Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488
Rev. Colomb. Entomol. vol.41 no.1 Bogotá Jan./June 2015
Growth regulator insecticides for the control of the lesser mealworm beetle Alphitobius diaperinus (Coleoptera: Tenebrionidae)
Insecticidas reguladores del crecimiento para el control del escarabajo de la cama Alphitobius diaperinus (Coleoptera: Tenebrionidae)
JANAINA ZORZETTI1,2, KELLY CONSTANSKI1,2, PATRICIA H. SANTORO3, INÊS C. B. FONSECA2,4 and PEDRO M. O. J. NEVES2,4
1 Ph. D. jzorzetti@hotmail.com. Corresponding author. kconstanski@hotmail.com.
2 Universidade Estadual de Londrina, Centro de Ciencias Agrárias Rod. Celso Garcia Cid, PR 445, km 380, CP: 6001, CEP: 86051-9901. Londrina, PR, Brazil.
3 Ph. D. Instituto Agronómico do Paraná, Rod. Celso Garcia Cid, PR 445, km 375, CP: 481 CEP: 86047-902, Londrina, PR, Brazil. patriciasantoro@iapar.br.
4 Ph. D. pedroneves@uel.br; inescbf@uel.br.
Abstract: The purpose of this study was to evalúate the efficiency of the insecticides methoxyphenozide, teflubenzuron, pyriproxyfen, chlorantraniliprole and also Azadirachta indica in the control of Alphitobius diaperinus (Coleoptera: Tenebrionidae). The study was carried out in the Microbial Control laboratory of Universidade Estadual Londrina (Brazil) and consisted of two bioassays. In the first, A. diaperinus larvae were fed with corn feed treated with the insecticides in seven dosages. In the second bioassay, the different treatments were sprayed on the larvae only at the three highest dosages. Evaluations were performed daily, quantifying larval mortality, and on the 30th day, the number of pupae and adults were evaluated. Teflubenzuron, chlorantraniliprole and A. indica were responsible for the greatest percentage of mortalities in larvae fed with treated feed. The treatments with methoxyphenozide and pyriproxyfen caused the lowest mortalities; however, with pyriproxyfen the larvae were not transformed into pupae and adults up to the 30th day of assessment. The treatment with teflubenzuron showed the greatest toxicity, with the lowest survival time (ST50) When the products were applied directly on to the larvae, the most efficient treatments were teflubenzuron, chlorantraniliprole and A. indica; however, there was a reduction in the mortality percentages with teflubenzuron and chlorantraniliprole in relation to the feed bioassay. For A. indica, the application method did not affect the mortality levels. Pyriproxyfen, which had not led to mortality through feed ingestion, obtained an increase of up to 30% after being applied directly on to the larvae.
Key words: Growth regulator insecticides. Chlorantraniliprole. Azadirachta indica. Toxicity. Survival analysis.
Resumen: El objetivo del trabajo fue evaluar la eficiencia de los insecticidas metoxifenozide, teflubenzuron, piriproxifeno, clorantraniliprole y Azadirachta indica en el control del escarabajo de la cama Alphitobius diaperinus. Se realizaron dos bioensayos en el laboratorio del control microbiano de la Universidade Estadual Londrina (Brasil). En el primero, las larvas de A. diaperinus fueron alimentadas con pienso de maíz tratado con los insecticidas en siete dosis (i.e. dosis recomendada - DR y porcentajes del 50; 25; 12,5; 6,25; 3,12 y 1,65 de la misma). En el segundo bioensayo, lós diferentes tratamientos fueron aplicados en las larvas pero sólo en las tres más altas dosis. La cuantificación de la mortalidad de larvas se hizo diariamente hasta el día 30, cuando se evaluóel número de pupas y adultos. Las mayores mortalidades de larvas se presentaron en larvas alimentadas con raciones con teflubenzuron, clorantraniliprole y A. indica. Los tratamientos con metoxifenozide y piriproxifeno causaron menor mortalidad, sin embargo, con este último, las larvas no alcanzaron a formar pupas ni adultos. Teflubenzuron presentóla mayor toxicidad con menor TL50. Cuando los productos fueron aplicados directamente en las larvas, los tratamientos más eficientes fueron teflubenzuron, clorantraniliprole y A. indica, sin embargo, hubo reducción en los porcentajes de la mortalidad con teflubenzuron y clorantraniliprole. Para A. indica, el método de aplicación no interferiócon los niveles de mortalidad. Piriproxifeno, que no había causado mortalidad en el consumo de ración, aumentóhasta el 30% cuando fue aplicado a la larva.
Palabras clave: Insecticidas reguladores del crecimiento. Clorantraniliprole. Azadirachta indica. Toxicidad. Análisis de supervivencia.
