Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488
Rev. Colomb. Entomol. vol.41 no.1 Bogotá Jan./June 2015
Quality of Beauveria bassiana conidia after successive passages through Alphitobius diaperinus (Coleóptera: Tenebrionidae)
Calidad de conidios de Beauveria bassiana después de sucesivos pasajes por el huésped Alphitobius diaperinus (Coleoptera: Tenebrionidae)
PATRICIA H. SANTORO1, JANAINA ZORZETTI2, KELLY CONSTANSKI2 and PEDRO M. O. J. NEVES3
1 Pesquisadora Doctora en Agronomía, Instituto Agronômico do Paraná, Rodovia Celso Garcia Cid, km 375, Três Marcos 86047-902, Caixa Postal 481, CEP 86051-990, Londrina, PR 86047-902, Brasil. patriciasantoro@iapar.br. Corresponding author.
2Estudiantes de doctorado en Agronomía, Universidade
Estadual de Londrina, Centro de Ciências Agrárias, Rodovia Celso Garcia Cid, km 380, Caixa Postal 6001, CEP 86051-990, Londrina, PR 445, Brasil. jzorzetti@ hotmail.com; kconstanski@hotmail.com.
3 Professor Doctor en Agronomía, Universidade Estadual de Londrina, Centro de Ciências Agrárias, Rodovia
Celso Garcia Cid, km 380, Caixa Postal 6001, CEP 86051-990, Londrina, PR 445, Brasil. pedroneves@uel.br.
Abstract: The vegetative growth, conidial production, conidial yield on rice, virulence, and heat and UV radiation tolerances of Beauveria bassiana after successive passages through Alphitobius diaperinus were investigated. Three strains of B. bassiana were cultivated in a culture medium, and passed them up to 15 times through the host insect. The conidia of each strain, derived from dead insects, were cultivated in a culture medium. We used conidia corresponding to the first, fifth, tenth, and fifteenth passages in our experiments. Our results showed that successive passages of the fungus through the host insect affected the quality of the conidia; moreover, the effect on conidial quality varied among the strains. For strains Unioeste 4 and Unioeste 40, successive passages through the host insect resulted in reduced vegetative growth and conidial production. In contrast, vegetative growth and conidial production of strain CG 152 were unaffected by successive passages through the host insect. For all 3 strains, successive passages through the host insect resulted in a higher conidial yield on rice and increased virulence, especially after the tenth and fifteenth passages. In addition, an increase in the number of passages through the host insect led to a decrease in the UV radiation tolerance, but an increase in the heat tolerance, especially after the tenth and fifteenth passages. Our results indicate that the conidial yield on rice, virulence, and heat tolerance of B. bassiana are favored by successive passages through A. diaperinus.
Key words: Biological control. Conidial production. Entomopathogenic fungus. Heat tolerance. UV radiation tolerance.
Resumen: El objetivo de este trabajo fue evaluar el efecto de pasajes sucesivos de Beauveria bassiana por Alphitobius diaperinus en relación al crecimiento vegetativo, a la producción de conidios, a la virulencia, a la sensibilidad frente a la temperatura y a la radiación UV. Fueron utilizados tres aislados inicialmente multiplicados en medio de cultivo y pasados 15 veces por los insectos. Los conidios de cada aislado, provenientes de los insectos muertos, correspondientes al primer, quinto, décimo y décimo quinto pasaje fueron multiplicados en medio de cultivo y empleados en los experimentos. Los pasajes del hongo por el huésped afectaron la calidad de los conidios, lo que varió en los diferentes aislados. De manera general, hubo reducción del crecimiento vegetativo y de la producción de conidios para los aislados Unioeste 4 y Unioeste 40 a medida que el hongo fue pasando por el huésped. En contraste, el crecimiento vegetativo y la producción de conidios del aislado CG 152 no fueron afectados. Para los tres aislados, la producción de conidios en arroz y la virulencia aumentaron después de los pasajes por el huésped, principalmente en el décimo y décimo quinto pasaje. La sensibilidad a la radiación UV fue reducida con el aumento del número de pasajes en los tres aislados, mientras que la sensibilidad a la temperatura aumentó principalmente en los décimo y décimo quinto pasajes. La calidad de los conidios de B. bassiana en relación a la producción de conidios en arroz, virulencia y sensibilidad frente a la temperatura, fue favorecida por los pasajes del hongo por el huésped.
Palabras clave: Control biológico Producción de conidios. Hongo entomopatógeno.Tolerancia al calor. Tolerancia a la radiación UV.
Introduction
Entomopathogenic fungi exhibit considerable genetic varia-bility. By using appropriate techniques, it is possible to select highly virulent strains for pest control (Alves 1998). Alves et al. (2004, 2005) and Steinkraus et al. (1991) reported the natural occurrence of B. bassiana and Metarhizium anisopliae on Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae), an important pest in poultry houses. Never-theless, strain selection of B. bassiana and M. anisopliae indicates that A. diaperinus, especially the adult stage, has a high tolerance to fungi; thus, many fungal strains are not pathogenic or show only low virulence (Rohde et al. 2006; Santoro et al. 2008). Steinkraus et al. (1991) demonstrated an increase in the virulence and higher susceptibility of B. bassiana toward A. diaperinus larvae, after a single passage through the host insect.
