Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488
Rev. Colomb. Entomol. vol.41 no.1 Bogotá Jan./June 2015
Adult longevity and reproductive capacity in Cochliomyia macellaria (Díptera: Calliphoridae) reared on an alternative diet
Longevidad del adulto y capacidad reproductiva de Cochliomyia macellaria (Diptera: Calliphoridae)criada con una dieta alternativa
DÉBORA CARDOSO DA SILVA1, VALÉRIA MAGALHÃES AGUIAR2, SANDRA LÚCIA DA CUNHA E. SILVA1, RAFAELA PEREIRA DE CARVALHO2, ALEXANDRE SILVA3 y GONZALO EFRAIN MOYA-BORJA4
1 Ph. D. Departamento de Estudos Básicos e Instrumentais, Universidade Estadual do Sudoeste da Bahia. Praça Primavera s/n, Bairro Primavera, 45700- 000, Itapetinga, BA, Brasil. dcardoso_rj@hotmail.com. Corresponding author.
2 Ph. D. Laboratório de Estudo de Dípteros, Departamento Microbiologia e Parasitologia, Universidade Federal do Estado do Rio de Janeiro. Rua Frei Caneca, 94, Centro, 20211-040, Rio de Janeiro, RJ, Brasil.
3 Ph. D. Centro de Ciências Exatas e Tecnologia, Departamento Matemática e Estatística, Universidade Federal do Estado do Rio de Janeiro. Av Pasteur, 458, Urca, 22290-240, Rio de Janeiro, RJ, Brasil.
4 Ph. D. Departamento de Parasitologia. Instituto de Veterinária, Universidade Federal Rural do Rio de Janeiro. Rod. BR 465, km 7, 23890-000, Seropédica, RJ, Brasil.
Abstract: The search for alternative diets that are cheap and maintain rearing stock quality is very important. The objective of the present study was to assess the adult longevity and reproductive capacity of Cochliomyia macellaria derived from juveniles reared on a chicken gizzard diet compared to those fed beef diet. Couples were formed shortly after adult emergence and distributed in four cages, totaling 40 couples per treatment, and maintained at 30 °C day/28 °C night, 70 ± 10% RH, anda12 hour light period. Chicken gizzards or beef were offered according to the treatment to stimulate oviposition. The mean weight of the egg mass (1.063 g; 1.12 g), mean weight of the egg mass/day (0.0658 g; 0.0698 g), and the mean weight of the egg mass/female/day (0.0118 g; 0.0125 g) did not differ significantly between the chicken gizzard and beef diets, respectively. The mean number of eggs/g (8221.23; 8569.29) and the mean viability of eggs (99%; 94%) did not differ significantly by a Student t-test, (α = 5%) for the chicken gizzard and beef diets, respectively. The Kaplan-Meier non-parametric method and the Weibull parametric regression method found no differences (in days) in the mean total longevity (37 vs. 38) and mean estimated longevity of males (41.08 vs. 40.04) and females (33.79 vs. 36.29) fed chicken gizzards and beef, respectively. The maximum longevity was 74 days for both diets. The chicken gizzard diet is an efficacious and cheap alternative for rearing C. macellaria in the laboratory.
Key words: Blowfly. Forensic entomology. Insect rearing. Laboratory biology.
Resumen: La búsqueda de dietas alternativas con bajo costo para mantener la calidad de la cría de insectos en laboratorio es esencial. Este estudio tuvo como objetivo evaluar la longevidad de los adultos y capacidad reproductiva de Cochliomyia macellaria, provenientes de formas inmaduras, criadas con dieta de mollejas de pollo en comparación con aquellas alimentadas con carne. Poco después de la aparición de los adultos, se formaron parejas, distribuidas en cuatro jaulas, para un total de 40 pares por tratamiento y se mantuvieron a 30 °C día/28 °C noche, a 70 ± 10% HR y fotoperiodo de 12 h. Fue ofrecida molleja/carne, dependiendo del tratamiento, para estimular la ovoposición. El peso promedio de la masa de huevos (1,063 g; 1,12 g), el peso promedio de la masa de huevos/día (0,0658 g; 0,0698 g) y el peso promedio de la masa de huevos/ hembra/día (0,0118 g; 0,0125 g) no difirieron significativamente entre las dietas de molleja y carne, respectivamente. Igualmente, el número promedio de huevos/g (8221,23; 8569,29) y la media de viabilidad de los huevos (99%; 94%), no difirieron de manera significativa entre las dietas (prueba t de Student α = 5%). Se demostró a través del método no paramétrico Kaplan-Meier y del método paramétrico de regresión de Weibull, que no se presentaron diferencias (en días) entre el tiempo mediano total de longevidad (37 vs. 38), el tiempo promedio estimado para la longevidad de machos (41,08 vs. 40,04) y hembras (33,79 vs. 36,29) alimentados con molleja y carne, respectivamente. El máximo de longevidad fue de 74 días para ambas dietas. Los Resultados demostraron que la dieta de mollejas de pollo es una alternativa eficaz y económica de criar C. macellaria en laboratorio.