Introduction
The lesser mealworm Alphitobius diaperinus (Panzer, 1797) (Coleoptera: Tenebrionidae) is considered the main pest in poultry houses around the world, found in large quantities in poultry litter and manure (Pfeiffer and Axtell 1980; Lambkin et al. 2007). Its presence is also observed in the compacted soil floor of broiler houses and it may reach a depth of 0.8 m (Chernaki-Leffer et al. 2001). Moreover, it can be found under feeders, feeding on chicken feed and also consuming dead or moribund chicks (Axtell and Arends 1990).
This pest can be a source and vector of several pathogens like bacteria, viruses, fungi, protozoa and platyhelminthes parasites that cause harmful diseases to poultry and humans (Despins and Axtell 1995; Mcallister et al. 1995; Goodwin and Waltman 1996; Chernaki-Leffer et al. 2002; Vittori et al. 2007). Also, A. diaperinus can be harmful to poultry, since the poultry can consider it to be an alternative source of food, but eating it may result in lesser weight gain compared to those poultry which feed on the nutrient-balanced feed (Axtell and Arends 1990; Matias 2000).
The expansion of the poultry industry and the current breeding systems contributed to the development of an ideal habitat for lesser mealworms. These systems reuse the chicken litter with each lot exchange, providing the environment with suitable temperature and moisture for rapid population growth and the spread of new areas of infestation from one lot to another (Salin 2000).
The difficulty in controlling this pest leads to significant economic losses and sanitary problems in poultry production.
Henee studies are encouraged to obtain Solutions for the management of its populations (Bates et al. 2004).
The control of A. diaperinus populations is done through environment management, such as frequent cleaning of the poultry house and removal of the litter after each lot; however, it is an expensive and laborious activity (Axtell and Arends 1990). Another method is the use of chemical products at the end of the poultry cycle such as: pyrethroids (Hamm et al. 2006); organophosphorus compounds, chlorates and carbamates (Morales 1991); macrocyclic lactones (Miller 1990); and boric acid (Dufor et al. 1992). One negative result is the resistance factors directly multiply with numbers of insecticide applications (Lambkin and Rice 2006).
In addition to these insecticides, other methodologies are being studied for the control of the lesser mealworm, such as bioinsecticides and products with a differentiated mode of action. In this context, insect growth regulator (IGR) insecticides are an alternative because they act in a more specific way and they are less toxic to mammals (Silva and Mendes 2002). In the IGR group, the benzoylphenyl ureas (e.g., teflubenzuron), the diacyl-hydrazines (e.g., methoxyfenozide) and pyridyloxypropyl ether (pyriproxyfen) stand out.
The benzoylphenylureas (chitin synthesis inhibitors) are active during larvae ecdysis, specifically affecting chitin deposition, preventing it from secreting a new cuticle and freeing it from the exocuticle (Silva et al. 2003). These products have already been cited as efficient in lesser mealworm control (Weaver 1996). The diacyl hydrazines act as ecdysone antagonists, promoting acceleration in the ecdysis process (Dhadialla et al. 1998; Omoto 2000). The juvenoids (pyridyloxypropyl ether) are juvenile hormone analogues and cause development disorders in the insects (Ferreira 1999).
Azadirachtin, a botanical compound extracted from the neem plant, Azadirachta indica A. Juss. (Meliaceae), is also considered to be a growth regulator because it is active in ecdysis, probably competing with ecdysteroids at the larvae's receptor site (Sharma 1992; Dev and Koul 1997; Adel and Sehnal 2000).
The anthranilic diamides (e.g., chlorantraniliprole), even though they are not included in the IGR group, exhibit a different mode of action against the insects. They are ryano-dine receptor activators and cause insects to lose control of muscular activity (Cordova et al. 2006).
This new generation of insecticides may be considered as an alternative in pest control because they have more specific action and cause less toxicity to warm-blooded animals. Edwards and Abraham (1985) observed low toxicity to vertebrates of some IGR in their study, such as methoprene and fenoxycarb, and suggested that these compounds could be administered via feed in poultry raising, remaining biologically active even after passing through the poultry digestive tract.
The purpose of this study was to evaluate the effect of growth regulator insecticides (methoxyphenozide, tefluben-zuron, pyriproxyfen), chlorantraniliprole and A. indica in the control of A. diaperinus larvae.
Materials and methodsInsects. Chicken litter containing A. diaperinus larvae was collected in a commercial poultry house in Londrina, Paraná. This material was taken to the laboratory where the larvae were separated from the litter and from the adult insects for later use in the bioassays.
