Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488
Rev. Colomb. Entomol. vol.41 no.1 Bogotá Jan./June 2015
Sección Básica
Artículos de investigación
Cardinal distribution of sucking insects in Caryocar brasiliense (Caryocaraceae)in e Cerrado (Brazil)
Insectos chupadores in Caryocar brasiliense (Caryocaraceae) en el Cerrado (Brasil)
GERMANO LEÃO DEMOLIN LEITE1, RONNIE VON DOS SANTOS VELOSO2, JOSÉ COLA ZANUNCIO2, GERALDO WILSON FERNANDES3, CHRYSTIAN IEZID MAIA ALMEIDA1, PAULO SÉRGIO FIÚZA FERREIRA2,JATNEL ALONSO4 and JOSÉ EDUARDO SERRÃO5
1 D. Sc. and D. Sc., respectively. Insetário G. W. G. Moraes, Instituto de Ciencias Agrárias, Universidade Federal de Minas Gerais, Av. Universitária n. 1000, B. Universitário, 39404-006, Caixa Postal 135. Montes Claros, MG, Brazil. gldleite@ig.com.br. Autor para correspondencia.
2 D. Sc., Ph. D., and Ph. D., respectively. Departamento de Entomologia, Universidade Federal de Viçosa, 36571-000 Viçosa, MG, Brazil.
3 Ph. D. Ecologia Evolutiva y Biodiversidade, Caixa Postal 486, ICB/Universidade Federal de Minas Gerais, 30161-970 Belo Horizonte, MG, Brazil.
4 D. Sc. Instituto de Ciencia Animal, Apartado Postal 24, San José de las Lajas, La Habana, Cuba. D. Sc.
5 Departamento de Biologia Geral, Universidade Federal de Viçosa, 36571-000 Viçosa, MG, Brazil.
Abstract: Caryocar brasiliense (Caryocaraceae), a tree characteristic of e Cerrado, is widely distributed and can reach a height of 10 meters with a six-meter-wide canopy. The objective of this study was to study the distribution of sucking insects (Hemiptera) and their natural enemies in the canopies of C. brasiliense trees in the Brazilian Cerrado. One rare, nine common, and one constant species of sucking insects and three rare, seven common, and four constant species of natural enemies were observed on C. brasiliense trees. The diversity, number of individuals, and species of Hemiptera and their natural enemies were similar in all four cardinal directions of branches of host trees. Abundance of the natural predators Crematogaster sp. (Hymenoptera: Formicidae) and Zelus armillatus (Hemiptera: Reduviidae) was highest on leaves of the east and north sides of the C. brasiliense trees, respectively. A large number of Crematogaster sp. was observed on C. brasiliense, with a predominance of Dikrella caryocar n. sp. (Hemiptera: Cicadellidae) and Pseudoccocus sp. (Hemiptera: Pseudococcidae) also being observed. The predators Trybonia sp. (Thysanoptera: Phlaeothripidae) and Chrysoperla sp. (Neuroptera: Chrysopidae) showed the highest numbers, while the number of D. caryocar n. sp. and Aphis gossypii (Aphididae) decreased, respectively, in comparison to the control. An increase in the number of sucking insects also increased the number of their natural enemies, and this differential distribution negatively influenced sucking insects. The speed and direction of wind may have affected the distribution of sucking insects on different sides of C. brasiliense trees, as higher populations were found on the sides without prevailing winds.
Key words: Canopy. Insect distribution. Leafhoppers. Natural enemies. Pequi.
