar investigaciones sobre la complejidad de redes con el fin de comprender, además de la riqueza taxonómica y funcional, la dinámica de las interacciones en estos agrosistemas.
Palabras clave: Riqueza de especies; Abundancia; Sistemas agrícolas; Revisión
Introduction
The establishment of modern agriculture produces simplification of the structure of the environment, in which the natural diversity is replaced with a small number of crop species. These semi-artificial ecosystems require constant human intervention to regulate their functioning (Altieri 1995;1999). For this reason, modern conventional agro-systems exhibit difficulties such as cyclical outbreaks of pests, water contamination, salinization and soil erosion. Increases in pest problems have also been associated with the expansion of monocultures, which reduce vegetation complexity, an essential component of the landscape that provides key ecological services, including the protection of crops (Altieri and Letourneau 1982).
The so-called "conventional" agricultural model was largely adopted after the green revolution (García 1991). Its intensification and expansion represents a threat to global biodiversity because it causes the homogenization of agricultural landscapes, habitat loss and reduction, and increased use of pesticides and synthetized chemical fertilizers (Bengtsson et al. 2005). The role of conventional agriculture in the modification of ecosystems has been studied and documented (Wilson et al. 1999; Tilman et al. 2001, among others). For example, Hole et al. (2005) reported a dramatic decline in the abundance of several species associated with farms in Europe during the last quarter century.
Some farmers and professionals related to the fields of biology, ecology and agriculture have called attention to the deleterious environmental, economic and social effects of the practices employed in conventional agriculture (Céspedes 2005). Thus, there is an increasing search for alternative crop systems based on ecological principles that would allow agriculture to benefit from biodiversity, the use of more friendly and environmentally safe technologies, the production of products with reduced pollutants, and in consequence a more sustainable agriculture. Within this tendency, organic agriculture, which began around the 1970s, became an alternative based in safer and sustainable principles for the environment and for human societies (IFOAM a-b 2009; Rigby and Cáceres 2001). This form of agriculture reduces the use of external inputs such as fertilizers, synthetic pest control chemicals and genetically modified organisms. In addition, it promotes the maintenance of natural enemies of pest insects (Paoletti et al. 1992; Hole et al. 2005).
Organic crop production has increased in recent years (García 2002). According to the Research Institute of Organic Agriculture (FiLB) and The International Federation of Organic Agriculture Movements (IFOAM), in 2011, there were 37.2 million ha of organic crops grown worldwide. The regions with the largest areas are Oceania (12.2 million ha, corresponding to 33% of the total organic crop area in the world) and Europe (10.6 million ha, equivalent to 29%). Latin America comprises 6.9 million ha (18.4%), followed by Asia (3.7 million ha, 10%), North America (2.8 million ha, 7.5%) and Africa (1.1 million ha, 3%) (FiLB-IFOAM 2011).
Organic productive agrosystems are founded on two bases: the first is the minimization of the impacts of the crop on the natural equilibrium of the ecosystem, generating food of high quality without residues that could be harmful to the health of humans and other animals. The second is the implementation of water recycling and management practices (Mondelaers et al. 2009). Among the advantages of these practices is increased biodiversity (Dritschilo and Wanner 1980; Pfinner and Niggli 1996; Power and Stout 2011). Birds, mammals, arthropods and plants benefit from organic crop production, which also exhibits better pest control by maintaining natural enemies and pollinators (Hole et al. 2005; Garratt et al. 2011).
Because insects are the most diverse and conspicuous taxonomic group in transformed rural ecosystems, they have been subject to studies that measure the effects of such transformations on their diversity (Morris 1979; Rushton et al. 1989; Di Giulio et al. 2001; Vickery et al. 2001; Kruess and Tscharntke 2002). As a result of strong insect-plant relationships, they are susceptible to changes caused by anthropogenic transformations such as the establishment of monocultures. Those changes usually produce a loss of diversity of insects and transformations of trophic and ecological networks (Garrat et al. 2011). Several authors have documented the advantages of organic farming for the biodiversity of insects. These advantages are related to the increased taxonomic diversity (Feber et al. 2007; Salazar and Salvo 2007) and functional diversity (Letourneau and Goldstein 2001) as well as the generation of more complex pollinator-plant networks (Power and Stout 2011).
Despite the published case studies on the effects of organic and conventional crop production on the diversity of insects, we are not aware of any reviews that would allow generalizations on the impact of organic agriculture on the taxonomic and functional diversity of insects and the question of whether organic agriculture promotes higher diversity than conventional agriculture. For example, the meta-analyses by Hole et al. (2005) and Bengtsson et al. (2005), which utilize a variety of methodologies and scales, suggest that organic crops are associated with higher abundance and richness of a variety of taxonomic groups (plants, invertebrates, predators and birds); Büchs et al. (2003) show that the diversity and richness of several taxa are higher in organic crops; Garratt et al. (2011) found that organic crops increase the abundance of natural enemies, which favors pest management; and Sandhu et al. (2010) concluded that organic crops maintain ecosystem services such as pollination and biological control. Thus, this review analyses experimental studies published as journal articles between 2001 and 2013 that compare organic and conventional crops to determine whether organic practices effectively improve the conservation of insects compared to conventional practices. To do this, differences in the patterns of abundance and taxonomic diversity of insects on organic vs. conventional crops were analyzed. A comparison of functional diversity is also provided.
Key concepts
Organic agriculture, understood as agriculture practiced from a holistic perspective, considers that there is a deep and strong relationship between food production and the environment (Cáceres 2002). It promotes soil and crop protection by using crop practices such as nutrient and organic recycling, crop rotation, and biological and mechanical control of weeds and insect pests. It also eschews the use of synthetic pesticides, herbicides and fertilizers. The organic agriculture concept is closely related to the concepts of agroecology (Altieri 1987; Altieri and Nicholls 2000) and biodynamic agriculture (Koepf 1976; Childs 1995).
Conventional agriculture refers to the dominant common practices of farming. Since World War II, especially within the industrialized world, conventional agriculture is a form of agriculture characterized by mechanization, monocultures, the use of synthetic fertilizers and pest control chemicals and the cultivation of genetically modified organisms. It focuses on reaching the maximum productivity of the crop and the maximum economic benefit. It also considers crops as merchandise. The organic community uses the term "conventional agriculture" to refer to all agriculture systems that are not organic as defined above (Parra et al. 2004).
Trophic guild: A group of species that share a food resource and use it in a similar way. For example, insectivores, granivores, etc. (Root 1967). A trophic guild may contain species that are not taxonomically related.
Effect size: In a meta-analysis, the effect size expresses how much of the dependent variable can be controlled, predicted or explained by the independent variable (Snyder and Lawson 1993). It also defines the extent to which the null hypothesis is false (Cohen 1988). The effect size allows discussion of large or small differences in terms of the relevance of the differences found.