Insecticide. The active ingredients evaluated were methoxyfenozide (INTREPID®), teflubenzuron (NOMOLT®), pyriproxyfen (TIGER®), chlorantraniliprole (PREMIO®) and A. indica (NIMAZAL®). Since there was no reference to their use for A. diaperinus control, the dosages used were based on the highest recommended dosage (RD) for other pests, obtained from the instructions of the commercial products (Agrofit 2012). The commercial products were diluted in distilled water to obtain seven different concentrations of each active ingredient (recommended dosage-RD, and variables percentages of this RD - i.e. 50; 25; 12.5; 6.25; 3.12 and 1.56).
Bioassays. The bioassays were run in the Microbial Control laboratory of Universidade Estadual Londrina (Brazil) with 4th instar (± 0.5 cm) A. diaperinus larvae (Silva et al. 2005). Two bioassays were performed, evaluating indirect and direct contact with the products. For the first assay, called "Insecticides Applied on the Feed", 8 mL of each concentration were sprayed on 12 g of sterilized corn feed with the airbrush sprayer connected to a Fanen-Diapump vacuum pump-compressor at a pressure of 0.8 kgf.cm-1. The dosage in ppm of active ingredient for grams of feed was 1000 ppm for methoxyfenozide, 350 ppm for teflubenzuron, 233 ppm for chlorantraniliprole, 133 ppm for pyriproxyfen and 120 ppm for A. indica. After its complete homogenization, it was distributed in six acrylic boxes (2.5 cm diameter x 1.5 cm height) composed of 20 PET bottle caps glued side-by-side. Into these boxes, the larvae were subsequently individualized to avoid cannibalism.
In the second assay, called "Insecticides Applied on A. diaperinus Larvae", the insecticide solutions were sprayed directly on the larvae. The products used were the same as the previous assay; however, they were tested only at the three highest doses (RD, and percentages of this RD - i.e. 50; 25). Twenty 4th instar larvae, in a petri dish were sprayed with 0.5 mL. Them they were individualized in the caps and fed with sterilized corn feed.
For each bioassay, six replications with 20 larvae were performed, for a total of 120 larvae per treatment, plus a control group where the insecticides were substituted by distilled water. The insects were kept in an incubator (25 ± 1 °C, 12-hour photophase and relative humidity of 75 ± 10%) for 30 days. Mortality was assessed daily, and the number of live larvae, pupae and adults was determined only on the 30th day of evaluation.
Statistical analysis. Mortality data were analyzed by probit analysis (Finney 1971) (POLO-PC, Leora Software 1987) to obtain the median lethal concentration (LC50) its 95% confidence intervals and the slopes of dose-mortality curves. The median survival times (ST50) for all products were estimated using the Kaplan-Meier Product-Limit estimator method (JMP, SAS Institute 2008) and were compared using the log-rank test (Kabfleisch and Prentice 1980). The data of larvae mortality, live larvae, pupae and adults, in percentage, did not meet the requirements for a parametric test; thus, they were subjected to the Kruskal-Wallis test and the mean rank values compared by the Dunn test at 5% probability (Ayres et al. 2007).
Results and discussion
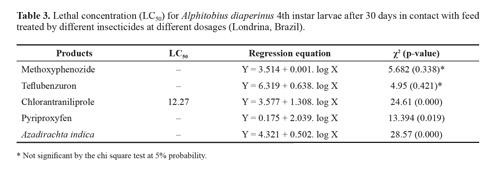
Insecticides applied on the feed. The highest mortalities of A. diaperinus larvae were observed for teflubenzuron, which led to a mean mortality rate from 96 to 100%. Chlorantraniliprole also caused high mortality which increased with higher dosages. A. indica base product also led to increased mortality with an increase in dosages; however, the highest percentage was obtained at 12.5 RD, whereas in RD, the mortality was reduced. This fact may have occurred due to the inhibiting action caused by the main compound of this plant, azadirachtin, which, in addition to being toxic to insects, has a feeding deterrent and repellent activity (Mordue (Luntz) and Nisbet 2000). In higher concentration in the feed, it may have inhibited insect feeding and, consequently, a smaller quantity of the product was ingested. In spite of the high mortalities caused by teflubenzuron, there was no significant difference between chlorantraniliprole and A. indica, but it differed from the methoxyphenozide and pyriproxyfen treatments, as these latter two led to the lowest levels of mortality, no different from the control (Table 1).
Due to the high mortality caused to the larvae fed on teflubenzuron treated feed, the percentage of live pupae, adults and larvae was practically null. After 30th day of evaluation, the highest number of live larvae, pupae and adults was lower than the control. For chlorantraniliprole and A. indica, increasing the dosages led to a low number of larvae transforming into pupae and adults (Table 1).
The benzoylphenyl ureas (teflubenzuron) inhibit the formation of chitin synthetase (Retnakaran et al. 1985). Hence, larvae treated with these insecticides cannot shed their exocuticle. This might be what led to the mortality of larvae fed with teflubenzuron treated feed in the present study.