Resumen:
Caryocar brasiliense (Caryocaraceae), un árbol símbolo de el Cerrado, tiene una amplia distribución y puede alcanzar los 10 m de altura y 6 m de ancho de copa. El objetivo fue estudiar la distribución de insectos chupadores (Hemiptera) y sus enemigos naturales en la copa de C. brasiliense en El Cerrado brasileño. La agrupación de las especies encontrada en la copa de los árboles de C. brasiliense mostró 1 y 3 especies raras, 9 y 7 especies comunes y 1 y 4 especies constantes para los insectos chupadores y sus enemigos naturales, respectivamente. La diversidad, el número de individuos y la riqueza de especies de Hemiptera y sus enemigos naturales fueron similares en todas las ramas del árbol huésped, según el muestreo en los puntos cardinales. Los depredadores Crematogaster sp. (Hymenoptera: Formicidae) y Zelus armillatus (Hemiptera: Reduviidae) fueron más abundante en la ramas orientadas hacia el este y el norte, respectivamente. Crematogaster sp. fue dominante en los árboles de C. brasiliense con predominio de Dikrella caryocar n. sp. (Hemiptera: Cicadellidae) y Pseudoccocus sp. (Hemiptera: Pseudococcidae). Los depredadores Trybonia sp. (Thysanoptera: Phlaeothripidae) y Chrysoperla spp. (Neuroptera: Chrysopidae) fueron mayores y disminuyeron el número de D. caryocar n. sp. y Aphis gossypii (Aphididae), respectivamente. El incremento de insectos chupadores aumentó la presencia de los enemigos naturales y la distribución diferencial de éstos influyó negativamente en los insectos chupadores. Se concluye que la velocidad y dirección de los vientos pueden afectar la distribución de insectos chupadores en la copa de los árboles de C. brasiliense, encontrándose las poblaciones más numerosas en la zona que no es afectada por el viento.Palabras clave: Copa. La distribución de los insectos. Saltamontes. Enemigos naturales. Pequí.
Introduction
The Brazilian savanna Cerrado, which is characterized by a high diversity and endemism of plants and insects (Bridgewater et al. 2004), occupies approximately 23% of the Brazilian territory (Da Silva and Bates 2002). As a result of increasing threats to its biodiversity, this biome has been designated a biodiversity hotspot (Myers et al. 2000). The Cerrado is used primarily for grain and cattle production (Aguiar and Camargo 2004), in addition to forest cultivation with exotic species, primarily eucalyptus (Zanuncio et al. 2002). Government incentives have facilitated the deforestation of the Cerrado, and its vegetation has been reduced to 20% of the original amount (Klink and Machado 2005). It has a complex mosaic of phytophysiognomies that range from open savanna ("campo limpo") to tall and woody forests of 10-15 meters high, called "cerradao" (Oliveira and Marquis 2002). Large patches of rich Cerrado in southeastern Brazil are used for agriculture (primarily soybean and sugar cane), cattle farms and cities (urbanization), with similar uses to those of the Montes Claros region in northern Minas Gerais State.
Caryocar brasiliense Camb. (Caryocaraceae) or "pequí" is a characteristic tree of the Cerrado. It has a wide distribution (Brandao and Gavilanes 1992; Bridgewater et al. 2004; Leite et al. 2006a) and can reach a height of 10 meters with a six-meter-wide canopy (Leite et al. 2006a, 2011a, 2012a). Its fruits have an internal mesocarp that is rich in oil, vitamins, and proteins as well as compounds of medicinal importance. The fruits are used as food, in production of cosmetics and lubricants, and in the pharmaceutical industry (Segall et al. 2005; Ferreira and Junqueira 2007; Garcia et al. 2007; Khouri et al. 2007). Besides, they represent the main income source of many communities that remain in the deforested areas of the Brazilian Cerrado (Leite et al. 2006a). Fruit collectors favor leaves, flowers and fruits, and sucking insects (Hemiptera) with a high number of species incur higher damage on isolated trees (Freitas and Oliveira 1996; Oliveira 1997; Boiga et al. 2004; Leite et al. 2009, 2011a,b,c,d,e, 2012a) and in tree seedlings (Leite et al. 2006b). It is necessary to understand the ecology of the insects on this valuable tree, which both occurs naturally and is planted in the Brazilian Cerrado.
The position of branches on a tree affects the abundance of insects due to 1) the wind direction (Feng et al. 2005; Leite et al. 2011c, d, e), which causes leaf desiccation and the fall of flowers and fruits (Leite et al. 2006a); 2) the sun exposure, which may influence the quality of the host plant tissues (Unsicker and Mody 2005); 3) the creation of microclimates, and 4) the impact of herbivores, which prefers the parts of plants that have the smallest presence of natural enemies (enemy-free space) (Unsicker and Mody 2005). The identification of potential pests and their spatial distribution on the crown of forest and agriculture plants is important for sampling plans (Nichols-Orians 1991; Casas and Aiuja 1997; Villanueva and Childers 2005; Evans and Gregoire 2007). Sucking insects are abundant among the fauna in the Cerrado (Pinheiro et al. 2002). For the present study, they were reared on C. brasiliense to support management programs for pests of this plant and to study their spatial distribution at tree level. The objective was to study the distribution of sucking insects (Hemiptera) and their natural enemies in the canopy of C. brasiliense trees in the Brazilian Cerrado.