The quality of the entomopathogen is important for efficient pest control. Therefore, entomopathogens must be handled appropriately so as to maintain their virulence, or to improve this virulence by using genetic, physical, or chemical methods, or biological processes, such as successive passa-ges through target insects (Alves and Pereira 1998; Azevedo 1998; Serafini et al. 2001). Indeed, successive passages through a host insect may represent a valuable means of maximizing the efficiency of entomopathogenic fungi in the control of A. diaperinus.
The efficacy of entomopathogenic fungi in the field is also dependent on environmental conditions (Zimmermann 1982). However, most investigations regarding the effectiveness of fungal passage through a host insect have assessed only changes in virulence, and have not considered the response of fungi to abiotic factors, such as UV radiation and heat; such factors may compromise the efficacy of control measures.
In the present study, the vegetative growth, conidial production, conidial yield on rice, virulence, and heat and UV radiation tolerances of B. bassiana were assessed after successive passages through A. diaperinus.
Materials and methods Successive passages of the fungus through the host insect.
Adults of A. diaperinus, collected in poultry houses 1 day before the bioassays were used. The insects were disinfected with a 2% sodium hypochlorite solution, and washed steri-lized distilled water. Three strains of B. bassiana (CG 152, Unioeste 4, and Unioeste 40) previously selected by Santoro et al. (2008) for control of A. diaperinus were used (Table 1). The strains are maintained in the entomopathogenic collection of Londrina State University. The conidia were subcultured on a sporulation medium (SPM) (agar, 20 g; potassium chloride, 1.0 g, dextrose, 10 g; yeast extract, 5 g; potassium phosphate, 0.36 g; sodium phosphate, 1.05 g; sodium nitrate, 1.58 g; and magnesium sulfate, 0.6 g) (Alves 1998) in Petri dishes, and incubated for 10 days at 25 ± 1 °C, with a 12 h photophase. Unless otherwise specified, the temperatura and photophase conditions for all of the experiments were identical.
Successive in vivo passages of the fungal strains were made by placing 100 live and healthy insects in contact with dead and contaminated insects, the bodies of which were covered with the sporulated fungus. The insects were fed with crushed and sterilized maize, and maintained in an incubator. After 5 days -the time required for killing more than 50% of the insects- the dead insects were disinfected and placed in a moist incubator for a further 5 days, to allow fungal development and sporulation. A total of 10 insects, the bodies of which were fully covered with the sporulated fungus, were used to inoculate a second group of 100 insects. The remaining dead insects, the bodies of which were also covered with the sporulated fungus, were placed in a sealed tube and identified as "insects with conidial strains of the first passage"; these insects were stored, as the form of pure conidia, at -6 °C for subsequent usage.
Twenty-four hours after inoculation of the second group of insects -the time required for contamination- the B. bassiana killed insects were removed, such that only live insects remained. The same procedures described for the first group of insects were carried out, and successively repeated until the fifteenth passage of the fungus through the host insect.
The conidia produced on the insects (stored at -6 °C), corresponding to the first, fifth, tenth, and fifteenth passages through the host insect, were cultivated in SPM and placed in an incubator for 10 days. The conidia produced were removed from the medium by using a spatula, and stored in sterilized tubes at -6 °C for use in all assays.
Vegetative growth and conidial production. The fungal strains were inoculated onto a central point of a Petri dish (9 cm diameter) containing SPM medium, by using a pointed platinum loop to obtain a single colony per plate. The Petri dishes were placed in an incubator for 10 days. Vegetative growth was determined by calculating the colony area based on the average of 2 opposing diameters. Conidial production was assessed by using the same colonies. The conidia were removed from the medium by using a spatula, suspended and diluted in Tween 20 aqueous solution at 0.005% (v/v), and quantified by using a hemocytometer. A completely randomized experimental design was used in a factorial arrangement (4 x 3; fungal passages through host insect * strains), with 5 replications.
Conidial yield on rice. A total of 500 g of parboiled rice (Tio Joao) was added to 1 L of boiling distilled water, and cooked for 3 min in a microwave oven, until a "rubbery" consistency was obtained. Next, 65 g of cooked rice was placed in 500 mL glass bottles. The bottles were covered with paper towels, to allow gaseous exchange, and sterilized in an autoclave for 30 min. After cooling, each bottle was inoculated with 1.5 mL of a 1.0 x 107 conidia mL-1 suspension, and placed in an incubator for 15 days. To avoid the agglomeration of rice grains during fungal growth, the bottles were shaken daily. Conidial production was evaluated by adding 300 mL of tween 20 aqueous solution at 0.005% (v/v) to each bottle, and shaking the bottles to release the conidia; after the necessary dilutions, the conidia were quantified by using a hemocytometer. A completely randomized experimental design was used in a factorial arrangement (4 x 3; fungal passages through host insect x strains), with 5 replications.