Materials and methods
Source of data. Data were compiled from studies published as journal articles that compare the taxonomic, trophic and functional diversity of insects between organic and conventional agriculture. A literature search was performed using ISI Web of Science with the key words "organic farming", "conventional farming", "multitrophic interactions", "insects", "insect biodiversity", "organic agriculture" and "pest and natural enemies". In addition, the references of the papers found in this search were also reviewed. Only papers between 2001 and 2013 were included. The criteria for including a study in this review were as follows: (1) published journal article, (2) compares at least one conventional to one organic crop, (3) explicitly presents data on the diversity (richness and abundance) of the insects in these two agrosystems and (4) compares trophic guilds between the two agrosystems. The initial search using different combinations of key words produced a total of 99 papers. Of those, 35 met the above criteria and thus were used for this review. The extent of this analysis includes studies performed worldwide.
Analysis of data. To determine whether organic agriculture effectively promotes better conservation of the taxonomic and functional diversity of insects than conventional agriculture, a descriptive analysis of the type included in traditional and classic reviews was combined with the tools of meta-analysis. The use of these additional tools allowed for the quantitative and statistical analysis of the data provided by the individual studies. It also provided an estimate of the effect size that represents confident and significant difference in small samples, allowing for easy comparison and synthesis of the results. In contrast to the classic narrative review, meta-analysis provides more rigor in the process of the selection of studies and in the integration and analysis of the results (Teagarden 1989).
The treatments in the analysis were the two types of agriculture: organic and conventional. The studies were organized in a matrix of data that contained, for each study, the following information: geographic location, climatic category according to the Köppen climate classification, size of the crop system, and sampling area and method (fields, plots, collecting traps, transects, etc.).
Species richness was used as the measure of diversity (Noss 1990). Abundance was considered as the total number of individuals for the study as well as the totals per trophic guild and per sampling unit.
Descriptive analysis. This analysis was performed with the 25 studies that reported data on species richness and abundance for each crop system (Table 1). Because proportions are a good way to make comparisons between studies that consider samples with different areas and sampling techniques, the proportional richness and abundance per treatment were estimated. Richness differences between trophic guilds were compared in the same way.
This part was performed with the 14 studies that reported mean richness and the 10 studies that reported mean abundance. No other studies were used for this analysis because the statistical procedure of meta-analysis requires this type of information, which was not provided by the remaining studies. After this, a matrix containing the average value, standard deviation (SD) and sampling size (N) for each treatment was developed for each paper. Effect sizes were estimated for comparisons made at three landscape scales: (1) plot; (2) farm; and (3) the landscape matrix. The effect size was calculated with the Hedge algorithm (g) (Hedges and Olkin 1985). This is calculated as the difference between the average values of the treatments divided by the SD and multiplied by a correction factor for bias in small samples, as indicated by the following algorithm (van Zandt and Mopper 1998):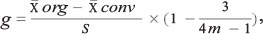
where m = (n org + n conv) - 2.
The magnitude of the effect size was classified as small, moderate or large. For this determination, the valuations were based on Hopkins (2013), who considered the relationship between g and the coefficient of correlation (r), where
g = 0.20 is equal to r = 0.10 and considered a small difference,
g = 0.63 is equal to r = 0.30 and considered a moderate difference, and
g = 1.15 is equal to r = 0.50 considered a large difference.
In addition, a mixed model of meta-analysis was used because it is preferred for synthesizing ecological data (Gurevitch and Hedges 1993). The confidence interval (CI) was used to evaluate the significance of the effect size. An effect size is determined to be significant if the limits of the 95% confidence interval do not include zero (Cooper and Hedges 1994; Prieto-Benitez and Mendez 2011).
The heterogeneity of the effect size for richness and abundance among studies, within the three landscape scales described above, was calculated using the Q test for a model of random effects (DerSimonian and Laird 1986). This test calculates the weighted sum of the differences between the effects determined for each of k studies and for the global average:
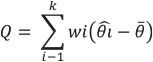
where
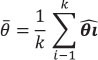
The significance is obtained by a chi2 test (Harrison 2011). If Q is significant, the effect size is heterogeneous, that is, there are differences among studies.
Additionally, an I2 test was performed to describe the percentage of heterogeneity that is due to differences among studies beyond the differences expected due to randomness. Values of less than 20% indicate minimum heterogeneity, values between 20 and 50% moderate heterogeneity, and values of 50% or more high heterogeneity.
All calculations for this section were performed with the software Comprehensive Meta-analysis Version 2 (Borenstein et al. 2005) and confirmed with the web page "Effect size calculator" (Ellis 2009).
Results and discussion
Of the 35 studies included in this review, 77% were conducted in countries with temperate/mesothermal climates, 22% in continental/microthermal climates, and 1% in countries with tropical/megathermal climates. Only two studies from tropical Central America were included (from Nicaragua and Costa Rica), and only one from South America (Argentina). The crops most frequently studied were the cereals (36%), followed by annual herbs (15%), pastures (12%), olives (9%), grapes (6%), and tomatoes, apples, canola, mangoes, strawberries, almonds, cashews and bananas (approximately 1% each) (Table 1).
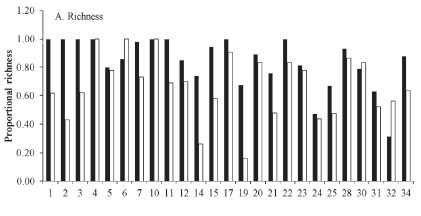
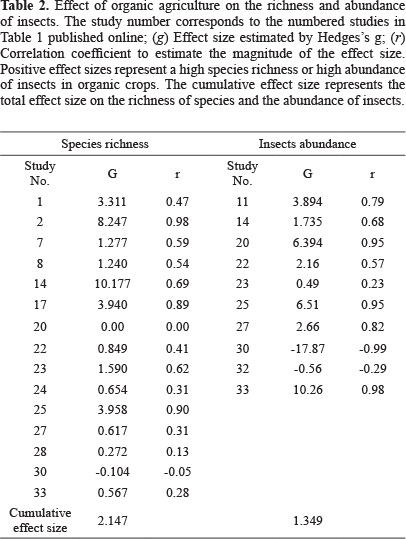
Taxonomic richness. Organic crops were associated with a higher richness of insects. Of the 26 studies that recorded quantitative data on richness (Table 1), 21 (83%) reported a higher richness of insects on organic crops (Fig. 1). In the same way, the global data on accumulated effect size (Table 2) revealed a significant increase in species richness associated with the organic agrosystem. In addition, the effect size calculated as the log ratio indicates that organic crops are 39% richer in insect species than conventional crops despite the heterogeneity among studies (Q = 737.79; I2 = 98.102; P < 0.05).