Material and methodsStudy area. This study was conducted in the Municipality of Montes Claros, Minas Gerais State, Brazil (43°55'7.3"W 16°44'55.6"S), at 943 masl, from January to December 2010. The region, called Cerrado, has a dry winter and rainy summer. According to Koppen, its climate is categorized as Aw (tropical) (Vianello and Alves 2000). The vegetation is typical of cerrado (savanna), and the region has a dystrophic yellow red latosoil (sandy texture). In previous studies, a density of thirteen C. brasiliense trees was found per hectare in the study area (size around of 50 ha) (Leite et al. 2006a, 2011b). "Cerrado" refers to the biome, and "cerrado" refers to the physiognomy (vegetation type); latter is the subject of this paper.
System. The system of study was the "pequi"-herbivores-enemies. The adult Caryocar brasiliense trees were 4.07 ± 0.18 m (average ± standard error) high and had an average crown width of 2.87 ± 0.13 m (Leite et al. 2006a). The leaves of C. brasiliense are alternate and trifoliate and have high trichome density; the flowers are hermaphrodite, but they are mostly cross-pollinated. Fruit production is annual; C. brasiliense blooms between July and September (dry period) and fructifies from October to January (rainy season) (Leite et al. 2006a). Fruits are drupes with 1-4 seeds, and they weigh 158.49 ± 8.14 g (fresh weigh), with a volume of 314.90 ± 20.93 cm3 (Leite et al. 2006a).
Study design. The design was completely randomized, with 25 replications (25 trees) in the cerrado sensu stricto. The distribution of the sucking insects and their arthropod natural enemies was recorded on three leaves (fully expanded), three curl of flowers, and three fruits per cardinal orientation of the branches using a compass (north, south, west, and east) in each tree, monthly, in the morning (7-11 h) by direct visual observation (Horowitz 1993). The distance between the C. brasiliense trees evaluated was approximately 50 m, and they were located at least 100 m from the edge; for details of the floristic diversity in this area, see Leite et al. (2012a).
The insects were collected from the leaves, flowers and fruits with tweezers, brushes and aspirators and were preserved for identification in vials with 70% alcohol and 30% water.
A total of 900 leaves (samples), 225 flowers (samples) from July to September and 240 fruits (samples) from September to January of C. brasiliense were evaluated per cardinal orientation.
Statistical analyses. The abundance, species richness, and diversity of the sucking insects and their natural enemies were calculated per cardinal orientation (average). The diversity was calculated using the formula of Hill (Hill 1973), and the abundance and species richness were calculated using Simpson indices (Townsend et al. 2006; Lazo et al. 2007). The species of sucking insects and their natural enemies were classified according their frequencies as a) constant (> 50%), b) common (< 49%), or c) rare (< 10%) (Siqueira et al. 2008).
The correlations between the diversity index, number of individuals, and species of sucking insects and the abundance and species richness of natural enemies (ants, predator thrips, bugs, spiders, ladybirds, and green lacewings) on the numbers of sucking insects were submitted to analysis of variance (ANOVA) (P < 0.05), using a simple regression analysis (P < 0.05), and the curves were adjusted for the quadratic function, as required. The effect of the cardinal orientation of the branches on the ecological indices, the number of individuals per species of sucking insects and the natural enemies was tested with ANOVA (P < 0.05) and Tukey's test (P < 0.05).
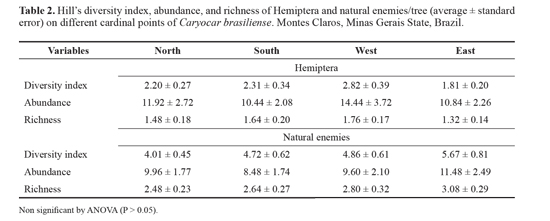
ResultsOne rare, nine commons, and one constant species of sucking insects and three rare, seven common, and four constant species of natural enemies were found on the C. brasiliense trees (Table 1). Using Hill's diversity index, the number of individuals and species of sucking insects and their natural enemies were similar (P > 0.05) among the cardinal sides of C. brasiliense trees (Table 2). There were collected 988 and 1191 total individuals of natural enemies and sucking insects, respectively, and 13 species of natural enemies plus spiders (19 species) and 10 species of sucking insects.