Virulence toward A. diaperinus. A total of 50 adult insects were placed in 6 cm diameter polystyrene crystal dishes, and sprayed with 0.5 mL of a 1 x 106 conidia mL-1 suspension, by using a Fanem-Diapump vacuum pump-compressor at a pressure of 0.8 kgf cm-1. In the control treatment, insects were sprayed with Tween 20 aqueous solution at 0.005% (v/v). The insects were fed with sterilized corn feed, and maintained in an incubator for 10 days, after which dead insects were placed in a moist incubator for 5 days to confirm mortality caused by the fungus (confirmed mortality). A completely randomized experimental design was used in a factorial arrangement (4 x 3; fungal passages through host insect x strains) plus control, with 5 replications of 50 insects.
Heat tolerance. To preserve their original characteristics, conidia were not dried before the experiments. The drying process is normally used to study the effect of temperature on viability. However, drying standardizes the conidial water content, and may interfere with characteristics derived from successive passages through a host insect. The conidia were stored in sterilized test tubes, and placed in an incubator at 30 °C for 15 days. Conidial viability was assessed by using a germination test, in which 0.1 mL of a 1 x 107 conidia mL-1 suspension was spread over the surface of the SPM. The dishes were placed in an incubator for 20 h, after which germination (%) was quantified. Germinated conidia were considered as those for which the germ tube extended to 3 times the size of the conidia. We also evaluated the viability of conidia that had not been exposed to heat. The experimental design was completely randomized in a factorial arrangement (4 x 3; fungal passages through host insect x strains), with 5 replications.
UV radiation tolerance. Exposure of conidia to UV radiation may delay germination (Alves et al. 1998; Moore et al. 1993; Nascimento et al. 2010). Therefore, the UV radiation tolerance was assessed by using the colony forming unit test; this enabled to evaluate the conidial viability regardless of a delay in the germination process. First, 0.1 mL of a 1 x 103 conidia mL'1 suspension was spread over the surface of the SPM by using a sterile glass spreader. Next, the open dishes were exposed to a germicidal lamp (253.7 nm, Philips TUV, low pressure 30 W) located in a central position at a distance of 52 cm, for 1 min. The dishes were covered and transferred to an incubator for 5 days, after which the number of colonies formed was assessed. We also evaluated the viability of conidia that had not been exposed to UV radiation. The experimental design was completely randomized in a fac' torial arrangement (4 x 3; fungal passages through host insect x strains), with 5 replications.
Statistical analyses. Data from the treatments with a com-pletely randomized design in a factorial arrangement were submitted to analysis of variance (ANOVA), and the mean values were compared by using Tukey's test (P < 0.05), using the SISVAR statistical software (Ferreira 2011). Comparison of the factorial mean values with each control was carried out by using Dunnett's test (P < 0.05), using the SAS statistical software (SAS Institute 1997).
Results and discussionVegetative growth and conidial production. Stability of conidial production is important for the development of commercial products based on entomopathogenic fungi (Vandenberg and Cantone 2004). However, successive in vitro and in vivo cultures may alter some phenotypic traits (Crecy et al. 2009; Scully and Bidochka 2005; Vandenberg and Cantone 2004).
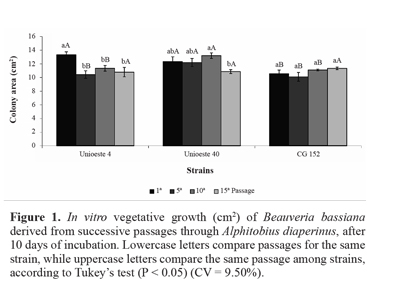
In the present study, vegetative growth of strain Unioeste 4 on SPM was reduced by 15-22% after the fifth, tenth, and fifteenth passages, in comparison with the first passage. Further, vegetative growth of strain Unioeste 40 was reduced by 18% after the fifteenth passage, in comparison with the tenth passage. In contrast, vegetative growth of strain CG 152 was unaffected by successive passages through the host insect. Comparison among the strains revealed that after the first passage, vegetative growth of strains Unioeste 4 and Unioeste 40 was significantly greater than that of strain CG 152, while after the fifth and tenth passages, vegetative growth of strain Unioeste 40 was significantly greater than that of strains Unioeste 4 and CG 152. However, after the fifteenth passage, vegetative growth did not differ significantly among the 3 strains (Fig. 1).
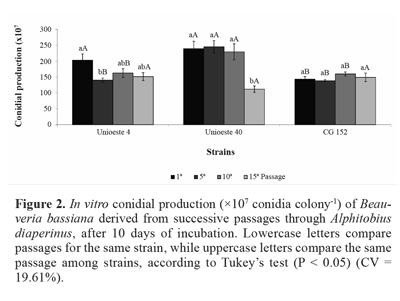
Conidial production of strain Unioeste 4 was reduced by 31% after the fifth passage, in comparison with the first passage. Further, conidial production of strain Unioeste 40 was reduced by 50% after the fifteenth passage, when compared with the first, fifth, and tenth passages. In contrast, conidial production of strain CG 152 was unaffected by successive passages through the host insect. Comparison among the strains revealed that after the first passage, conidial production of strains Unioeste 4 and Unioeste 40 was significantly greater than that of strain CG 152, while after the fifth and tenth passages, conidial production of strain Unioeste 40 was significantly greater than that of strains Unioeste 4 and CG 152, However, after the fifteenth passage, conidial production did not differ significantly among the 3 strains (Fig. 2).