The higher species richness on organic crops could be due to characteristics of this agriculture type that better emulate the characteristics of semi-natural habitats, making these environments more attractive to a larger variety of species (Wickramasinghe et al. 2003). In contrast, in conventional systems, the presence of synthetized pest and weed control chemicals has deleterious effects on the neurophysiology and metabolism of insects. In addition to pests, these chemicals also affect beneficial organisms such as natural enemies and pollinators and propitiate the development of pest resistance. In turn, increased pest resistance leads to an increase in the dosage used to kill the pests, with negative effects on human health (Lannacone and Lamas 2003; Desneux et al. 2007). In organic agriculture, the less aggressive system of soil management for organic crops has a positive effect on the dynamics of insects inhabiting the soil environment. In comparison, the techniques used by conventional systems to turn over the soil and mix the soil layers and the organisms they contain cause the disruption of ecological networks, of vegetation residues, and of nutrient contents (Moreby et al. 1994; Castro et al. 1996). In addition, organic crops include a larger variety of plants cultivated in the same plot along with herbs that grow freely (no weeds under this type of agriculture). This helps to maintain better microclimates inside the plots, which facilitates the establishment and maintenance of larger numbers of arthropods and microarthropods (Moreby et al. 1994; Paoletti 1995; Stopes et al. 1995; Castro et al. 1996, Dunning et al. 1999) because it provides them with more food and habitat resources. For example, Marino and Landis (1996) demonstrated that increases in the diversity of plants and the complexity of vegetation architecture in agroecosystems increases the diversity of parasitoids. Weibull (2000) reported an increase in the diversity of butterflies as a consequence of landscape heterogeneity inside farms. Similarly, Kerr (2001) showed that the number and type of land covers in an area influence the spatial distribution of the diversity of butterflies. By comparison, Hole et al. (2005) found that the habitat modification produced by conventional agriculture results in the reduction of plant and insect diversity as a consequence of the use of synthetic herbicides and pesticides.
Despite the robust results from this study, organic crops were not always associated with increased species richness. Studies 6, 10, 30 and 32 (17%), recorded higher species richness on conventional crops (Fig. 1A). In addition, the results from the meta-analysis show that in study 20, there was no significant effect of agriculture type on the richness of insects, and study 30 presented a higher richness of insects on conventional crops (Table 2). The studies that exhibited these conflicting results were conducted in areas where farming is performed within small land cover mosaics in which the cropland is surrounded by natural and seminatural habitats, live fences, trees and forests. This condition favors landscape heterogeneity, which increases both pest species and their natural enemies because, as mentioned before, this mosaic offers refuge and easy dispersal of insects as a consequence of the vicinity of a variety of landscape elements (Benton et al. 2003; Weibull et al. 2003). In addition, in conventional crops, the non-cultivated areas have a deleterious effect in that they help maintain pest species, but at the same time, they have positive impacts by maintaining natural enemies and pollinators. This phenomenon has been documented by authors such as Varchola and Dunn (1999; 2001), who studied the influence of live fences and pastures on the richness and diversity of Carabidae in corn fields. They concluded that the surrounding habitats maintain the abundance and diversity of these insects during most of the growing season. Girma et al. (2000) reported similar results for live fences surrounding corn and red bean fields in Kenya.
On the other hand, the high heterogeneity of the effect size reported here indicates that there may be other variables influencing the results. Among these are the differences in climatic zones, crop species, and the methodological designs of the studies analyzed (Colditz et al. 1995). For example, a large majority of the studies were conducted in countries with temperate climates; however, many of them pertained to a variety of geographical regions; Some European regions are close to each other but differ from other regions, such as North America, South America and South Africa.
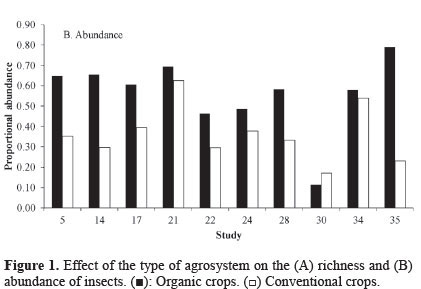
Taxonomic abundance. The results indicate that organic crops also increase insect abundance. Of the 10 studies that reported these data (Table 1; Fig. 2), nine (87.5%) found a higher abundance of insects on organic crops. Moreover, the global data on cumulative effect size were significant (Table 2), indicating that organic crops have a positive effect on abundance. The cumulative effect size estimated as log ratio shows that insect abundance was 34% higher in organic agrosystems. A high heterogeneity among studies was also found (Q = 628.95; I2 = 99.857; P < 0.05). This could be caused, as suggested earlier, by the effects of other variables.
The large positive effects of organic agriculture could be related to the combined effects of more sustainable practices of pest control and soil nutrition and the structure of the crop field. Compared to conventional agriculture, organic farms do not use synthetic herbicides or fertilizer, generating more heterogeneous crop densities within farms, which facilitates a variety of microclimatic and ecological conditions that favor a larger range of species and individuals who can find refuge and food there (Altieri 1992; Feber et al. 1997; Freeman et al. 1998; Landis et al. 2000).
Some conflicting results are reported, with study 30 differing from the descriptive study (Fig. 1B) and studies 30 and 32 differing from the meta-analysis; these studies reported a higher abundance of insects on conventional crops (Table 2). This could be explained by the type of organisms under study. Studies 30 and 32 analyzed aphids. Aphids are more abundant in conventional systems, which are constantly provided with fertilizers and mineral herbicides that increase the development of the aphids due to the higher content of nitrogen available in the plants (Schütz et al. 2008).
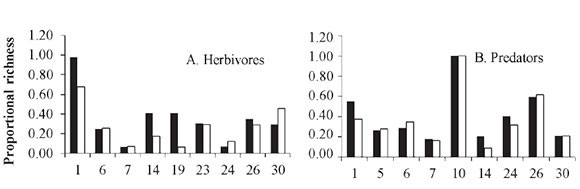
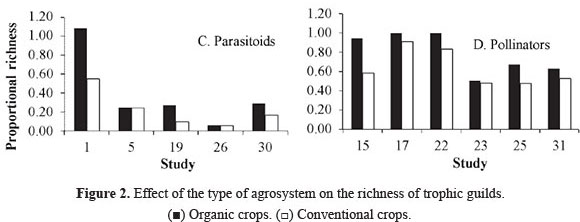
Richness of trophic guilds. The terrestrial communities related directly to plants are composed of at least three interacting trophic levels: plants, herbivores and the natural enemies of herbivores (Price et al. 1980). Compared to conventional crops, organic crops supported a higher richness of trophic guilds (Fig. 2A-D). Table 3 (supplement on line, see citation in end article) lists the insect species that were recorded by some studies, classified by family and trophic guild. The highest proportion of species is grouped within predators (58%), followed by pollinators (20.3%), herbivores (16.5%), coprophages (3.6%) and parasitoids (1.6%). In addition, some species were found to be exclusive to a particular type of crop system. However, organic crops supported a higher species richness in all trophic guilds.