Palabras clave: Mosca barrenadora. Entomología forense. Cría de insectos. Biología de laboratorio.
Introduction
Establishing insect colonies in the laboratory is essential for different entomological studies, making sure to meet the biological aspects of the species while also being economi-cally viable. For species in the Calliphoridae family, several studies have been developed seeking alternative diets that meet these two requirements (Taylor and Mangan 1987; Cunha-Silva and Milward-de-Azevedo 1994; Green et al. 2003; Barbosa et al. 2004; Silva et al. 2008; Mendonga 2009; Pires et al. 2009; Ferraz et al. 2011).
The natural diets used in there a ring of Calliphoridae in the laboratory include beef, horse meat, fish, sheep meat, and other foods with high protein values (Greenberg and Szyska 1984; Marckenko 1985; Queiroz and Milward-de-Azevedo 1991; Cunha-Silva and Milward-de-Azevedo 1994; Chaudhury et al. 2000; Day and Wallman 2006; Barbosa et al. 2008). Chicken gizzards have similar nutritional cha-racteristics to beef (Esposito et al. 2009), are readily available in the market, cost approximately 40% less than beef, and are easy to handle. This has triggered the interest of the scientific community, as shown by studies on rearing juvenile calliphorids, such as Chrysomya albiceps (Wiedemann, 1819) and Cochliomyia macellaria (Fabricius, 1775), with promising results in terms of post-embryonic development (Ferraz et al. 2012; Silva et al. 2012).
Cochliomyia macellaria is an important species from a medical and veterinary point of view because it can cause secondary myiasis in cows, sheep, horses, dogs, pigs, and chickens (Bermudez et al. 2007) and can be associated with human myiasis, making it an agent of a neglected disease (Marquez et al. 2007; Ferraz et al. 2011). This species can be a vector of Dermatobia hominis (Linnaeus Jr., 1781) eggs, which are responsible for cutaneous furuncular myiasis (Guimaraes and Papavero 1999; Moya-Borja 2003), and it is also a pathogen carrier in humans (Thyssen et al. 2004; Graczyk et al. 2005; Ribeiro et al. 2011) and animals (Greenberg 1971). This species has also been mentioned in studies on ecological succession in animal carcasses (Batan et al. 2007; Gomes et al.2009; Biavati et al. 2010), and it has been found invading human corpses, allowing it to be used to estimate the post death interval (IPM) (Byrd and Butler 1996; Barreto et al.2002; Oliveira-Costa and Mello-Patiu 2004).
Females necrobiontophageous blow flies are anauto-genous, i.e., they require a protein meal in order to produce eggs. They require regular ingestion of amino acids, vitamin C, and mineral salts for normal ovule production (Zucoloto 2000). Observed feeding habits of adult C. macellaria include necrophagy and ingestion of body exudates and urban food residues (Laake et al. 1936; Ferreira1983; Guimaraes and Papavero 1999). They have been observed to feed on flower nectar and attracted to plants that emit odors similar to putrefied meat, like Aristolochia sp. and Iris foetidissima, Linnaeus (Greenberg 1971; Tompkins and Bird 1988). However, it is the quantity and quality of food consumed in the larval stage that may affect growth and development, resulting in a reproductively competitive adult (Parra et al.2009).
Thus the objective of the present study was to compare the reproductive potential and longevity of C. macellaria adults from juveniles reared on natural chicken gizzards to those reared on beef, under controlled conditions.