The abundance of most of the sucking species on leaves, flowers, and fruits of C. brasiliense trees were similar between the cardinal points (Table 3). The predators, Crematogaster sp. (Hymenoptera: Formicidae) (df = 72, F = 4.269, P = 0.00785) and Zelus armillatus (Lep. and Servi, 1825) (Hemiptera: Reduviidae) (df = 72, F = 2.794, P = 0.04633) presented the highest abundance on leaves from the east and north sides of C. brasiliense trees, respectively (Table 3).
The ant Crematogaster sp. was more frequent on C. brasiliense trees that had larger numbers of Dikrella caryocar n. sp. (Coelho, Leite and Da Silva, 2014) (Hemiptera: Cicadel-lidae) and Pseudoccocus sp. (Hemiptera: Pseudococcidae).
The numbers of the predators Trybonia sp. (Thysanoptera: Phlaeothripidae) and Chrysoperla sp. (Neuroptera: Chrysopi-dae) was inversely correlated to those of D. caryocar n. sp. and Aphis gossypii (Glover, 1877) (Aphididae), respectively. This pattern was also observed for the number of individuals of sucking insects and those of their natural enemies (Fig. 1).
DiscussionThe similar diversity index and number of individuals and species of Hemiptera and their natural enemies on the different sides of C. brasiliense may be explained by the low number of constant species of sucking insects (9.10%) and their natural enemies (28.6%) on this tree. In other words, most of the species were found at low population densities.
It was not possible to detect significant differences among the number of individuals and diversity on different sides in this plant, which may have been due to the higher floristic diversity of the cerrado area. This varies with the insect group considered because the environmental complexity and host plants can influence the diversity of the herbivore arthropods and their natural enemies (Coyle et al. 2005; Espírito Santo et al. 2007; Lazo et al. 2007; Leite et al. 2011a, 2012a). In less complex environments, the number of species associated with a given host species may be lower and the abundance of each species is generally higher, thereby increasing the likelihood that herbivores that feed on economically valuable plants will become significant pest (Gratton and Denno 2003; Coyle et al. 2005; Lazo et al. 2007).
Althrough we did not detect significant differences, the tendency (P > 0.05) for a higher diversity index value and more individuals and species of Hemiptera on the west side of C. brasiliense trees may be explained by 1) a prevailing wind from the northeast and eastern (Leite et al. 2006a, 2009, 2011c, d, e), 2) more sunlight on the north side in the Southern Hemisphere (Vianello and Alves 2000) and 3) avoiding ants and predators, which presented higher numbers in the east and north sides of C. brasiliense trees, respectively. The direction of the wind may have influenced the dispersion of insects (Pathak et al. 1999; Tixier et al. 2000; Auslander et al. 2003; Schooley and Wiens 2003; Feng et al. 2004, 2005; Leite et al. 2009, 2011c, d, e) and pollination. A wind speed greater 2.0 m/seconds reduced the visits of bees to flowers (Dutra and Machado 2001). The desiccant effect of wind is the semi-arid north of Minas Gerais State, Brazil, and can higher in regions that have a low relative humidity and high reduce the fruit production and photosynthesis and causetemperature, which is typical of the cerrado vegetation in the malformation or fall of flowers and fruits (Leite et al. 2006a), both of which influence insect populations. The higher desiccant effect of the wind on the east and north sides of C. brasiliense trees may be explained by the sun exposure influencing the quality of the host plants for insects (Fernandes 1990; Unsicker and Mody 2005). The number of species and individuals of insects was lower on the sunny side of the Australian Melaleuca trees (Richardson et al. 1999).
The lower populations of ants and predators may explain the tendency (P > 0.05) of higher ecological parameters of sucking insects on the west sides of C. brasiliense trees as spaces that are free of natural enemies. This could make the colonization by sucking insects on north and east sides of the C. brasiliense canopy difficult. Predators can respond to a local increase in vegetation complexity and alternative prey with higher efficiency against herbivores (Auslander et al. 2003).