Five studies recorded a higher richness of herbivores in organic crops and four in conventional crops (Fig. 2A), indicating that both types of systems have a similar richness. Feber et al. (1997) reported similar abundances of pest butterflies in organic and conventional systems. However, the nitrogen content in plants, which is a limiting factor for insects, is higher in conventional crops (Schütz et al. 2008). In the case of organic crops, this supply of nitrogen could be provided by crop rotation with legume plants and/or the addition of organic compost or manure. This would be an interesting hypothesis that needs to be evaluated.
Regarding predators, four studies indicated higher richness on organic crops and three on conventional crops. Two did not find a difference between crop systems (Fig. 2B). In the case of parasitoids, all studies reported higher species richness on organic crops. These two results combined imply that organic crops increase the richness of natural enemies of crop pests. This can be supported by the fact that natural enemies are more susceptible to agrochemicals than their prey, which are absent from organic crops (Klein et al. 2002; Langhof et al. 2003; Symington 2003). In addition, the "natural enemies hypothesis" predicts that ecosystems with a large variety of plants will support more predators, which exert a top-down control of herbivores (Root 1973). This synergic association in response to prey is described by Evans (2008), who examined how the availability of prey such as aphids and other herbivores affects the numeric response (aggregative and reproductive) and the functional response of predators.
The same response was found for pollinators. They exhibited higher proportional richness in organic crops (Fig. 2D). Altieri and Nicholls (2000) showed that diversified agrosystems such as organic ones contain resources that provide a large variety of food resources (pollen and nectar) to adult pollinators. Moreover, recent studies report a decrease of pollinators in conventional crops due to their sensitivity to pesticides (Biesmeijer et al. 2006; Potts et al. 2010).
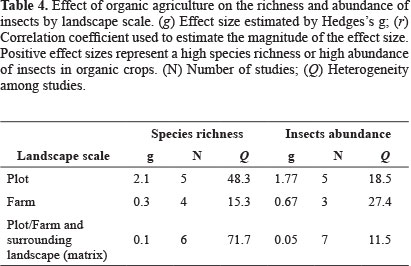
Effect size by landscape category. The meta-analysis showed a higher richness and abundance of insects on organic crops in all cases (Table 4). However, there was a larger effect size at the plot level, followed by the farm scale, and last the landscape matrix (Table 4). This could be caused by the fact that in small plots, the positive effects are more conspicuous due to the individual behavior of insects such as preferences for some host plants or food resources (Peterson and Parker 1998; Bommarco and Banks 2003; Bengtsson et al. 2005). As with the above results from the meta-analysis, there was also strong heterogeneity among studies (Table 4).
The conservation of diversity in agroecosystems depends on the system of agriculture in use as well as the landscape surrounding the farms. The former facilitates soil conservation and plant diversity within the planted area, and the second corresponds to non-planted areas (side roads, pastures, live fences and other small habitats), which provide important refuges and food sources for many invertebrate groups. Thus, two components of biodiversity can be recognized in agrosystems: the first one is planned biodiversity, i.e., the managed crops and livestock that are intentionally included in the agrosystem. These vary according to the temporality and planning of the farmer. The second component, the associated biodiversity, includes all organisms from the soil, herbivores, carnivores, decomposers, etc. that colonize the agrosystem from the surrounding environments and flourish in it due to the management of the area (Vandermeer and Perfecto 1995). These two components complement each other in such a way that the conservation of biodiversity depends on the preservation, restoration and management of both components (Stopes et al. 1995; Baudry et al. 2000; Tscharntke et al. 2002).
Limitations of the study. When considering only published journal articles that are accessible online, it is likely that selection and publication biases will occur. In the case of selection bias, it is clear that information included in thesis documents and as project reports is very difficult to find and obtain. The vast majority of this grey or non-conventional literature is stored in libraries or offices with no access beyond a very small region (the university, the city, etc.), making its access impossible. The second case, publication bias, is common in studies such as meta-analyses that analyze secondary information because very often researchers and journal editors are reluctant to publish results with no statistical significance. Thus, such out-of-hand results are very distant from what has been called the "accessible population" (Letelier et al. 2005). Because it would be a very long, labor-intensive effort to include this type of studies, the vast majority of reviews and meta-analyses, such as the one performed here, include only published journal papers, which also ensures the validity of the studies analyzed given that all of them have been subject to the peer review process, which is not the case for some studies in the non-conventional literature.
Conclusions and recommendations
This review, based on journal articles published between 2001 and 2013, found that organic crops certainly increase the taxonomic richness and abundance of insects as well as the richness of insects within trophic guilds (herbivores, predators, pollinators and parasitoids). Thus, the belief that organic agriculture contributes to the conservation of biodiversity is supported by the analyses performed here for the case of insects. An additional and important result that emerged from this study is that both the agrosystem and the surrounding landscape are relevant to the conservation of biodiversity. Thus, both the planned and incidental vegetal and insect biodiversity in an agroecosystem have important consequences for the conservation of biodiversity, contributing to ecosystem functioning, the recycling of nutrients, and the increase of productivity and crop health.
On the other hand, too few studies performed in tropical areas were found that passed the rigorous evaluation for the review and the meta-analysis. This indicates a need to perform a large amount of experimental studies with large sample sizes that would allow more homogenous and precise generalizations about what is occurring in the region that supports the highest biodiversity on the planet but at the same time suffers from a high rate of conversion of natural landscapes to agriculture. In addition, it is necessary to advance beyond conventional studies of biodiversity based on species diversity and abundance by developing studies that analyze the structure and complexity of ecological networks. This will allow a more detailed comprehension of the functioning, relationships and variation of the insect communities.
Finally, from the area of policy definition, this analysis justifies the continuation of support from governments and NGOs of the maintenance and increase of organic farming as a way to preserve biodiversity in transformed areas. In the case of Colombia and other tropical countries, as proposed by Altieri and Nicholls (2000), agroecological farms including organic crops should be able to produce food using fewer external resources and support the conservation of biodiversity and more sustainable food production that would directly benefit the farmers and the environment that supports our production systems.
Acknowledgements
The authors give their thanks to Pontificia Universidad Javeriana, which provided logistical, economic and bibliographic resources. Neidy Clavijo provided helpful comments on a preliminary version of the manuscript. Two anonymous reviewers also provided comments that enhanced the quality of the final version of the manuscript.