These results contrast with those of Chernaki-Leffer et al. (2006), which, in spite of having used products from the benzoylphenyl urea group (triflumuron and diflubenzuron at 10 ppm), obtained a reduced percentage of mortality of A. diaperinus larvae. Nevertheless, it is important to highlight that in the study cited, the larvae were exposed to the treated food for three days only, afterwards being fed with untreated feed. In the present study, the larvae remained with the treated food throughout the entire period of evaluation (30 days), which allowed the constant ingestion of the active ingredient. The A. indica insecticide action on A. diaperinus adults and larvae in laboratory was previously shown in other studies (Szczepanik 2001; Marcomini et al. 2009). This effect on larvae may be caused by the azadirachtin, which acts as a chitin synthesis inhibitor, interfering in cuticle formation and resulting in deformation and death of insects (Casida and Quistad 1998).
In addition to being efficient in diverse pest control, the complexity and diversity of the mode of action of A. indica could avoid the selection of resistant insects (Chaieb et al. 2007; Khatter 2011). This characteristic is of great importance in A. diaperinus control, in which resistance to cyfluthrin (pyrethroid) and fenitrothion (organophosphate) has already been reported (Lambkin and Rice 2006).
Also chlorantraniliprole may be used as an alternative for A. diaperinus control and in resistance management strategies because, besides exhibiting high mortality rates, it has a different mode of action that would reduce the chance of the development of resistant populations. It acts when its molecules bond to the ryanodine receptors in the myofibrils of muscle cells, and causes uncontrolled release of calcium. Consequently, insects lose muscle control, leading to rapid cessation of feeding, regurgitation and failure of the heart muscle (Cordova et al. 2006).
Methoxyphenozide is a diacyl hydrazine group product and acts as an agonist of the ecdysteroids, causing acceleration in the ecdysis process (Omoto 2000). However, it doesn't appear to be efficient in A. diaperinus larvae control (Chernaki-Leffer et al. 2006). These results corroborate with those observed in the present study where methoxyphenozide exhibited the lowest larvae mortality.
Kostyukovsky et al. (2000) evaluated the tebufenozide effect on Tribolium castaneum (Herbst) (Coleoptera: Tene-brionidae) and also observed low mortality. This product belongs to the same chemical group as methoxyphenozide, and its insecticide action may have occurred due to some characteristic of the product in relation to the coleopteran. According to Sousa (2003) they are more efficient in Le-pidoptera control, interfering in the development of cater-pillars, with no action on pupae and with sub-lethal action in adults.
Pyriproxyfen, also included in the IGR group, is a ju-venoid which acts by prolonging the nymph larval stages (Ferreira 1999). Some studies report that in addition to affecting adult emergence, the compound also affects adult weight, sexual ratio, and insect deformation (Dhadialla et al. 1998; Sial and Brunner 2010). This may explain the results obtained in the present study, where on average 90% of the larvae fed with feed treated with this product remained in the early stage (larvae) up to the 30th day of evaluation. Therefore, pyriproxyfen may be seen as a promising agent in the control and management of A. diaperinus populations because it will cause a rupture in the insect cycle.
This same effect was obtained by Kostyukovsky et al. (2000), who evaluated the mortality and morphological characteristics of Rhyzopertha dominica (Fabricius, 1792) (Coleoptera, Bostrichidae), Sitophilus oryzae (Linné, 1763) (Coleoptera: Curculionidae) and T. castaneum larvae after the consumption of food treated with pyriproxyfen by adult parents. The authors observed the hatching of deformed larvae, and soon after a long larval period, they died while still in this stage.
The mortality of larvae by teflubenzuron was already observed on the third day of evaluation; nevertheless, the period of feeding in order to achieve 50% mortality was 10 days in all doses, except in RD, where the ST50 was 15 days. This may be due to feeding inhibition caused by the insecticide, which at elevated concentration, made the larvae take more time to begin their feeding and to become intoxicated by the product. The treatment with teflubenzuron showed higher toxicity when compared with other products because it showed the lowest ST50. Nevertheless, in 25RD and 50RD, it did not differ from chlorantraniliprole and in RD fromA. indica, which indicates that at higher concentrations, these products act in a more rapid way (Table 2).
For A. indica, the ST50 was high for the 25RD and 50RD; however, with the increase of concentration, the lethal time was reduced (Table 2). This slower action was also reported by Szczepanik (2001), where 1st instar larvae fed with poultry feed treated with a neem-based commercial insecticide (Neem-Azal-TTM) at the concentrations of 0.01 and 0.1% died after 25 and 45 days, respectively.