The greater number of Trybonia sp. and Chrysoperla sp. reduced the abundance of D. caryocar n. sp. and A. gossypii, respectively, on the C. brasiliense trees, but the higher populations of Crematogaster sp. increased the abundance of D. caryocar n. sp. and Pseudoccocus sp. Ants and other predators reduced the infestation of Eunica bechina Talbot 1928 (Lepidoptera: Nymphalidae) and Edessa rufomarginata (De Geer, 1773) (Hemiptera: Pentatomidae), Prodiplosis floricola Felt 1907 (Diptera: Cecidomyiidae), petiole gall insects (Hymenoptera: Chalcidoidea) (Freitas and Oliveira 1996; Oliveira 1997), and beetle defoliators (Leite et al. 2012b) on this plant. Green lacewings are important predators of aphids as A. gossypii (Leite et al., 2006b, 2012c). The positive associations among ants, leafhoppers and aphids are common food-for-protection mutualisms (Moya-Raygoza and Larsen, 2014; Shik et al. 2014). The east side of the trees was apparently more unfavorable to insect herbivores in the African Savanna, as greater leaf damage occurred on the west and north sides. This finding can be explained by the species-specific reactions (plants and herbivores) and the biotic environment conditions (Unsicker and Mody 2005). The distribution of herbivores could also reflect the avoidance of predators in addition to reactions to the chemical composition of host plants or the microclimate (Unsicker and Mody 2005).
The sucking insect species that have greater potential to become pests in commercial C. brasiliense plantations under natural conditions are Aethalium reticulatum L., 1767 (Aetalionidae), E. rufomarginata; D. caryocar n. sp. and A. gossypii due to of their high abundance and status as a common species. Aethalium reticulatum and E. rufomarginata are pests in C. brasiliense trees (Leite et al. 2012c), and D. caryocar n. sp. and A. gossypii are pests in seedling in this culture (Leite et al. 2006b). Predators affected these insects on this plant. The high diversity of sucking insects shows the necessity of studying the population dynamics of these organisms in the arboreal systems of the Brazilian savanna.
AcknowledgementsWe thank A.D. Brescovit (Instituto Butanta) (Aracnidae), A.M. Bello (Coleoptera), I.C. Nascimento (EMBRAPA-ILHÉUS-Centro de Pesquisas do Cacau, CEPLAC, Itabuna, BA) (Formicidae), and R.C. Monteiro (Thysanoptera) for the identification of the specimens, and C. Barbosa, O.M. da Silva and F.M. Ruas for supplying climate data. We also thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundagao de Amparo á Pesquisa do Estado de Minas Gerais and Secretaria de Ciencia e Tecnologia do Estado de Minas Gerais for financial support.
Literature citedAGUIAR, L. M. S.; CAMARGO, A. J. A. 2004. CERRADO: ECOLOGIA E CARACTERIZADO. EMBRAPA - CPAC, PLANAL-TINA. 464 p. [ Links ]
AUSLANDER, M.; NEVO, E.; INBAR, M. 2003. The effects of slope orientation on plant growth, developmental instability and suscep-tibility to herbivores. Journal of Arid Environments 55 (3): 405-416. [ Links ]
BOIQA, J. R.; ARLINDO, L.; TEREZINHA, S. M.; PASSILONGO, J. 2004. Trígona spinipes (Fabr.) (Hymenoptera: Apidae) in passion fruit species: seasonal fluctuation, visitation time and flower damage. Neotropical Entomology 33 (2): 135-139. [ Links ]
BRANDÁO, M., ANDGAVILANES, M. L. 1992. Espécies padroni-zadoras do cerrado mineiro e sua distribuigao no estado. Informe Agropecuário 16 (173): 5-11. [ Links ]
BRIDGEWATER, S.; RATTER, J. A.; RIBEIRO, J. F. 2004. Biogeo-graphic patterns, p-diversity and dominance in the cerrado biome of Brazil. Biodiversity and Conservation 13 (12): 2295-2318. [ Links ]