Literature cited
ALTIERI, M. A. 1987. Agroecology. The scientific basis of alternative agriculture. Boulder, Colorado: Westview Press. 214 p. [ Links ]
ALTIERI, M. A. 1992. Biodiversidad, agroecología y manejo de plagas. CETAL Ediciones, Valparaíso, 162 p. [ Links ]
ALTIERI, M. A. 1995. Agroecology: The science of sustainable agriculture. Boulder, Colorado. Westview Press. 433 p. [ Links ]
ALTIERI, M. A. 1999. The ecological role of biodiversity in agroecosystems. Agriculture Ecosystem Environment 74 (1-3): 19-31. [ Links ]
ALTIERI, M.; LETOURNEAU, D. K. 1982. Vegetation management and biological control in agroecosystems. Crop Protection 1 (4): 405-430. [ Links ]
ALTIERI, M.; NICHOLLS, C. I. 2000. Agroecología. Teoría y práctica para una agricultura sustentable. Programa de las Naciones Unidas para el Medio Ambiente (PNUMA), México. 257 p. [ Links ]
ALVAREZ, T.; FRAMPTON, G. K.; GOULSON, D. 2001. Epigeic Collembola in winter wheat under organic, integrated and conventional farm management regimes. Agriculture, Ecosystem and Environment 83 (1-2): 95-110. [ Links ]
ANDERSSON, G. K. S.; RUNDLÖF, M.; SMITH, H. G. 2012. Organic farming improves pollination success in strawberries. PLoS ONE 7 (2): e31599. [ Links ]
ASTERAKI, E. J.; HART, B. J.; INGS, T. C.; MANLEY, W. J. 2004. Factors influencing the plants and invertebrate diversity of arable field margins. Agriculture Ecosystem Environment 102 (2): 219-231. [ Links ]
BAUDRY, J.; BUREL, F.; THENAIL, C.; LE COEUR, D. 2000. A holistic landscape ecology study of the interactions between activities and ecological patterns in Brittany, France. Landscape and Urban Planning 50 (1-3): 119-128. [ Links ]
BENGTSSON, J.; AHNSTRÖM, J.; WEIBULL, A. 2005. The effects of organic agriculture on biodiversity and abundance: a meta-analysis. Journal of Applied Ecology 42 (2): 261-269. [ Links ]
BENTON, T. G.; VICKERY, J. A.; WILSON, J. D. 2003. Farmland biodiversity: is habitat heterogeneity the key? Review Article. Trends in Ecology and Evolution 18 (4): 182-188. [ Links ]
BIESMEIJER, J. C.; ROBERTS, S. P. M.; REEMER, M.; OHLEMÜLLER, R.; EDWARDS, M.; PEETERS, T.; SCHAFFERS, A. P.; POTTS, S. G.; KLEUKERS, R.; THOMAS, C. D.; SETTELE, J.; KUNIN, W. E. 2006. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313 (5785): 351-354. [ Links ]
BIRKHOFER, K.; FLIEßBACH, A.; WISE, D. H.; SCHEU, S. 2008. Generalist predators in organically and conventionally managed grass-clover fields: implications for conservation biological control. Annals of Applied Biology 153 (2): 271-280. [ Links ]
BOMMARCO, R.; BANKS, J. E. 2003. Scale as a modifier in vegetation diversity experiments: effects on herbivores and predators. Oikos 102 (2): 440-448. [ Links ]
BORENSTEIN, M.; HEDGES, L.; HIGGINS, J.; ROTHSTEIN, H. 2005. Comprehensive Meta-analysis Version 2, Biostat, Englewood NJ. [ Links ]
BRITTAIN, C.; BOMMARCO, R.; VIGHI, M.; SETTELE, J.; POTTS, S. G. 2010. Organic farming in isolated landscapes does not benefit flower-visiting insects and pollination. Biological Conservation 143 (8): 1860-1867. [ Links ]
BRUGGISSER, O. T.; SCHMIDT-ENTLING, M. H.; BACHER, S. 2010. Effects of vineyard management on biodiversity at three trophic levels. Biological Conservation 143 (6): 1521-1528. [ Links ]
BÜCHS, W.; HARENBERG, A.; ZIMMERMANN, J.; WEIB, B. 2003. Biodiversity, the ultimate agri-environmental indicator? Potential and limits for the application of faunistic elements as gradual indicators in agroecosystems. Agriculture, Ecosystems and Environment 98 (1-3): 99-123. [ Links ]
CABALLERO-LÓPEZ, B.; BLANCO-MORENO, J. M.; PÉREZ-HIDALGO, N.; MICHELENA-SAVAL, J. M.; PUJADE-VILLAR, J.; GUERRIERI, E.; SANCHEZ-ESPIGARES, J. A.; SANS, F. X. 2012. Weeds, aphids, and specialist parasitoids and predators benefit differently from organic and conventional cropping of winter cereals. Journal Pest Science 85 (1): 81-88. [ Links ]
CÁCERES, D. 2002. Agricultura orgánica versus agricultura industrial. Su relación con la diversificación productiva y la seguridad alimentaria. Agroalimentaria (Venezuela) 16 (16): 29-39. [ Links ]
CASTILLO, F. X.; VERA, L. O. 2000. Comparación de la biodiversidad de la macrofauna de suelos bananeros con manejo convencional y orgánico en EARTH. Trabajo de grado Ingeniero Agrónomo. Universidad EARTH. Guácimo, Costa Rica. 66 p. [ Links ]
CASTRO, J.; CAMPOS, P.; PASTOR, M. 1996. Influencia de los sistemas de cultivo empleados en olivar y girasol sobre la composición de la fauna de artrópodos en el suelo. Boletín de Sanidad Vegetal, Plagas (Spain) 22 (3): 557-570. [ Links ]
CARVALHEIRO, L. G.; SEYMOUR, C. L.; VELDTMAN, R.; NICOLSON, S. W. 2010. Pollination services decline with distance from natural habitat even in biodiversity-rich areas. Journal of Applied Ecology 47 (4): 810-820. [ Links ]
CÉSPEDES, M. C.; OVALLE, C.; HIRZEL, J. 2005. Agricultura orgánica: principios y prácticas de producción. Instituto de Investigaciones Agropecuarias (INIA), Chile. 20 p. [ Links ]
CHILDS, G. 1995. Rudolf Steiner: his life and work. Anthroposophy Press, New York. 111 p. [ Links ]
CLOUGH, Y.; KRUESS, A., TSCHARNTKE, T. 2007. Local and landscape factors in differently managed arable fields affect the insect herbivore community of a non-crop plant species. Journal of Applied Ecology 44 (1): 22-28. [ Links ]
COHEN, J. 1988. Statistical power analysis for the behavioral sciences. Segunda edición. Academic Press, New York. 567 p. [ Links ]
COLDITZ, G.A.; BURDICK, E.; MOSTELLER, F. 1995. Heterogeneity in meta-analysis of data from epidemiologic studies: a commentary. Annual Journal Epidemiology 142 (4): 371-82. [ Links ]
COOPER, H.; HEDGES, L.V. 1994. The Handbook of Research Synthesis. Russel Sage Foundation, New York. 592 p. [ Links ]
COTES, B.; CAMPOS, M.; PASCUAL, F.; GARCÍA, P.A.; RUANO, F. 2010. Comparing taxonomic levels of epigeal insects under different farming systems in Andalusian olive agroecosystems. Applied Soil Ecology 44 (3): 228-236. [ Links ]
DERSIMONIAN, R.; LAIRD, N. 1986. Meta-analysis in clinical trials. Control Clinical & Trials 7 (3): 177-188. [ Links ]
DESNEUX, N.; DECOURTYE, A.; DELPUECH, J. 2007. The sublethal effects of pesticides on beneficial arthropods. The Annual Review of Entomology 52 (1): 81-106. [ Links ]
DI GIULIO, M.; EDWARDS, P.; MEISTER, E. 2001. Enhancing insect diversity in agricultural grasslands: the roles of management and landscape structure. Journal of Applied Ecology 38 (2): 310-319. [ Links ]
DÖRING, T.F.; HILLER, A.; WEHKE, S.; SCHULTE, G.; BROLL, G. 2003. Biotic indicators of carabid species richness on organically and conventionally managed arable fields. Agriculture, Ecosystems and Environment 98 (1-3): 153-161. [ Links ]
DRITSCHILO, W.; WANNER, D. 1980. Ground beetle abundance in organic and conventional corn fields. Environmental Entomology 9 (5): 629-631. [ Links ]
DUNNING, J. B.; DANIELSON, B. J.; PULLIAM, H. R. 1999. Ecological processes that affect populations in complex landscapes. Oikos 65 (1): 169-175. [ Links ]
ELLIS, P. D. 2009. "Effect size calculators". Available at: http://www.polyu.edu.hk/mm/effectsizefaqs/calculator/calculator.html [Date review: 20 September 2013] [ Links ].