Materials and methodsThe experimental part was carried out in the Fly Study Laboratory of the Department of Microbiology and Parasi-tology at the Universidade Federal do Estado do Rio de Janeiro (UNIRIO), Brazil.To establish a colony, adults were collected in the municipality of Seropédica, Rio de Janeiro (22°45'48"S 43°41'23"W). The trap used followed the model by Mello et al. (2007), baited with fresh fish (Lycengraulis grossidens) and exposed for about six hours during the day. Adults were identified using the taxonomic key of Mello (2003). The rearing methodology followed the orientation developed by Cunha-e-Silva and Milward-de-Azevedo (1996) and Barbosa et al. (2008).
The adults (third-generation) used in the present study came from juveniles reared on two natural diets, chicken gizzards and beef (rump steak). The larvae rearing methodology was described by Silva et al. (2012) and Aguiar-Coelho and Milward-de-Azevedo (1996). Shortly after abandoning the diet, the larvae were transferred to test tubes containing sterilized wood chips that served as a pupation substrate, sealed with nylon fabric and fastened with elastic. After emergence, the adults from each diet (chicken gizzard and beef) were sexed and randomly formed couples, which were transferred to1L polyethylene cages with nylon screens on the sides (Barbosa et al. 2004). Ten couples were used per cage, with four cages per treatment, totaling 40 couples. The protein source, 30 g chicken gizzard or 30 g beef, according to the treatment, was offered until the fourth day of age to stimulate oogenesis. After the 12th day, to standardize the start of the oviposition phase, the protein source was offered again, kept for a period of 24 hours, and reintroduced every two days until all the females died. A 50% honey and water solution was offered without interruption throughout the experiment. The experiment was carried out in a rearing chamber regulated to 30 °C day/28 °C night, 70 ± 10% RH, and 12 hour light period. The observations were made daily at 09:00 hours until the death of all the adults.
After oviposition, the egg masses were weighed and each egg mass was divided randomly. One part continued in the rearing chamber for a further 12 hours, and, after eclosion, egg viability was observed using a stereoscopic microscope from the eclosion of the larva. The other part of the egg mass was again weighed and taken to the refrigerator (-5 °C) in a Petri dish for later egg counting. A solution of sodium hypochlorite solution (0.5%) diluted in 50% distilled water was used to help separate the egg mass. After removing from the refrigerator, about 3 ml of the solution was placed on the mass and observed under the microscope, where a stiletto was used to loosen the eggs from the mass to be counted.
The reproductive potential results were analyzed by a Student t-test (P < 0.05). The longevity was described by the Kaplan-Meier non-parametric method and the Weibull parametric regression method. A complete randomized experimental design was used.
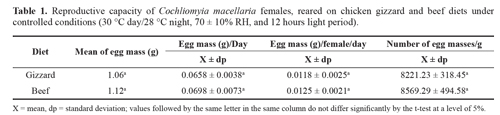
Results and discussionThe mean weight of the C. macellaria eggs from the females fed chicken gizzards was 1.06 g and 1.12 g for those fed beef (Table 1), and there was no significant difference (P = 0.774). Cunha-e-Silva and Milward-de-Azevedo (1996) worked with groups of 20 and 40 couples per replication fed with horsemeat and obtained a mean of 1.11 g and 1.68 g of eggs, respectively, suggesting that there was not necessarily a tendency for exponential growth in egg production with increasing couples. Paes et al. (2005) analyzed the reproductive performance of Lucilia cuprina (Wiedemann, 1830) fed horsemeat and observed that females of isolated couples laid more eggs than grouped females.
There was no significant difference in the mean weight of eggs laid per day (P = 0.02) between the chicken gizzard and beef treatments (0.0658 g and 0.0698 g, respectively), the same was true for the mean daily weight of eggs/female, 0.0118 g and 0.0125, respectively (P = 0.9582) (Table 1). According to Hall (1948), C. macellaria lays 49-250 eggs/ female, while Greenberg and Skyska (1984) observed 75150 eggs/female using fish to stimulate oviposition, but these authors did not record the oviposition weights. Cunha-e-Silva and Milward-de-Azevedo (1996) used horsemeat to stimulate oviposition and observed that the egg mass/female varied from 0.017 to 0.027 g, corroborating the data of the present study.
The mean numbers of eggs/g were 8,221.23 and 8,569.2 with chicken gizzard and beef diets, respectively, which were not significantly different (P = 0.5981) (Table 1). Similar data were reported by Cunha-e-Silva and Milward-de-Azevedo (1994), who observed that 1 g of eggs corresponded to an average of 8,457.5 eggs. A lack of micronutrients can affect egg production without damaging insect body mass and size (Colegrave 1993; Vamosi 2005).Thus, experimental confirmation is important for analyzing the reproductive potential of adiet.