CASAS, J.; AIUJA, M. 1997. The geometry of search movements of insects in plant canopies. Behavioral Ecology 8 (1): 57-45. [ Links ]
COELHO, L. B. N.; LEITE, G. L. D.; DA-SILVA, E. R. 2014. A new species of Dikrella Oman, 1949 (Hemiptera: Cicadellidae: Typhlocybinae) found on Caryocar brasiliense Cambess. (Caryo-caraceae) in Minas Gerais State, Brazil. Psyche 2014: 1-5. [ Links ]
COYLE, D. R.; NEBEKER, T. E.; HART, E. R.; MATTSON, W. J. 2005. Biology and management of insect pests in North American intensively managed hardwood forest systems. Annual Review of Entomology 50 (1): 1-29. [ Links ]
DA SILVA, J. M. C.; BATES, J. M. 2002. Biogeographic patterns and conservation in the South American Cerrado: a tropica Savanna hotspot. BioScience 52 (3): 225-234. [ Links ]
DUTRA, J. C. S.; MACHADO, V. L. L. 2001. Entomofauna visitante de Stenolobium stans (Juss.) Seem (Bignoniaceae), durante seu período de floragao. Neotropical Entomology 30 (1): 43-53. [ Links ]
ESPÍRITO-SANTO, M. M.; NEVES, F. S.; ANDRADE-NETO, F. R.; FERNANDES, G. W. 2007. Plant architecture and meristem dynamics as the mechanisms determining the diversity of gall-inducing insects. Oecologia 153 (2): 353-364. [ Links ]
EVANS, A. M.; GREGOIRE, T. G. 2007. The tree crown distribution of hemlock woolly adelgid, Adelges tsugae (Hem., Adelgidae) from randomized branch sampling. Journal of Applied Entomology 131 (1): 26-33. [ Links ]
FENG, H. G.; WU, K. M.; CHENG, D. F.; GUO, Y. Y. 2004. North-ward migration of Helicoverpa armigera (Lepidoptera: Noctuidae) and other moths in early summer observed with radar in northern China. Journal of Economic Entomology 97 (6): 1874-1883. [ Links ]
FENG, H. G.; WU, K. M.; NI, Y. X.; CHENG, D. F.; GUO, Y. Y. 2005. High-altitude windborne transport of Helicoverpa armigera (Lepidoptera : Noctuidae) in mid-summer in northern China. Journal of Insect Behavior 18 (3): 335-349. [ Links ]
FERNANDES, G. W. 1990. Hypersensitivity: a neglected plant resistance mechanism against insect herbivores. Environmental Entomology 19 (5): 1173-1182. [ Links ]
FERREIRA, L. C.; JUNQUEIRA, R. G. 2007. Microbiological evaluation of pequi (Caryocar brasiliense Camb.) preserves made from a typical Brazilian fruit. World Journal of Microbiology and Biotechnology 23 (8): 1179-1181. [ Links ]
FREITAS, A. V. L.; OLIVEIRA, P. S. 1996. Ants as selective agents on herbivore biology: Effects on the behaviour of a nonmyrme-cophilous butterfly. Journal of Animal Ecology 65 (2): 205-210. [ Links ]
GARCIA, C. C.; FRANCO, B. I. P. M.; ZUPPA, T. O.; ANTONIOSI FILHO, N. R.; LELES, M. I. G. 2007. Thermal stability studies of some Cerrado plant oils. Journal of Thermal Analysis and Calorimetry 87 (3): 645-648. [ Links ]
GRATTON, C.; DENNO, R. F. 2003. Seasonal shift from bottom-up to top-down impact in phytophagous insect populations. Ecologia 134 (4): 487-95. [ Links ]
HILL, M. O. 1973. Diversity and evenness: a unifying notation and its consequences. Ecology 54 (2): 427-432. [ Links ]
HOROWITZ, A. R. 1993. Control strategy for the sweetpotato white-fly, Bemisia tabaci, late in the cotton-growing season. Phytopara-sitica 21 (4): 281-291. [ Links ]
KHOURI, J.; RESCK, I. S.; POQAS-FONSECA, M.; SOUSA, T. M. M.; PEREIRA, L. O.; OLIVEIRA, A. B. B.; GRISOLIA, C. K. 2007. Anticlastogenic potential and antioxidant effects of an aqueous extract of pulp from the pequi tree (Caryocar brasiliense Camb). Genetics and Molecular Biology 30 (2): 442-448. [ Links ]