Other studies have shown the great amount of time spent in controlling these insects with the IGR. Weaver (1996) obtained larval control of A. diaperinus seven days after applications with hexafluron and triflumuron. These products normally do not show an immediate effect because mortality only occurs after larval ecdysis. Thus, the author recommends its association with pyrethroids, which act in a rapid way and show good results in adult control, while the IGR will act efficiently in larvae control.
The differences obtained in the results did not allow the estimate of the lethal concentration of each product because they did not fit the Probit model. Even for chlorantraniliprole, which obtained LC50 of 12.27, it was not possible to check the fit of the values since the p-value for the chi-square test was significant (less than 0.005), indicating that the adjustment to the model is not appropriate. In addition, some products like methoxyphenozide and pyriproxyfen caused mortality below 50% (Table 3).
Insecticides applied on A. diaperinus larvae. There was a difference in the results when the insecticides were applied directly on the larvae in relation to the results observed for application on the feed. In spite of teflubenzuron still proving to be the most efficient product, the mortality percentages decreased compared with the product that was applied directly on the feed. For chlorantraniliprole there was also a reduction in the mortality percentages in relation to the first bioassay because, even with reduction in the product efficiency, chlorantraniliprole applied on the insects was one of the most effective treatments, not differing from teflubenzuron. For A. indica, this application method did not affect the mortality levels and caused a mean mortality that did not differ from teflubenzuron and chlorantraniliprole. However, methoxyphenozide, in spite of causing the lowest mortality, not differing from the control, obtained an increase of up to 30% in relation to the larvae that ingested the treated feed (Tables 1 and 4).
It was observed that when the product pyriproxyfen was applied directly on A. diaperinus larvae, mortality percentages were greater than when the product was applied directly on the feed in all dosages tested (Tables 1 and 4). This mortality percentage increase shows that direct contact between the product and the insect increases control efficiency, which probably occurs due to greater penetration capability of the insecticide through its cuticle. This may explain the results obtained in most of the studies with pyriproxyfen, where the highest mortalities are observed after topical application of the product on adults, nymphs and eggs of Eurygaster integriceps Puton (Heteroptera: Scutelleridae) (Chen and Borden 1989; Mojaver and Bandani 2010).
Some compounds may be toxic to insects through contact as well as ingestion, like that which occurred with A. indica in this study. This was also observed by Kavillieratos et al. (2007), for T. castaneum and S. oryzae reared in corn and
wheat. Nevertheless, for Gonipterus scutellatus Gyllenhal (Coleoptera: Curculionidae), lufenuron and flufenoxuron, that belong to the same Chemical group as teflubenzuron (benzoyl ureas), contact resulted in low toxicity (Molina and Carbone 2010). These results agree with the data obtained in this current study, where teflubenzuron was more efficient when ingested than when in direct contact with the larvae. This may be due to the particularities of the insects and how the product is metabolized by them, and also the compounds and mode of action of the insecticide. Some authors cited that the action of these insecticides is mainly through ingestion in the larval stage (Dhadialla et al. 1998; Tunaz and Uygun 2004).
Pyriproxyfen, despite causing a higher mortality rate when applied on the larvae, maintained the low number of pupae and adults observed in the ingestion bioassay, confirming its effect as a juvenoid, prolonging the larval stages. Interference in the life cycle of the insects was also reported in other studies, where the emergence of adults of A. diaperinus and Ostrinia nubilalis (HÜbner) (Lepidoptera: Pyralidae) was suppressed when treated with fenoxicarb, an insecticide belonging to the same chemical group as pyriproxyfen (Edwards and Abraham 1985).
After spraying the larvae with the different insecticides, the shortest time leading to death of 50% of the population was with teflubenzuron and chlorantraniliprole, which took 20 days with 25RD and 50RD, and 15 days at RD, proving to be more efficient in control of A. diaperinus. Pyriproxyfen was the least toxic product, taking 30 days at the 50RD and 25 days at RD (Table 5)
In conclusion, in all these cases it was observed that the insecticides used in this study need more time to act when compared to conventional products, such as the pyrethroids (Dhadialla et al. 1998). However, they are less toxic to poultry and humans (Silva and Mendes 2002). Therefore, a strategy for A. diaperinus population control is necessary, taking into account various factors, such as lethal time, the best application period, toxicity for poultry and the environment and resistance management. So the association of these products with contact insecticides, for adult control, mainly in treatment between poultry lots, is a valid alternative.
Henee, larvae might be controlled afterwards by the growth regulator insecticides.
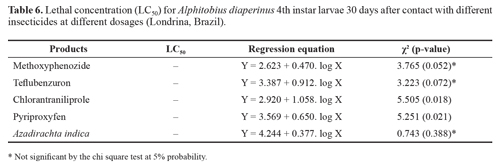
In spite of mortalities above 50% being observed in most of the tested products as well as in the bioassays with insecticides applied on the feed, the heterogeneity of the results did not allow the estimate of the lethal concentration of each product, and the data did not fit the Probit model (Table 6).