The mean egg viability with the chicken gizzard diet was 99%, while that with the beef diet was 94%, which were not significantly different (P = 0.3805). The protein substrate is important for ovocyte maturation (Wall et al. 2002) and the stimulation of mating and oviposition (Barton-Browne et al. 1976) in necrobiontphageous blowflies, so the gizzard substrate was shown to be efficient.
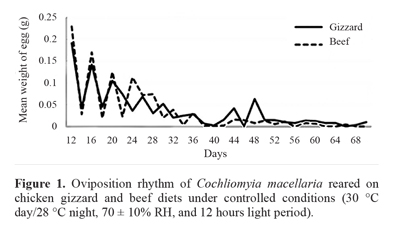
C. macellaria oviposition was the highest between the 12th and 20th day and the females made intermittent ovipositions, exceeding 68 days after emergence, on both diets (Fig. 1). Cunha-e-Silva and Milward-de-Azevedo (1996) analyzed C.macellaria fed with horsemeat where the females were stimulated to daily oviposition twice a day, and they observed peaks on the 11th and 22nd day, similar to the present study. However, oviposition occurred within 40 days, after which there was no oviposition, shorter than the oviposition time in this study. This may have occurred because, in the present study, the stimulus to oviposition may have been late, after the 12th day.
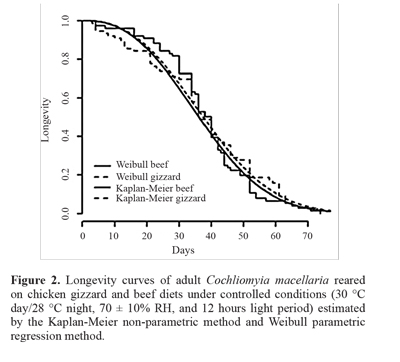
The estimated longevity curves were presented by the Kaplan-Meier non-parametric method and Weibull parametric regression method (Fig. 2). In both cases, the chicken gizzard and beef diets were considered co-variables.
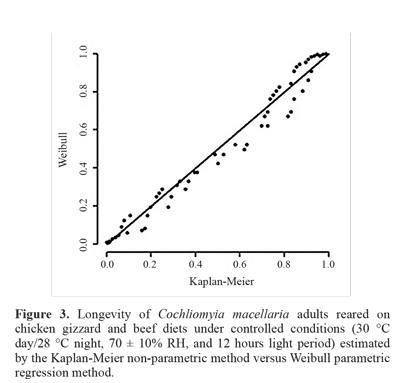
According to Colosimo and Giolo (2006), the suitability of a model can be verified by a graph comparing the estimates obtained by the Kaplan-Meier non-parametric method with the estimates obtained by the model. If the fit is good, the curve should follow the x = y line. In this sense, figure 3 pre-sents the estimated longevity by the Kaplan-Meier non-pa-rametric method versus the estimates obtained by the Weibull parametric regression method. As the curve follows a straight line, the Weibull parametric regression method was a good fit to the data.
According to the Kaplan-Meier estimator, which expres-ses a graphic representation of the real data (Carvalho et al. 2011), the average longevity for flies on the beef diet was 38 days, while for the chicken gizzard diet it was around 37 days. It was also observed that, for both diets, all the flies were dead within74 days. A log-rank test found no difference between the longevity curves for the chicken gizzard and beef diets, considering a confidence level of 95% (P = 0.573). Cunha-e-Silva and Milward-de-Azevedo (1996) worked with groups of 20 and 40 couples and observed a maximum longevity of 52 days for females and 49 for males and 65 days for females and 70 days for males, respectively.
From the Weibull regression (Fig. 2) (P = 0.58) it was concluded that there was no difference between the diets. The estimates of maximum likelihood for the form and scale parameters were 2.51 and 40.37, respectively. According to Reis and Haddad (1997), the survival curve was type I, that is, the mortality rate increased with time as the shape parameter was greater than 1.