KLINK, C. A., ANDMACHADO, R. B. 2005. A conservasao do cerrado brasileiro. Megadiversidade 1 (1): 147-155. [ Links ]
LAZO, J. A.; VALDES, N. V.; SAMPAIO, R. A.; LEITE, G. L. D. 2007. Zoological diversity associated to a silvopastural system leucaena-guinea grass with different establishment times. Pesquisa Agropecuária Brasileira 42 (12): 1667-1674. [ Links ]
LEITE, G. L. D.; VELOSO, R. V. S.; ZANUNCIO, J. C.; FERNANDES, L. A.; ALMEIDA, C. I. M. 2006a. Phenology of Caryocar brasiliense in the Brazilian Cerrado Region. Forest Ecology and Management 236 (2-3): 286-294. [ Links ]
LEITE, G. L. D.; VELOSO, R. V. S.; REDOAN, A. C. M.; LOPES, P. S. N.; MACHADO, M. M. L. 2006b. Arthropods associated to "piquizeiro" tree seedlings. Arquivos do Instituto Biológico 73 (3): 365-70. [ Links ]
LEITE, G. L. D.; VELOSO, R. V. S.; SILVA, F. W. S.; GUANABENS, R. E. M.; FERNANDES, G. W. 2009. Within tree distribution of a gall-inducing Eurytoma (Hymenoptera: Eurytomidae) on Caryo-car brasiliense (Caryocaraceae). Revista Brasileira de Entomologia 53 (4): 643-648. [ Links ]
LEITE, G. L. D.; ALVES, S. M.; NASCIMENTO, A. F.; LOPES, P. S. N. ; FERREIRA, P. S. F.; ZANUNCIO, J. C. 2011a. Identification of the wood borer and the factors affecting its attack on Caryocar brasiliense trees in the Brazilian Savanna. Acta Scientiarum Agronomy 33 (4): 589-566. [ Links ]
LEITE, G. L. D.; VELOSO, R. V. S.; ZANUNCIO, J. C.; ALVES, S. M.; AMORIM, C. A. D.; SOUZA, O. F. F. 2011b. Factors affecting Constrictotermes cyphergaster (Isoptera: Termitidae) nesting on Caryocar brasiliense trees in the Brazilian savanna. Sociobiology 57 (1): 165-180. [ Links ]
LEITE, G. L. D.; CERQUEIRA, V. M.; DAVILA, V. A.; MAGA-LHÁES, C. H. P.; FERNANDES, G. W. 2011c. Distribution of a leaf vein gall in Caryocar brasiliense (Caryocaraceae) tree. Revista Caatinga 24 (4): 186-190. [ Links ]
LEITE, G. L. D.; DAVILA, V. A.; CERQUEIRA, V. M.; NASCIMEN-TO, A. F.; FERNANDES, G. W. 2011d. Spatial distribution of a spherical gall (Hymenoptera: Eulophidae) on Caryocar brasiliense (Caryocaraceae). Revista Brasileira de Entomologia 55 (3): 396-400. [ Links ]
LEITE, G. L. D.; NASCIMENTO, A. F.; JESUS, F. M.; ALVES, S. M. ; FERNANDES, G. W. 2011e. Within tree distribution of a discoid gall on Caryocar brasiliense. Revista Colombiana de Entomología 37 (2): 289-293. [ Links ]
LEITE, G. L. D.; NASCIMENTO, A. F.; ALVES, S. M.; LOPES, P. S. N. ; SALES, N. L. P.; ZANUNCIO, J. C. 2012a. The mortality of Caryocar brasiliense in northern Minas Gerais State, Brazil. Acta Scientiarum. Agronomy 34 (2): 131-137. [ Links ]
LEITE, G. L. D.; VELOSO, R. V. S.; ZANUNCIO, J. C.; ALMEIDA, C. I. M.; FERREIRA, P. S. F.; SERRÁO, J. E.; RAMALHO, F. S. 2012b. Seasonal damage caused by herbivorous insects on Caryo-car brasiliense (Caryocaraceae) trees in the Brazilian savanna. Revista Colombiana de Entomología 38 (1): 35-40. [ Links ]
LEITE, G. L. D.; VELOSO, R. V. S.; ZANUNCIO, J. C.; FERNANDES, G. W.; ALMEIDA, C. I. M.; FERREIRA, P. S. F.; LAZO, J. A.; SERRÁO, J. E. 2012c. Seasonal abundance of hemipterans on Caryocar brasiliense (Malpiguiales: Caryocaraceae) trees in the Cerrado. Florida Entomologist 95 (4): 862-872. [ Links ]
MOYA-RAYGOZA, G.; LARSEN, K. J. 2014. Response of ants to the leafhopper Dalbulus quinquenotatus DeLong & Nault (Hemiptera: Cicadellidae) and extrafloral nectarines following fire. Sociobiology 61 (2): 136-144. [ Links ]
MYERS, N.; MITTERMEIER, R. A.; MITTERMEIER, C. G.; FONSECA, G. A. B.; KENT, J. 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853-858. [ Links ]