EVANS, E. W. 2008. Multitrophic interactions among plants, aphids, alternate prey and shared natural enemies-a review. European Journal of Entomology 105 (3): 369-380. [ Links ]
FIBL-IFOAM Report. The world of organic agriculture. statistics and emerging trends. 2011. Willer Helga and Lukas Kilcher (Eds.). Available in: www.intracen.org/WorkArea/DownloadAsset.aspx? [Date review: 06 July 2014] [ Links ].
FEBER, R. E.; FIRBANK, L. G.; JOHNSON, P. J.; MACDONALD, D. W. 1997. The effects of organic farming on pest and non-pest butterfly abundance. Agriculture, Ecosystems and Environment 64 (2): 133-139. [ Links ]
FEBER, R.; JOHNSON, P.; FIRBANK, L.; HOPKINS, A.; MACDONALD, D. 2007. A comparison of butterfly populations on organically and conventionally managed farmland. Journal of Zoology 273 (1): 30-39. [ Links ]
FREEMAN LONG, R.; CORBETT, A.; LAMB, C.; REBERG-HORTON, C.; CHANDLER, J.; STIMMANN, Y.M. 1998. Beneficial insects move from flowering plants to nearby crops. California Agriculture 52 (5): 23-26. [ Links ]
GABRIEL, D.; TSCHARNTKE, T. 2007. Insect pollinated plants from organic farming. Agriculture, Ecosystems and Environment 118 (1-4): 43-48. [ Links ]
GABRIEL, D.; SAIT, S. M.; HODGSON, J. A.; SCHMUTZ, U.; KUNIN, W. E.; BENTON, T. G. 2010. Scale matters: the impact of organic farming on biodiversity at different spatial scales. Ecology Letters 13 (7): 858-869. [ Links ]
GAIGHER, R.; SAMWAYS, M. J. 2010. Surface-active arthropods in organic vineyards, integrated vineyards and natural habitat in the Cape Floristic Region. Journal of Insect Conservancy 14 (6): 595-605. [ Links ]
GARCÍA, J. E. 2002. Situación actual y perspectivas de la agricultura orgánica en y para Latinoamérica. Latinoamerica 38 (13): 21-34. [ Links ]
GARRATT, M.; WRIGHT, D.; LEATHER, S. 2011. The effects of farming system and fertilizers on pests and natural enemies: A synthesis of current research. Agriculture, Ecosystems and Environment 141 (3-4): 261-270. [ Links ]
GIRMA, H.; RAO, M. R.; SITHANANTHAM, S. 2000. Insect pests and beneficial arthropod populations under different hedgerow intercropping systems in semiarid Kenya. Agroforestry Systems 50 (3): 279-292. [ Links ]
GUREVITCH, J.; HEDGES, L.V. 1993. Meta-analysis: combining the results of independent experiments. Chapman and Hall, New York. 398 p. [ Links ]
HARRISON, F. 2011. Getting started with meta-analysis. Methods in Ecology and Evolution 2 (1): 1-10. [ Links ]
HEDGES, L. V.; OLKIN, I. 1985. Statistical methods for meta-analysis. Academic Press, Orlando, Florida. 369 p. [ Links ]
HOLE, D.; PERKINS, A.; WILSON, J.; ALEXANDER, I.; GRICE, P.; EVANS, A. 2005. Does organic farming benefit biodiversity?. Biological Conservation 122 (1): 113-130. [ Links ]
HOPKINS, WILL G. 2013. A new view of statistics. Available in: http://www.sportsci.org/resource/stats/index.html. [Date review: 03 October 2013] [ Links ].
HUTTON, S. A.; GILLER, P. S. 2003. The effects of the intensification of agriculture on northern temperature dung beetle communities. Journal of Applied Ecology 40 (6): 994-1007. [ Links ]
JIMÉNEZ-MARTÍNEZ, E.; GÓMEZ-MARTÍNEZ, J. 2012. Insectos plagas y benéficos asociados al Marañon (Anacardium occidentale L.) orgánico y convencional, en León, Nicaragua. La Calera, Revista Científica (Nicaragua) 12 (18): 09-17. [ Links ]
IFOAM, International Federation of Organic Agriculture Movements. 2009a. The principles of organic agriculture. Available in: http://www.ifoam.org/about_ifoam/principles/index.html [Date review: 08 October 2012] [ Links ].
IFOAM, International Federation of Organic Agriculture Movements. 2009b. Definitions of organic agriculture. Available in: http://www.ifoam.org/growing_organic/definitions/doa/index.html [Date review: 08 October 2012] [ Links ].