The effect of successive in vitro and in vivo cultures on conidial production is highly dependent on the intraspecific strain characteristics. Vandenberg and Cantone (2004) obser-ved distinct behaviors among strains of Isaria fumosorosea (Wise), and reported a reduction in conidial production of strain 4,461 after the fifth and tenth passages through Diuraphis noxia (Kurdjumov) (Hemiptera: Aphididae). On the other hand, conidial production remained stable after 15 successive passages through Plutella xylostella (Linnaeus) (Lepidoptera: Plutellidae) or 30 successive in vitro subcultures. Further, conidial production of strain 4,481 was unaffected by successive in vitro and in vivo subcultures, whereas conidial production of strain 4,491 was reduced after 30 successive in vitro passages, and increased after 15 successive passages through D. noxia.
Reductions in vegetative growth and biomass production of Aspergillus flavus (Link) on PDA medium after several passages through Galleria mellonella (Linnaeus) (Lepi-doptera: Pyralidae) have been reported, indicating that the capacity of a fungus to grow as a saprobiont may be reduced by forced passage through the host insect (Scully and Bidochka 2005). In the present study, the host insect may have exercised selection pressure on strains Unioeste 4 and
Unioeste 40. A greater number of passages through the host insect may also have favored adaptation of these strains to the nutrients contained within the host insect, thereby reducing the capacity for saprophytic development in the culture medium.
Conidial yield on rice. The use of fungi as biological control agents is dependent on a number of biological variables, including the economical viability to produce high concentrations of infective and stable propagules (Jaronski 1986; Latgé et al. 1986). A major advantage of B. bassiana and other entomopathogenic fungi for large-scale production is the facility for in vitro cultivation Leite et al. (2003), thereby enabling the enhancement or maintenance of productive capacity. In Brazil, solid substrates (especially rice grains) are the most frequently used materials for large-scale production of entomopathogenic fungi, mainly because of low cost (Alves and Pereira 1998; Leite et al. 2003). Therefore, elucidation of the conidial yield on rice after successive fungal passages through the host insect is of fundamental importance.
In the present study, the conidial yield on rice showed a different trend to that observed for vegetative growth and conidial production on SPM (Fig. 3). For strain Unioeste 4, the conidial yield on rice approximately doubled after the tenth passage, but subsequently remained stable after the fifteenth passage. For strain Unioeste 40, the conidial yield on rice approximately tripled after the fifteenth passage. Meanwhile, for strain CG 152, the differences were less pronounced, and the conidial yield on rice differed significantly only between the fifth and fifteenth passages (Fig. 3).
Comparison among the strains revealed that the conidial yield on rice did not differ significantly among the 3 strains after the first and fifth passages, while the conidial yield on rice for strains Unioeste 4 and Unioeste 40 did not differ significantly after the tenth passage. However, after the fifteenth passage, the conidial yield on rice for strain Unioeste 40 was significantly greater than that for strains Unioeste 4 and CG 152 (Fig. 3).
Growth, conidial production, and morphology of entomopathogenic fungi may be affected not only by nutritional composition, but also by nutrient availability (Kamp and Bidochka 2002). These effects may vary among strains of a single species Barbosa et al. (2002); Damir (2006); Loureiro et al. (2005); Monteiro et al. (2004); moreover, they are difficult to predict because the complex relationship between strains and culture media leads to considerable variability regarding nutrient types and concentrations.
In the present study, an individual nutrient, or a combi-nation of various rice nutrients, may have been responsible for the increase in conidial production after successive fungal passages through the host insect.
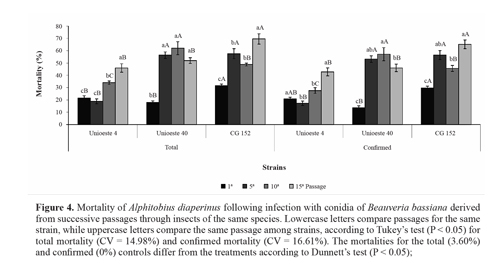
Virulence toward A. diaperinus. In the present study, successive fungal passages through the host insect increased the virulence ofall 3 strains by more than 100%. For strain Unioeste 4, the total mortality increased after the tenth passage, while the confirmed mortality increased after the fifteenth passage. For strain Unioeste 40, the total and confirmed mortalities increased after the fifth passage, and thereafter remained high. For strain CG 152, the total and confirmed mortalities were highest after the fifteenth passage. Comparison among the strains revealed that after the fifth passage, the total and confirmed mortalities for strains CG 152 and Unioeste 40 did not differ from each other, but were higher than that of strain Unioeste 4. After the tenth and fifteenth passages, the highest total and confirmed mortalities were determined for strains Unioeste 40 and CG 152, respectively (Fig. 4).