The growth regulator insecticides, teflubenzuron, chlo-rantraniliprole and A. indica, affected the survival and de-velopment of A. diaperinus larvae in the laboratory. Py-riproxyfen did not allow the larvae to reach the adult phase and Methoxyphenozide was not efficient in controlling A. diaperinus.
When applied as a contact insecticide, teflubenzuron caused lower mortality rates than when ingested with feed, in contrast to pyriproxyfen, which obtained higher mortality rates when applied directly on the insects.
AcknowledgementsThe authors wish to express their thanks to Anízio Fecchio, the producer and owner of the poultry houses where the insect collections were made and to the CNPq for the scholarship.
Literature citedADEL, M. M.; SEHNAL, F. 2000. Azadirachtin potentiates the action of ecdysteroid agonist RH-2485 in Spodoptera littoralis. Journal of Insect Physiology 46: 267-274. [ Links ]
AGROFIT. Sistema de agrotóxicos fitossanitários. 2012. Available from:;http://agrofit.agricultura.gov.br/agrofit_cons/principal_ agrofit_cons. Review date: 04 April 2013. [ Links ]
AXTELL, R. C.; ARENDS, J. J. 1990. Ecology and management of arthropod pests of poultry. Annual Review of Entomology 35: 101-126. [ Links ]
AYRES, M.; AYRES JÚNIOR, M.; AYRES, D. L.; SANTOS, A. A. 2007. BIOESTAT - Aplicares estatísticas nas áreas das ciencias biomédicas. Ong Mamiraua. Belém, PA. [ Links ]
BATES, C. 2004. Relationship of Campylobacter isolated from poultry and from darkling beetles in New Zealand Avian Diseases 48: 138-147. [ Links ]
CASIDA, J. E.; QUISTAD, G. B. 1998. Golden age of insecticide: Past, present, or future? Annual Review of Entomology 43: 1-16. [ Links ]
CHAIEB, I.; HALIMA-KAMEL, B. M.; TRABELSI, M.; HLAWA, W.; RAOUANI, N. 2007. Pesticidal potentialis os Cestrun parqui saponis. International Journal of Agricultural Research 2: 275-281. [ Links ]
CHEN, N. M.; BORDEN, J. H. 1989. Adverse effects of fenoxycarb on reproduction by the California fivespined ips, Ips paraconfusus Lanier (Coleoptera: Scolytidae). Canadian Entomologist 121: 1059-1068. [ Links ]
CHERNAKI-LEFFER, A. M.; BIESDORF, S. M.; ALMEIDA, L. M. 2001. Exigencias térmicas, período de desenvolvimento e sobrevivencia de imaturos Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae). Neotropical Entomology 30: 365-368. [ Links ]
CHERNAKI-LEFFER, A. M.; BIESDORF, S. M.; ALMEIDA, L. M.; LEFFER, E. V. B.; VIGNE, F. 2002. Isolamento de enterobactérias em Alphitobius diaperinus e na cama de aviários no Oeste do Estado do Paraná, Brasil. Revista Brasileira de Ciencia Avícola. 4: 243-247. [ Links ]
CHERNAKI-LEFFER, A. M.; SOSA-GOMES, D. R.; ALMEIDA L. M. 2006. Suscetibilidade de Alphitobius diaperinus (Panzer, 1797) (Coleoptera: Tenebrionidae) a reguladores de crescimento de insetos (RCI). Arquivos do Instituto Biológico 73 (1): 51-55. [ Links ]
COLLET, D. 1994. Modelling survival data in medical research. Chapman and Hall, London. [ Links ]
CORDOVA, D.; BENNER, E. A.; SACHER, M. D.; RAUH, J. J.; SOPA, J. S.; LAHM, G. P. 2006. Anthranilic diamides: a new class of insecticides with a novel mode of action, ryanodine receptor activation. Pesticide Biochemistry and Physiology 84: 196-214. [ Links ]
DEV, S.; KOUL, O. 1997. Insecticides of natural origin. Hardwood Academic, Amsterdam. [ Links ]
DHADIALLA, T. S.; CARLSON, G. R.; LE, D. L. 1998. New insecticides with ecdysteroidal and juvenile hormone activity. Annual Review of Entomology 43: 545-569. [ Links ]
DESPINS J. L.; AXTELL, R. C. 1995. Feeding behavior and growth of broiler chicks fed larvae of the darkling beetle, Alphitobios diaperinus. Poultry Science 74: 331-336. [ Links ]