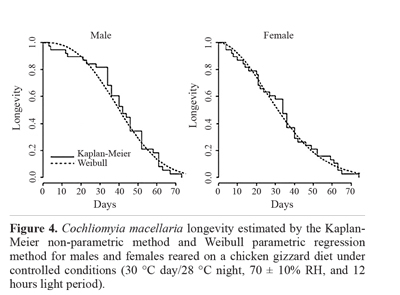
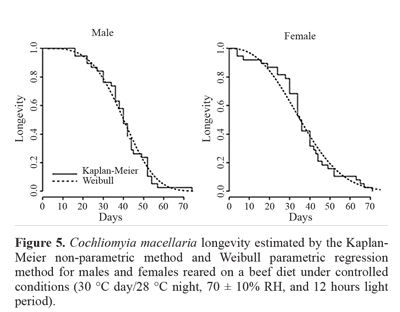
The log-rank test (P = 0.21) showed that there was no difference between the mean time estimated for male longevity (41.08 days) and female longevity (33.79 days) when fed a chicken gizzard diet (Fig. 4). The shape and scale parameters for the males were 2.63 and 46.23, respectively, and for the females, 1.89 and 38.08, respectively. The same occurred for the beef diet, where there was no significant difference between the sexes (P = 0.36). The estimated mean longevity was 40.04 days for males and 36.29 days for females (Fig. 5). The values of the estimates obtained for the fit of the Weibull parametric regression method were 3.67 for the shape parameter and 44.38 for the scale parameter. The estimated values were 2.48 and 40.91 for the females for the form and shape parameters, respectively. This showed that the survival curve was type I for both sexes on each diet. Cunha-e-Silva and Milward-de-Azevedo (1996), observed a mean longevity of 30 and 35 days for females and males of C. macellaria, respectively.
The function of the adult, in many cases, is related to dispersion and, especially, reproduction. These functions depend on the interaction of physiological and behavioral processes that are related to food intake and use. Egg or progeny production involves energy and nutrient accumu-lation by females, which makes them consume more and gain more weight, affecting egg production (Parra et al. 2009). In this sense, an efficient alternative diet should have the nutritional aspects that supply the needs of the insect, resulting in individuals with the same or greater longevity and/or reproductive capacity as those reared on a traditional natural diet.
From the observed results, chicken gizzards are an efficient natural diet for C. macellaria rearing. Furthermore, chicken gizzards are advantageous because they cost less than beef, are easy to buy in the market, and are easier to handle than beef.
AcknowledgementsWe would like to thank the Conselho Nacional de Desen-volvimento Científico e Tecnológico (CNPq), Financia-dora de Estudos e Projetos (FINEP), Fundayao de Amparo á Pesquisa do Estado da Bahia (FAPESB), Fundaqao de Amparo á Pesquisa do Estado do Rio de Janeiro (FAPERJ), Universidade Estadual do Sudoeste da Bahia (UESB), and Universidade Federal do Estado do Rio de Janeiro (UNIRIO) for financial support.
Literature citedAGUIAR-COELHO, V. M.; MILWARD-DE-AZEVEDO, E. M. V. 1996. Relayoes intra-específicas de Cochliomyia macelaria (Fabr), Chrysomya albiceps (Wied) e Chrysomya megacephala (Fabr) (Díptera, Calliphoridae) em condiyoes experimentais. Revista Brasileira de Entomologia 40 (1): 35-40. [ Links ]
BARBOSA, L. S.; JESUS, D. M. L.; COELHO, V. M. A. 2004. Longevidade e capacidade reprodutiva de casais agrupados de Chrysomya megacephala (Fabricius, 1794) (Diptera, Calliphoridae) oriundos de lavras criadas em dieta natural e oligídica. Revista Brasileira de Zoociéncias 6 (2): 207-217. [ Links ]
BARBOSA, L. S.; COURI, M. S.; AGUIAR-COELHO, V. M. 2008. Development of Nasonia vitripennis (Walker, 1836) (Hymenoptera: Pteromalidae) in pupae of Cochliomyia macelaria (Fabricius, 1775) (Diptera: Calliphoridae), using different densities of parasitoid. Biota Neotropica 8 (1): 49-54. [ Links ]