Hyphomycete fungi may adapt to a particular host insect after forced passages through the species (Ferron 1985). Adames et al. (2010) observed that M. anisopliae conidia became more virulent to Rhipicephalus microplus (Canestrini) (Acari: Ixodidae) after the fourth passage, and showed the highest virulence after the seventh passage. Song and Feng (2011) reported that strains of B. bassiana showed a 3- to 4-fold increase in virulence after the second passage through Nilaparvata lugens (Stal) (Hemiptera: Del-phacidae), and subsequently remained unchanged after the third passage. The observed increase in virulence was correlated with an increase in Pr1 protease production. Pr1 protease is responsible for degradation of the insect cuticle (Shah et al. 2005), and also for an increase in zeta potential and hydrophobicity (surface properties related to enhance adhesion of the conidia to the insect cuticle) (Boucias et al. 1988; Cho et al. 2007; Holder and Keyhani 2005).
Virulence stability after in vitro cultures is a desirable trait for commercial production of biological control agents (Vandenberg and Cantone 2004). Nevertheless, the possibility of increasing fungal virulence after successive passages through the host insect is an important strategy, particularly for species such as A. diaperinus, which have a high tolerance to fungal pathogens.
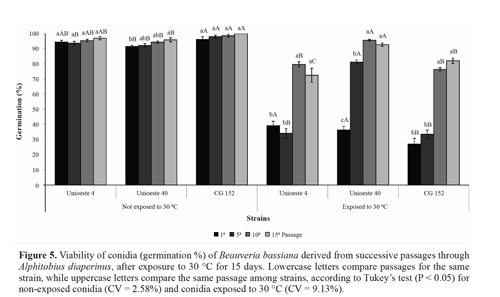
Heat tolerance. In the present study, for conidia that were not exposed to a temperature of 30 °C, successive fungal passage through the host insect affected only the viability of strain Unioeste 40; this strain showed a small increase in germination after the fifteenth passage. The viability of strain CG 152 was greater than that of strain Unioeste 40 after all passages, but greater than that of strain Unioeste 4 only after the fifth passage (Fig. 5). Nevertheless, the viability of all 3 strains after each passage was > 90%, which is satisfactory for fungal use in biological control programs. It is possible that the differences observed in the present study were derived from the intrinsic characteristics of each strain.
Successive fungal passages through the host insect increased the tolerance of conidia exposed to 30 °C for 15 days. For strain Unioeste 40, the heat tolerance increased gradually; after the tenth and fifteenth passages, the viability was comparable with that of conidia that were not subjected to heat stress. For strains Unioeste 4 and CG 152, the heat tolerance increased after the tenth passage, and subsequently remained unchanged after the fifteenth passage; after these passages, the viability of conidia was > 70%. Comparison among the strains revealed that strain Unioeste 40 showed a greater heat tolerance than strain CG 152 after all passages, and a greater heat tolerance than strain Unioeste 4 after all passages except the first one (Fig. 5).
The susceptibility of entomopathogenic fungi to heat is a limiting factor for biological control efficiency, but has rarely been studied. In particular, data regarding the effects of successive in vivo and in vitro cultures are lacking. Ento-mopathogenic fungi have been shown to adapt to different temperature conditions (Bidochka et al. 2001; Fargues et al. 1997; Rangel et al. 2005). The results of our present study indicate that forced passage of the fungus through the host insect may lead to an increase in the heat tolerance.
UV radiation tolerance. Solar radiation negatively affects pathogens, partly through the formation of cyclobutane pyrimidine dimers in the pathogen DNA (Chelico et al. 2005). Yao and Ying (2010) postulated that even M. anisopliae and B. bassiana strains with a high tolerance to UV radiation would be unable to survive a single day of exposure to solar light. Therefore, the use of photoprotectors in addition to strain selection has been suggested (Edgington et al. 2000; Inglis et al. 1995; Reddy et al. 2008). In the present study, the viability of conidia not exposed to UV radiation was unaffected by successive fungal passages through the host insect; moreover, the viability did not differ among the 3 strains. For conidia exposed to UV radiation, an increase in the number of passages led to a reduction in the UV radiation tolerance (Fig. 6).
Comparison among the strains revealed that the UV radiation tolerance of strain Unioeste 4 was reduced after the fifth passage, and further reduced after the tenth and fifteenth passages. The UV radiation tolerance of strain Unioeste 40 did not differ significantly after the first, fifth, and tenth passages, but was reduced after the fifteenth passage. The UV radiation tolerance of strain GC 152 was reduced after the tenth and fifteenth passages. The viability of strain CG 152 was higher than that of strain Unioeste 40 after all passages, and higher than that of strain Unioeste 4 after all passages except the first one. Meanwhile, the viability of strain Unioeste 4 was lower than that of strains Unioeste 4 and CG 152 after the tenth and fifteenth passages (Fig. 6). It is possible that the differing tolerances to UV radiation were derived from genetic varia-bility. Previous studies have demonstrated considerable variability regarding tolerance to solar radiation, even among strains of the same species (Fargues et al. 1996; Fernandes et al. 2007). This variability may be derived from natural adaptation to different environmental conditions (Nascimento et al. 2010).