KEHINDE, T.; SAMWAYS, M. J. 2012. Endemic pollinator response to organic vs. conventional farming and landscape context in the Cape Floristic Region biodiversity hotspot. Agriculture, Ecosystems and Environment 146 (1):162-167. [ Links ]
KERR, J. T. 2001. Butterfly species richness patterns in Canada: energy, heterogeneity, and the potential consequences of climate change. Conservation Ecology 5 (1): 10-18. [ Links ]
KLEIN, A. M.; DEWENTER, I. S.; TSCHARNTKE, T. 2002. Predator-prey ratios on cocoa along a land-use gradient in Indonesia. Biodiversity Conservation 11 (4): 683-693. [ Links ]
KLEIN, A.; BRITTAIN, C.; HENDRIX, S.D.; THORP, R.; WILLIAMS, N.; KREMEN, C. 2012. Wild pollination services to California almond rely on semi-natural habitat. Journal of Applied Ecology 49 (3): 723-732. [ Links ]
KOEPF, H. H.; PETTERSSON, B. D.; SCHAUMANN. 1976. Biodynamic agriculture: an introduction. Anthroposophy Press, New York. 429 p. [ Links ]
KRUESS, A.; TSCHARNTKE, T. 2002. Contrasting responses of plant and insect diversity to variation in grazing intensity. Biological Conservation 106 (3): 293-302. [ Links ]
LANDIS, D. A.; WRATTEN, S. D.; GURR, G. M. 2000. Habitat management to conserve natural enemies of arthropod pest in agriculture. Annual Review Entomology 45 (1): 175-201. [ Links ]
LANGHOF, M.; GATHMANN, A.; POEHLING, H. M.; MEYHÖFER, R. 2003. Impact of insecticide drift on aphids and their parasitoids: Residual toxicity, persistence and recolonisation. Agriculture Ecosystem Environment 94 (3): 265-274. [ Links ]
LANNACONE, J.; LAMAS, G. 2003. Efecto insecticida de cuatro extractos botánicos y del cartap sobre la polilla de la papa Phthorimaea operculella (Zeller) (Lepidoptera: Gelechiidae), en el Perú. Entomotrópica 18 (2): 95-105. [ Links ]
LETELIER, L. M.; MANRIQUEZ, J.; RADA, G. 2005. Revisiones sistemáticas y metaanálisis: ¿son la mejor evidencia?. Revista Médica de Chile 133 (2): 246-249. [ Links ]
LETOURNEAU, D.; GOLDSTEIN, B. 2001. Pest damage and arthropod community structure in organic vs. conventional tomato production in California. Journal of Applied Ecology 38 (3): 557-570. [ Links ]
MACFADYEN, S.; GIBSON, R.; POLASZEK, A.; MORRIS, R. J.; CRAZE, P. G.; PLANQUE, R.; SYMONDSON, W.; MEMMOTT, J. 2009. Do differences in food web structure between organic and conventional farms affect the ecosystem service of pest control?. Ecology Letters 12 (3): 229-238. [ Links ]
MARINO, P. C.; LANDIS D. A. 1996. Effect of landscape structure on parasitoid diversity and parasitism in agroecosystems. Ecological Applications 6 (1): 276-284. [ Links ]
MIÑARO, M.; ESPADALER, X.; MELERO, V. X.; SUAREZ-ÁLVAREZ, V. 2009. Organic versus conventional management in an apple orchard: effects of fertilization and tree-row management on ground-dwelling predaceous arthropods. Agricultural and Forest Entomology 11 (2): 133-142. [ Links ]
MONDELAERS, K.; AERTSENS, J.; VAN HUYLENBROECK, G. 2009. A meta-analysis of the differences in environmental impacts between organic and conventional farming. British Food Journal 111 (10): 1098-1119. [ Links ]
MORANDIN, L. A.; WINSTON, M. L. 2005. Wild bee abundance and seed production in conventional, organic, and genetically modified canola. Ecological Applications 15 (3): 871-881. [ Links ]
MOREBY S. J.; AEBISCHER N. J.; SOUTHWAY S. E.; SOTHERTON N. W. 1994. A comparison of the flora and arthropod fauna of organically and conventionally grown winter-wheat in southern England. Annals of Applied Biology 125 (1): 13-27. [ Links ]
MORRIS, M. 1979. Responses of grassland invertebrates to management by cutting. II. Heteroptera. Journal of Applied Ecology 16 (2): 417-432. [ Links ]
NOSS, R. F. 1990. Indicators for monitoring biodiversity, a hierarchical approach. Conservation Biology 4 (4): 355-364. [ Links ]
PAOLETTI, M. G.; PIMENTEL, D.; STINNER, B. R.; STINNER, D. 1992. Agroecosystem biodiversity: matching production and conservation biology. Agriculture, Ecosystems and Environment 40 (1-4): 3-23. [ Links ]
PAOLETTI, M. G. 1995. Biodiversity, traditional landscapes and agroecosystem management. Landscape and Urban Planning 31 (1-3): 117-128. [ Links ]
PARRA, C.; CALATRAVA, J.; DE HARO, T. 2004. Análisis multifuncional de sistemas agrarios: aplicación del método del proceso analítico jerárquico al olivar de producción convencional, ecológica e integrada en Andalucía. Málaga, Analistas Económicos de Andalucía. Analistas Económicos de Andalucía, Málaga. Spain. 301 p. [ Links ]
PETERSON, D. L.; PARKER, T. V. 1998. Ecological scale: Theory and Applications. Columbia University Press, New York. 608 p. [ Links ]
PFINNER, L.; NIGGLI, U. 1996. Effects of bio-dynamic, organic and conventional farming on ground beetles (Col. Carabidae) and other epigaeic arthropods in winter wheat. Biological Agriculture and Horticulture 12 (4): 353-364. [ Links ]
POTTS, S. G.; ROBERTS, S. P. M.; DEAN, R.; MARRIS, G.; BROWN, M.; JONES, R.; SETTELE, J. 2010. Declines of managed honeybees and beekeepers in Europe. Journal of Apicultural Research 49 (1): 15-22. [ Links ]
POVEDA, K.; STEFFAN-DEWENTER, I.; SCHEU, S.; TSCHARNTKE, T. 2006. Belowground effects of organic and conventional farming on aboveground plant-herbivore and plant-pathogen interactions. Agriculture, Ecosystems and Environment 113 (1-4): 162-167. [ Links ]
POWER, E.; STOUT, J. 2011. Organic dairy farming: impacts on insect-flower interaction networks and pollination. Journal of Applied Ecology 48 (3): 561-569. [ Links ]
PRICE, W. P.; BOUTON, C. E.; GROSS, P.; McPHERON, B. A.; THOMPSON, J. N.; WEIS, A. E. 1980. Interactions among three trophic levels: influence of plants on interactions between insect herbivores and natural enemies. Annual Review of Ecology and Systematics 11: 41-65. [ Links ]