NICHOLS-ORIANS, C. M. 1991. The effects of light on foliar che-mistry, growth and susceptibility of seedlings of a canopy tree to an attine ant. Oecologia 86 (4): 552-560. [ Links ]
OLIVEIRA, P. S. 1997. The ecological function of extrafloral necta-ries: herbivore deterrence by visiting ants and reproductive output in Caryocar brasiliense (Caryocaraceae). Functional Ecology 11 (3): 323-330. [ Links ]
OLIVEIRA, P. S.; MARQUIS, R. J. 2002. The cerrados of Brazil: ecology and natural history of a neotropical savanna. Columbia Univ. Press, New York. 398 p. [ Links ]
PATHAK, S. C.; KULSHRESTHA, V; CHOUBEY, A. K.; PARULEKAR, A. H. 1999. Insect drift over the northern Arabian Sea in early summer. Journal of Biosciences 24 (2): 233-240. [ Links ]
PINHEIRO, F.; DINIZ, I. R.; COELHO, D.; BANDEIRA, M. P. S. 2002. Seasonal pattern of insect abundance in the Brazilian cerrado. Austral Ecology 27 (2): 132-136. [ Links ]
RICHARDSON, B. J.; AZARBAYJANI, F. F.; SHELLEY, B.; RICHARDSON, S. 1999. Arboreal arthropod biodiversity in woo-dlands: Effect of collection procedures and geographic distance on estimates of diversity found on two species ofMelaleuca. Austral Ecology 24 (5): 544-554. [ Links ]
SCHOOLEY, R. L.; WIENS, J. A. 2003. Finding habitat patches and directional connectivity. Oikos 102 (3): 559-570. [ Links ]
SEGALL, S. D.; ARTZ, W. E.; RASLAN, D. S.; FERRAZ, V. P.; TAKAHASHI, J. A. 2005. Triacylglycerol analysis of pequi (Caryocar brasiliensis Camb.) oil by electrospray and tandem mass spectrometry. Journal of the Science of Food and Agriculture 86 (3): 445-452. [ Links ]
SHIK, J. Z.; KAY, A. D.; SILVERMAN, J. 2014. Aphid honeydew provides a nutritionally balanced resource for incipient Argentine ant mutualists. Animal Behaviour 95: 33-39. [ Links ]
SIQUEIRA, K. M. M.; KIILL, L. H. P.; MARTINS, C. F.; LEMOS, I. B.; MONTEIRO, S. P.; FEITOZA, E. A. 2008. Comparative study of pollination of Mangifera indica L. in conventional and organic crops in the region of the Submédio Sao Francisco valley. Revista Brasileira de Fruticultura 30 (2): 303-310. [ Links ]
TIXIER, M. S.; KREITER, S.; AUGER, P. 2000. Colonization of vine-yards by phytoseiid mites: their dispersal patterns in the plot and their fate. Experimental and Applied Acarology 24 (3): 191-211. [ Links ]
TOWNSEND, C. R.; BERGON, M.; ANDHARPER, J. L. 2006. Fundamentos em ecologia. Artmed, Porto Alegre. 592 p. [ Links ]
UNSICKER, S. B.; MODY, K. 2005. Influence of tree species and compass bearing on insect folivory of nine common tree species in the West African savanna. Journal of Tropical Ecology 21 (2): 227-231. [ Links ]
VILLANUEVA, R. T.; CHILDERS, C. C. 2005. Diurnal and spatial patterns of Phytoseiidae in the citrus canopy. Experimental and Applied Acarology 35 (4): 269-280. [ Links ]
VIANELLO, R. F., ANDALVES, A. R. 2000. Meteorologia básica e aplicares. UFV, Vifosa. 448 p. [ Links ]
ZANUNCIO, J. C.; LOPES, E. F.; ZANETTI, R.; PRATISSOLI, D.; COUTO, L. 2002. Spatial distribution of nests of the leaf cutting ant Atta sexdens rubropilosa (Hymenoptera: Formicidae) in plantations of Eucalyptus urophylla in Brazil. Sociobiology 39 (2): 231-242. [ Links ]
Received: 1-Dec-2013
Accepted: 1-May-2015
LEITE, G. L. D.; VON DOS SANTOS VELOSO, R.; ZANUNCIO, J. C.; FERNADES, G. W.; ALMEIDA, C. I. M.; FERREIRA, P. S. F.; ALONSO, J.; SERRáO, J. E. 2015. Cardinal distribution of sucking insects in Caryocar brasiliense (Caryocaraceae) in e Cerrado (Brazil). Revista Colombiana de Entomología 41 (1): 105-111. Enero-Junio 2015. ISSN 0120-0488.