Increased virulence after successive fungal passages through a host insect has been reported previously (Vandenberg and Cantone 2004; Adames et al. 2010; Song and Feng 2011;), and is an important strategy for improving control efficiency. However, under field conditions, control efficiency is affected by abiotic factors, particularly UV radiation (Braga et al. 2001; Cagan and Svercel 2001). Thus, elucidation of the effect of successive fungal passages through the host insect on UV radiation sensitivity is of fundamental importance. The results of the present study indicate that successive passages of B. bassiana through A. diaperinus have a detrimental impact on the UV radiation tolerance. This detrimental impact may compromise the control efficiency of this species, despite a related increase in virulence.
ConclusionsIn the present study, it was demonstrated variable responses among B. bassiana strains to successive passages through A. diaperinus. For strains Unioeste 4 and Unioeste 40, suc-cessive passages through the host insect resulted in reduced vegetative growth and conidial production on SPM; however, these effects varied according to the strain.
For all 3 strains, conidial yield on rice, virulence, and heat tolerance were favored by successive fungal passages. In contrast, UV radiation tolerance was negatively affected. Thus, in order to avoid compromising the benefits achieved via increases in conidial yield on rice, virulence, and heat tolerance, measures must be taken to protect the conidia from the deleterious effects of UV radiation.
Literature citedADAMES, M.; FERNÁNDEZ-RUVALCABA, M.; PENÁ-CHORA, G.; HERNÁNDEZ-VELÁSQUEZ, V. M. 2010. Effects of passages through a suitable host of the fungus, Metarhizium anisopliae, on the virulence of acaricide-susceptible and resistant strains of the tick, Rhipicephalus microplus. Journal of Insect Science 11: 1-13. [ Links ]
ALVES, R. T.; BATEMAN, R. P.; PRIOR, C.; LEATHER, S. R. 1998. Effects of simulated solar radiation on conidial germination of Metarhizium anisopliae in different formulations. Crop Protection 17: 675-679. [ Links ]
ALVES, L. F. A.; ALVES, V. S.; BRESSAN, D. F.; NEVES, P. M. O. J.; ALVES, S. B. 2004. Ocorrencia de Metarhizium anisopliae (Metsch) Sorok. (Moniliales: Moniliaceae) em adultos de cascudinho (Alphitobius diaperinus) (Panzer) (Coleoptera: Te-nebrionidae), em aviários comerciais em Cascavel, PR, Brasil. Neotropical Entomology 33: 793-795. [ Links ]
ALVES, L. F. A.; GASSEN, M. H.; PINTO, F. G. S.; NEVES, P. M. O. J.; ALVES, S. B. 2005. Ocorrencia natural de Beauveria bassiana (Bals.) Vuilleman (Moniliales: Moniliaceae) sobre o cascudinho (Alphitobius diaperinus) (Panzer) (Coleóptera: Tenebrionidae), em aviários comerciais de Cascavel, PR. Neo-tropical Entomology 34: 507-510. [ Links ]
ALVES, S. B. 1998. Fungos entomopatogenicos. pp. 289-382. In: Alves, S. B. (Ed.). Controle microbiano de insetos. Piracicaba. UFG. Goiánia. Brazil. 1163 p. [ Links ]
ALVES. S. B.; PEREIRA, R. M. 1998. Produjao de fungos ento-mopatogenicos. FEALQ. Piracicaba. pp. 845-867. In: Alves, S. B. (Ed.). Controle Microbiano de Insetos. UFG. Brasil. 1163 p. [ Links ]
AZEVEDO, J. L. 1998. Genética de microorganismos. UFG. Goia-nia. 490 p. [ Links ]
BARBOSA, C. C.; MONTEIRO, A. C.; CORREIA, A. C. B.; PEREIRA, G. T. 2002. Crescimento e esporulajao de isolados de Verticillium lecanii sob diferentes condijoes nutricionais. Pesquisa Agropecuária Brasileira 37: 821-829. [ Links ]
BIDOCHKA, M. J.; KAMP, A. M.; LAVENDER, T. M.; DEKON-ING, J.; DE CROSS, J. N. A. 2001. Habitat association in two genetic groups of the insect-pathogenic fungus Metarhizium anisopliae: uncovering cryptic species? Applied and Environ-mental Microbiology 67: 1335-1342. [ Links ]
BOUCIAS, D. G.; PENDLAND, J. C.; LATGÉ, J. P. 1988. Non-specific factors involved in the attachment of entomopathogenic deuteromycetes to host insect cuticle. Applied and Environmen-tal Microbiology 54: 1797-1805. [ Links ]
BRAGA, G. U. L.; FLINT, S. D.; MILLER, C. D.; ANDERSON, A. J.; ROBERTS, D. W. 2001. Variability in response to UV-B among species and strains of Metarhizium strain from sites at latitudes from 61°N to 54°S. Journal of Invertebrate Pathology 78: 98-108. [ Links ]