DUFOUR, L.; SANDER, J. E.; WYATT, R. D. 1992. Experimental exposure of broiler chickens to boric acid to assess clinical signs and lesion of toxicosis. Avian Diseases 36: 1007-1011. [ Links ]
EDWARDS, J. P.; ABRAHAM, L. 1985. Laboratory evaluation of two insect juvenile hormone analogues against Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae). Journal of Stored Product Research 21: 189-194. [ Links ]
FERREIRA, W. L. B. 1999. Inseticidas de uso domiciliar e controle de vetores de doenjas. pp. 403-452. In: Mariconi, F. A. M. (Eds.). Insetos e outros invasores de residencias. Fealq, Piracicaba. [ Links ]
FINNEY, D. J. 1971. Probit analysis. Cambridge University Press, London. [ Links ]
GOODWIN, M. A.; WALTMAN, W. D. 1996. Transmission of Eimeria, viruses, and bacteria to chicks: Darkling beetles (Alphitobius diaperinus) as vector of pathogens. Journal of Applied Poultry Research 5: 51-55. [ Links ]
HAMM, R. L.; KAUFMAN, P. E.; REASOR, C. A. 2006. Resistance to cyfluthrin and tetrachlorvinphos in the lesser mealworm, Alphitobius diaperinus, collected from the eastern United States. Pest Management Science 62: 673-677. [ Links ]
KABFLEISCH, J. D.; PRENTICE, R. L. 1980. The statistical analysis of time failure data. John Wiley & Sons, New York. [ Links ]
KAVILLIERATOS, N. G.; ATHANASSIOU, C. G.; SAITANIS, C. J.; KONTODIMAS, D. C.; ROUSSOS, A. N.; TSOUTSA, M. S.; ANASTASSOPOULOU, U. A. 2007. Effect of two azadirachtin formulations against adults of Sitophilus oryzae and Tribolium confusum on different grain commodities. Journal of Food Protection 70: 1627-1632. [ Links ]
KHATTER, N. A. 2011. Efficiency of azadirachtin, a chitin synthesis inhibitor on growth, development and reproductive potential of Tribolium confusum after adult treatment. Journal of Entomology 8: 440-449. [ Links ]
KOSTYUKOVSKY, M.; CHEN, B.; ATSMI, S.; SHAAYA, E. 2000. Biological activity of two juvenoids and two ecdysteroids against three stored product insects. Insect Biochemistry and Molecular Biology 30: 891-897. [ Links ]
LAMBKIN, T. A.; RICE, S. J. 2006. Baseline responses of Alphitobius diaperinus (Coleoptera: Tenebrionidae) to cyfluthrin and detection of b resistance in field populations in eastern Australia. Journal of. Economic Entomology 99: 908-913. [ Links ]
LAMBKIN, T. A.; KOPITTKE, R. A.; RICE, S. J.; BARTLETT, J. S.; ZALUCKI, M. P. 2007. Distributions of lesser mealworm in litter of a compacted earth floor broiler house in subtropical Queensland, Australia. Journal of Economic Entomology 100: 1136-1146. [ Links ]
LEORA SOFTWARE. 1987. Polo-PC: A user's guide to probit or logit analysis. Berkeley, USA. [ Links ]
MATIAS, R. S. 2000. Controle do cascudinho Novas Perspectivas. Simpósio Brasil Sul de Avicultura, Anais. Chapecó, SC. [ Links ]
MCALLISTER, J. C.; STEELMAN, C. D.; SKEELES, J. K.; NEWBERRY, L. A. 1995. Isolation of infectious bursal disease virus from the lesser mealworm, Alphitobios diaperinus (Panzer). Poultry Science 74: 45-49. [ Links ]
MARCOMINI, A. M.; ALVES, L. F. A.; BONINI, A. K.; MERTZ, J. C.; SANTOS, J. C. 2009. Atividade inseticida de extratos vegetais e do óleo de neem sobre adultos de Alphitobius diaperinus Panzer (Coleoptera, Tenebrionidae). Arquivos do Instituto Biológico 76: 413-420. [ Links ]
MILLER, R. W. 1990. Use of ivermectin to control the lesser mealworm (Coleoptera: Tenebrionidae) in a simulated poultry broiler house. Poultry Science 69: 1281-1284. [ Links ]
MOJAVER, M.; BANDANI, A. R. 2010. Effects of the insect growth regulator pyriproxyfen on immature stages of sunn pest, Eurygaster integriceps Puton (Heteroptera: Scutelleridae). Munis Entomology & Zoology 5: 187-197. [ Links ]
MOLINA, D. E.; CARBONE, S. S. 2010. Toxicity of synthetic and biological insecticides against adults of the Eucalyptus snout-beetle Gonipterus scutellatus Gyllenhal (Coleoptera: Curculionidae). Journal of Pest Science 83: 297-305. [ Links ]