PRIETO-BENÍTEZ, S.; MÉNDEZ, M. 2011. Effects of land management on the abundance and richness of spiders (Araneae): A meta-analysis. Biological Conservation 144 (2): 683-691. [ Links ]
PURTAUF, T.; ROSCHEWITZ, I.; DAUBER, J.; THIES, C.; TSCHARNTKE, T.; WOLTERS, V. 2005. Landscape context of organic and conventional farms: Influences on carabid beetle diversity. Agriculture, Ecosystem and Environment 108 (2): 165-174. [ Links ]
RIGBY, D.; CÁCERES, D. 2001. Organic farming and the sustainability of agricultural systems. Agricultural systems 68 (1): 21-40. [ Links ]
ROOT, R. B. 1967. The niche exploitation pattern of the blue-gray gnatcatcher. Ecological Monographs 37 (4): 317-350. [ Links ]
ROOT, R. S. 1973. Organization of a plant-arthropod association in simple and diverse habitats: the fauna of collards (Brassica oleracea). Ecological Monographs 43: 95-120. [ Links ]
RUNDLÖF, M.; SMITH, H. G. 2006. The effect of organic farming on butterfly diversity depends on landscape context. Journal of Applied Ecology 43 (1): 1121-1127. [ Links ]
RUNDLÖF, M.; NILSSON, H.; SMITH, H. G. 2008a. Interacting effects of farming practice and landscape context on bumble bees. Biological Conservation 141 (2): 417-426. [ Links ]
RUNDLÖF, M.; BENGTSSON, J.; SMITH, H. G. 2008b. Local and landscape effects of organic farming on butterfly species richness and abundance. Journal of Applied Ecology 45 (3): 813-820. [ Links ]
RUSHTON, S., LUFF, M.; EYRE, M. 1989. Effects of pasture improvement and management on the ground beetle and spider communities of upland grasslands. Journal of Applied Ecology 26 (2): 489-503. [ Links ]
SALAZAR, L.; SALVO A. 2007. Entomofauna asociada a cultivos hortícolas orgánicos y convencionales en Córdoba, Argentina. Neotropical Entomology 36 (5): 765-773. [ Links ]
SANDHU, H.; WRATTEN, S.; CULLEN, R. 2010. Organic agriculture and ecosystem services. Environmental science & policy 13 (1): 1-7. [ Links ]
SCHÜTZ, K.; BONKOWSKI, M.; SCHEU, S. 2008. Effects of Collembola and fertilizers on plant performance (Triticum aestivum) and aphid reproduction (Rhopalosiphum padi). Basic and Applied Ecology 9 (2): 182-188. [ Links ]
SHAH, P. A.; BROOKS, D. R.; ASHBY, J. E.; PERRY, J. N.; WOIWOD, I. P. 2003. Diversity and abundance of the coleopteran fauna from organic and conventional management system in southern England. Agricultural and Forest Entomology 5 (1): 51-60. [ Links ]
SNYDER, P.; LAWSON, S. 1993. Evaluating results using corrected and uncorrected effect size estimates. Journal of Experimental Education 61 (4): 334-349. [ Links ]
STOPES, C.; MEASURES, M.; SMITH, C.; FOSTER, L. 1995. Hedgerow management in organic farming impact on diversity. Llerema, J. J. Eds. Bonn, Germany. 125 p. [ Links ]
SYMINGTON, S. A. 2003. Lethal and sublethal effects of pesticides on the potato tuber moth, Phthorimaea operculella (Zeller) (Lepidoptera: Gelechiidae) and its parasitoid Orgilus lepidus Muesebeck (Hymenoptera: Braconidae). Crop Protection 22 (3): 513-519. [ Links ]
TEAGARDEN, J. R. 1989. Meta-analysis: whither narrative review? Pharmacotherapy 9 (5): 274-284. [ Links ]
TILMAN, D.; FARGIONE, J.; WOLFF, B.; ANTONIO, C.; DOBSON, A.; HOWARTH, R. 2001. Forecasting agriculturally driven global environmental change. Science 292 (5515): 281-284. [ Links ]
TSCHARNTKE, T.; STEFFAN-DEWENTER, I.; KRUESS, A.; THIES, C. 2002. Contribution of small habitat fragments to conservation of insect communities of grassland-cropland landscapes. Ecological Applications 12 (2): 354-363. [ Links ]
VAN ZANDT, P. A.; MOPPER, S. 1998. A meta-analysis of adaptive deme formation in phytophagous insect populations. American Naturalist 152 (4): 595-604. [ Links ]
VANDERMEER, J.; PERFECTO, I. 1995. Breakfast of biodiversity: the truth about rainforest destruction. Food First Books, Oakland. 185 p. [ Links ]
VARCHOLA, J. M.; DUNN, J. P. 1999. Changes in ground beetle (Coleoptera: Carabidae) assemblages in farming systems bordered by complex or simple roadside vegetation. Agriculture, Ecosystems and Environment 73 (1): 41-49. [ Links ]
VARCHOLA, J. M.; DUNN, J. P. 2001. Influence of hedgerow and grassy field borders on ground beetle (Coleoptera: Carabidae) activity in fields of corn. Agriculture, Ecosystems and Environment 83 (1-2): 153-163. [ Links ]
VICKERY, J.; TALLOWIN, J.; FEBER, R.; ASTERAKI, E.; ATKINSON, P.; FULLER, R.; BROWN, V. 2001. The management of lowland neutral grasslands in Britain: effects of agricultural practices on birds and their food resources. Journal of Applied Ecology 38 (3): 647-664. [ Links ]
WEIBULL, A. C.; BENGTSSON, J.; NOHLGREN, E. 2000. Diversity of butterflies in the agricultural landscape: the role of farming system and landscape heterogeneity. Ecography 23 (6): 743-750. [ Links ]
WEIBULL, A. C.; ÖSTMAN, Ö.; GRANQVIST, A. 2003. Species richness in agroecosystems: the effect of landscape, habitat and farm management. Biodiversity and Conservation 12 (7): 1335-1355. [ Links ]
WICKRAMASINGHE, L. P.; HARRIS, S.; JONES, G; VAUGHAN, N. 2003. Bat activity and species richness on organic and conventional farms: impact of agricultural intensification. Journal of Applied Ecology 40 (6): 984-993. [ Links ]
WICKRAMASINGHE, L. P.; HARRIS, S.; JONES, G.; VAUGHAN-JENNINGS, N. 2004. Abundance and species richness of nocturnal insects on organic and conventional farms: Effects of agricultural intensification on bat foraging. Conservation Biology 18 (5): 1283-1292. [ Links ]
WILSON, J.; MORRIS, A.; ARROYO, B.; CLARK, S.; BRADBURY, R. 1999. A review of the abundance and diversity of invertebrate and plant foods of granivorous birds in northern Europe in relation to agricultural change. Agriculture Ecosystems and Environment 75 (1-2): 13-30. [ Links ]
Received: 17-Apr-2014
Accepted: 3-Nov-2014
Support information:
This article has a supplement (Table 3) available in version Online http://www.socolen.org.co/_archivos/RCdE_40_2_2014_suplemento.pdf