CAGAN, L.; VERCEL, M. S. 2001. The influence of ultraviolet light on pathogenicity of entomopathogenic fungus Beauve-ria bassiana (Balsamo) Vuillemin to the European corn borer, Ostrinia nubilalis HBN. (Lepidoptera: Crambidae). Journal of Central European Agriculture 2: 228-232. [ Links ]
CHELICO, L. C.; HAUGHIAN, J. L.; WOYTOWICH, A. E.; KHA-CHATOURIANS, G. G. 2005. Quantification of ultraviolet-C irradiation induced cyclobutane pyrimidine dimers and their removal in Beauveria bassiana conidiospore DNA. Mycologia 97: 621-627. [ Links ]
CHO, E. M.; KIRKLAND, B. H.; HOLDER, D. J.; KEYHANI, N. O. 2007. Phage display cDNA cloning and expression analysis of hydrophobins from the entomopathogenic fungus Beauveria (Cordyceps) bassiana. Microbiology 153: 3438-3447. [ Links ]
CRECY, E. de; JARONSKI, S.; BENJAMIN, L.; LYONS, T. J.; NEMAT, O. K. 2009. Directed evolution of a filamentous fungus for thermotolerance. BMC Biotechnology 9: 1-11. [ Links ]
DAMIR, M. El. 2006. Effect of growing media and water volume on conidial production of Beauveria bassiana and Metarhizium anisopliae. Journal of Biological Sciences 6: 269-274. [ Links ]
EDGINGTON, S.; SEGURA, H.; LA ROSA, W.; WILLIAMS, T. 2000. Photoprotection of Beauveria bassiana: Testing simple formulations for control of the coffee berry borer. International Journal of Pest Management 46: 169-176. [ Links ]
FARGUES, J.; GOETTE, M. S. L; SMITS, N.; OUEDRAOGO, A.; ROUGIER, M. 1997. Effect of temperature on vegetative growth of Beauveria bassiana strains from different origins. Mycologia 89: 383-392. [ Links ]
FARGUES, J.; GOETTE, M. S. L; SMITS, N.; OUEDRAOGO, A.; VIDAL, C.; L. A. LACEY; LOMER, C. J.; ROUGIER, M. 1996. Variability in susceptibility to simulated sunlight of conidia among strains of entomopathogenic hyphomycetes. My-copathologia 135: 171-181. [ Links ]
FERNANDES, E. K. K.; RANGEL, D. E. N.; MORAES, A. M. L.; BITTENCOURT, V. R. E. P.; ROBERT, D. 2007. Variability in tolerance to UV-B radiation among Beauveria spp. strains. Journal of Invertebrate Pathology 96: 237-243. [ Links ]
FERREIRA, D. F. 2011. Sisvar: a computer statistical analysis sys tem. Ciencia e Agrotecnologia 35: 1039-1042. [ Links ]
FERRON, P. 1985. Fungal control. pp. 313-346. In: Kerkut, G. A.; L. I. Gilbert, (Eds.). Comprehensive insect physiology, bio-chemistry and pharmacology. Pergamon press. Oxford. UK. [ Links ]
HOLDER, D. J.; KEYHANI, N. O. 2005. Adhesion of the entomopathogenic fungus Beauveria (Cordyceps) bassiana to substrata. Applied and Environmental Microbiology 71: 5260-5266. [ Links ]
INGLIS, G. D.; GOETTEL, M. S.; JOHNSON, D. L. 1995. Influence of ultraviolet light protectants on persistence of the entomopathogenic fungus, Beauveria bassiana. Biologic Control 5: 581-590. [ Links ]
JARONSKI, S. T. 1986. Commercial development of deuteromy-cetous fungi of arthropods: a critical appraisal. pp. 653-656. In: Samson, R. A.; Vlak, J. M.; Peters, R. (Eds.). Fundamental and Applied Aspects of Invertebrate Pathology Netherlands: Foundation of the Fourth International Colloquium of Invertebrate Pathology, Wageningen. [ Links ]
KAMP, A. M.; BIDOCHKA, M. J. 2002. Conidium production by insect pathogenic fungi on commercially available agars. Letters in Applied Microbiology 35: 74-77. [ Links ]
LATGÉ, J. P.; HALL, R. A.; CABRERA, R. I.; KERWIN, J. C. 1986. Liquid fermentation of entomopathogenic fungi. Fundamental and Applied Aspects of Invertebrate Pathology. pp. 603-606. In: Samson, R. A.; Vlak, J. M.; Peters, R. (Eds.). Fundamental and Applied Aspects of Invertebrate Pathology Netherlands: Foundation of the Fourth International Colloquium of Invertebrate Pathology, Wageningen. [ Links ]
LEITE, L. G.; BATISTA FILHO, A.; ALMEIDA, J. E. M.; ALVES, S. B. 2003. Produjao de fungos entomopatogenicos. Ribeirao Preto, 92 p. [ Links ]
LOUREIRO, E. de S.; BATISTA FILHO, A.; ALMEIDA, J. E. M.; DE PESSOA, L. G. A. 2005. Produjao de isolados de Metarhi-zium anisopliae, selecionados para o controle de Mahanarva fimbriolata (Stál, 1854). Arquivos do Instituto Biológico 72: 469-472. [ Links ]