MORALES, A. 1991. Control químico del coleóptero Alphitobius diaperinus con malathion, carbaril y Dipterex en granjas avícolas. Revista Cubana de Ciencia Avícola 18: 205-209. [ Links ]
MORDUE (LUNTZ), A. J.; NISBET, A. J. 2000. Azadirachtin from the neem tree Azadirachta indica: its action against insects. Annais da Sociedade Entomológica do Brasil 29: 615-632. [ Links ]
OMOTO, C. 2000. Modo de ajao de inseticidas e resistencia de insetos a inseticidas. pp. 31-50. In: Guedes, C.; Costa, I. D.; Castiglioni, E. (Eds.). Bases e técnicas do manejo de insetos. Pallotti, Santa Maria, RS, Brazil. [ Links ]
PFEIFFER, R. W.; AXTELL, R. C. 1980. Coleoptera of poultry manure in caged-layer houses in North Carolina. Environmental Entomology 9: 21-28. [ Links ]
RETNAKARAN, A.; GRANETT, J.; ENNIS, T. 1985. Insect growth regulators. pp. 529-601. In: Kerkut, G. A.; Gilbert, L. I. (Eds.). Comprehensive insect physiology biochemistry and pharmacology. Pergamon, Oxford. [ Links ]
SALIN, C.; DELETTRE, Y. R.; CANNAVACCIUOLO, M.; VERNON, P. 2000. Spatial distribution of Alphitobius dia-perinus (Panzer) (Coleoptera: Tenebrionidae) in the soil of a poultry house along a breeding cycle. European Journal Soil Biology 36: 107-115. [ Links ]
SAS Institute Inc. 2004. SAS® 9.1.3 ETL Studio: User's Guide. SAS Institute, Cary, NC. [ Links ]
SHARMA, G. K. 1992. Growth-inhibiting activity of azadirachtin on Corcyra cephalonica. Phytoparasitica 20: 47-50. [ Links ]
SIAL, A. A; BRUNNER, J. F. 2010. Lethal and sublethal effects of an insect growth regulator, pyriproxyfen, on Obliquebanded leafroller (Lepidoptera: Tortricidae). Journal of Economic En-tomology 103: 340-347. [ Links ]
SILVA, A. S.; HOFF, G.; DOYLE, R. L.; SANTURIO, J. M.; MONTEIRO, S. G. 2005. Ciclo biológico do cascudinho Alphitobius diaperinus em laboratório. Acta Scientiae Veterinariae 33: 177-181. [ Links ]
SILVA, J. J.; MENDES, J. 2002. Effect of diflubenzuron on stages of Hematobia irritans (L.) (Diptera, Muscidae). In: Uberlándia, State of Minas Gerais, Brasil. Memórias do Instituto Oswaldo Cruz 97: 679-682. [ Links ]
SILVA, M. T. B.; COSTA, E. C.; BOSS, A. 2003. Control of Anticarsia gemmatalis Hübner (Lepidoptera: Noctuidae) larvae with insect growth regulators. Ciencia Rural 33: 601-605. [ Links ]
SOUSA, N. J. 2003. Importancia do manejo de resistencia de inseticidas no controle integrado dos pulgoes-gigantes-do-pi-nus. In Simpósio sobre Cinara em Pinus, Embrapa Florestas, Colombo, Curitiba. [ Links ]
SZCZEPANIK, M. 2001. Studies on the biological activity of azadirachtin lesser mealworm, Alphitobius diaperinus Panzer. pp. 228-233. In: Konopinska, D. (Eds.). Arthropods - Chemical, physiological and environmental aspects. University of Wro-claw, Poland. [ Links ]
TUNAZ, H.; UYGUN, N. 2004. Insect growth regulators for insect pest control. The Turkish Journal of Agriculture and Forestry 28: 377-387. [ Links ]
WEAVER, J. E. 1996. The lesser mealworm, Alphitobius diaperinus: Field trials for control in a broiler house with insect growth regulators and pyrethroids. Journal of Agricultural Entomology 13: 93-97. [ Links ]
VITTORI, J. 2007. Alphitobius diaperinus como veiculador de Clostridiumperfingens em granjas avícolas do interior paulista-Brasil. Ciencia Rural 37: 894-896. [ Links ]
Received: 16-Feb-2014
Accepted: 20-Apr-2015
Suggested citation:
ZORZETTI, J.; CONSTANSKI, K. C.; SANTORO, P. H.; FONSE-CA, I. C. B.; NEVES, P. M. O. J. 2015. Growth regulator insec-ticides for the control of the lesser mealworm beetle Alphitobius diaperinus (Coleoptera: Tenebrionidae). Revista Colombiana de Entomología 41 (1): 24-32. Enero-Junio 2015. ISSN 0120-0488.