MONTEIRO, A. C.; BARBOSA, C. C.; CORREIA, A. do C. B. 2004. Crescimento e esporulajao de isolados de Verticillium lenacii sob diferentes fatores ambientais. Pesquisa Agropecuária Brasileira 39: 561-565. [ Links ]
MOORE, D.; BRIDGE, P. D; HIGGINS, P. M.; BATEMAN, R. P; PRIOR, C. 1993. Ultraviolet radiation damage to Metarhizium flavoviride conidia and the protection given by vegetable and mineral oils and chemical sunscreens. Annals of Applied Bio-logy 22: 605-616. [ Links ]
NASCIMENTO, E.; SILVA, S. H DA.; MARQUES, E. dos R.; ROBERTS, D. W.; BRAGA, G. U. L. 2010. Quantification of Induced by UVB radiation in conidia of the fungi Aspergil-lus fumigatus, Aspergillus nidulans, Metarhizium acridum and Metarhizium robertsii. Photochemistry and Photobiology 20: 1-8. [ Links ]
RANGEL, D. E. N.; BRAGA, G. U. L.; FLINT, S. D.; ANDERSON, A. J.; ROBERTS, D. W. 2004. Variations in UV-B tole-rance and germination speed of Metarhizium anisopliae conidia produced on insects and artificial substrates. Journal of Inverte-brate Pathology 87: 77-83. [ Links ]
RANGEL, D. E. N.; BRAGA, G. U. L.; ANDERSON, A. J.; ROBERTS, D. W. 2005. Variability in conidial thermotolerance of Metarhizium anisopliae strains from different geographic origins. Journal of Invertebrate Pathology 88: 116-125. [ Links ]
REDDY, N. P.; KHAN, D. K. U.; VICTOR, J. S.; SHARMA, H. C. 2008. Assessment of the suitability of Tinopal as an enhancing adjuvant in formulations of the insect pathogenic fungus Beauveria bassiana (Bals.) Vuillemin. Pest Management Science 64: 909-915. [ Links ]
ROHDE, C.; ALVES, L. F. A.; BRESSAN, D. F.; NEVES, P. M. O. J.; SILVA, E. R. L.; ALVES, S. B.; ALMEIDA, J. E. M. 2006. Selejao de isolados de fungos para o controle do cascudinho Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae). Neotropical Entomology 35: 231-240. [ Links ]
SANTORO, P. H.; NEVES, P. M. O. J.; ALEXANDRE, T. M.; SARTORI, D.; ALVES, L. F. A.; FUNGARO, M. H. 2008. Selection of Beauveria bassiana strains to control Alphitobius diaperinus. Journal of Invertebrate Pathology 97: 83-90. [ Links ]
SAS INSTITUTE. 1997. SAS/STAT software: changes and enhan-cements through release 6.12. Cary: SAS Institute. 1116 p. [ Links ]
SCULLY, L. R.; BIDOCHKA, M. J. 2005. Serial passage of the opportunistic pathogen Aspergillus flavus through an insect host yields decreased saprobic capacity. Canadian Journal of Micro-biology 51: 185-189. [ Links ]
SERAFINI, L. A.; BARROS, N. M.; AZEVEDO, J. L. 2001. Biotecnologia na agricultura e na indústria. pp. 93-152. In. Aze-vedo, J. L. (Ed.). O uso dos fungos na biotecnologia, Guaíba. Agropecuária. Brazil. [ Links ]
SHAH, F. A.; WANG, C. S.; BUTT, T. M. 2005. Nutrition influences growth and virulence of the insect pathogenic fungus Metarhi-zium anisopliae. FEMS Microbiology Letters 251: 259-266. [ Links ]
SONG, T. T.; FENG, M. G. 2011. In vivo passages of heterologous Beauveria bassiana strains improve conidial surface properties and pathogenicity to Nilaparvata lugens (Homoptera: Delphaci-dae). Journal of Invertebrate Pathology 106: 211-216. [ Links ]
STEINKRAUS, D. C.; GEDEN, C. J.; RUTZ, D. A. 1991. Suscepti-bility of lesser mealworm (Coleoptera: Tenebrionidae) to Beau-veria bassiana: Effects of host stage, formulation, substrate and host passage. Journal of Medical Entomology 28: 314-321. [ Links ]
VANDENBERG, J. D.; CANTONE, F. A. 2004. Effect of serial transfer of three strains of Paecilomyces fumosoroseus on growth in vitro, virulence, and host specificty. Journal of Invertebrate Pathology 85: 40-45. [ Links ]
YAO, S.; YING, S.; FENG, M.; HATTING, J. L. 2010. In vitro and in vivo responses of fungal biocontrol agents to gradient doses of UV-B and UV-A irradiation. BioControl 55: 413-422. [ Links ]
ZIMMERMANN, G. 1982. Effect of high temperaturas and artificial sunlight on the viability of conidia of Metarhizium aniso-pliae. Journal of Invertebrate Pathology 40: 36-40. [ Links ]
Received: 1-May-2014
Accepted: 27-Apr-2015
SANTORO, P. H.; ZORZETTI, J.; CONSTANSKI, K.; NEVES, P. M. O. J. 2015. Quality of Beauveria bassiana conidia after suc-cessive passages through Alphitobius diaperinus (Coleoptera: Tenebrionidae). Revista Colombiana de Entomología 40 (1): 87-94. Enero-junio 2014. ISSN 0120-0488.