BARRETO, M.; BURBANO, M. E.; BARRETO, P. 2002. Flies (Calliphoridae, Muscidae) and beetles (Silphidae) from human cadavers in Cali, Colombia. Memorias do Instituto Oswaldo Cruz 97 (1): 137-138. [ Links ]
BARTON-BROWNE, L.; BARTEL, R. J.; VAN GERWEN, A. C. M.; LAWRENCE, L. A. 1976. Relationship between protein ingestion and sexual receptivity in females of the Australian sheep Luciliacuprina. Physiological Entomology 1 (4): 235-240. [ Links ]
BATTAN HORENSTEIN, M.; LINHARES, A. X.; ROSSO, B.; GARCIA, M. D. 2007.Species composition and seasonal succession of saprophagous calliphorids in a rural area of Cordoba, Argentina. Biological Research 40: 163-171. [ Links ]
BERMÚDEZ, S.; ESPINOSA, J.; CIELO, A. B.; CLAVEL, F.; SUBÍA, J.; BARRIOS, S.; MEDIANERO, E. 2007. Incidence of myiasis in Panama during the eradication of Cochliomyia hominivorax (Coquerel 1858, Diptera: Calliphoridae) (2002-2005). Memórias do Instituto Oswaldo Cruz 102 (6): 675-679. [ Links ]
BIAVATI, G. M.; ASSIS SANTANA, F. H.; PUJOL-LUZ, J. 2010. A checklist of Calliphoridae blowflies (Insecta, Díptera) associated with a pig carrion in Central Brazil. Journal of Forensic Sciences 55 (6): 1603-1606. [ Links ]
BYRD, J. H.; BUTLER, J. F. 1996. Effects of temperature on Cochliomyia macellaria (Diptera: Calliphoridae) development. Journal Medical Entomology 33: 901-905. [ Links ]
CARVALHO, M. S.; ANDREOZZI, V. L.; CODEQO, C. T.; CAMPOS, D. P.; BARBOSA, M. T. S.; SHIMAKURA, S. E. 2011. Análise de sobrevivencia: teoria e aplicares em saúde. Editora Fiocruz, Rio de Janeiro. 2nd ed., 434 p. [ Links ]
CHAUDHURY, M. F.; ALVAREZ, A. L. L.; VELAZQUEZ, L. A. 2000. New meatless diet for adult screwworm (Diptera: Calliphoridae). Journal of Economic Entomology 93 (4): 1398-1401. [ Links ]
COLEGRAVE, N. 1993 Does larval competition affect fecundity independently of its effect on adult weight? Ecology Entomology 18: 275-277. [ Links ]
COLOSIMO, E. A.; GIOLO, S. R. 2006. Análise de sobrevivencia aplicada. Edgard Blücher, Sao Paulo. 392 p. [ Links ]
CUNHA-E-SILVA, S. L.; MILWARD-DE-AZEVEDO, E. M. V. 1994. Estudo comparado do desenvolvimento pósembrionário de Cochliomyia macelaria (Fabricius) (Diptera, Calliphoridae) á base de carne em laboratorio. Revista Brasileira de Zoologia 11 (4): 659-668. [ Links ]
CUNHA-E-SILVA, S. L.; MILWARD-DE-AZEVEDO, E. M. V. 1996. Aspectos da biologia da reprodujo e longevidade de Cochliomyia macelaria(Fabricius) (Diptera, Calliphoridae), em condijoes experimentais. I. Casais agrupados. Revista Brasileira de Zoologia 13 (4): 883-889. [ Links ]
DAY, D. M.; WAlLMAN, J. F. 2006. Influence of substrate tissue type on larval growth in Calliphora augur and Lucilia cuprina (Diptera: Calliphoridae). Journal of Forensic Science 5 (13): 469-711. [ Links ]
ESPOSITO, A. B. M.; LIMA, C. C; SOUZA, F. N.; RIBAS, F.; KOROLHUK, J.; LUZ, K. C.; MUNARO, V; RIBAS, A. R.; BALBI, M. E. 2009. Avaliajao de miúdos de Gallus domesticus como fonte proteica.Visao Académica 10 (2): 59-74. [ Links ]
FERRAZ, A. C. P.; BOSISIO, D. D.; AGUIAR-COELHO, V. M. 2011. Dieta para larvas de Chrysomya megacephala, Chrysomya putoria e Cochliomyia macelaria (Diptera: Calliphoridae). EntomoBrasilis 4 (3): 125-129. [ Links ]
FERRAZ, A. C. P.; ALMEIDA, A. V. R. G.; JESUS, D. M.; ROTATORI, G. N.; NUNES, R.; PROENQA, B.; AGUIAR-COELHO, V. M.; LESSA, C. S. S. 2011. Epidemiological study of myiases in the Hospital do Andaraí, Rio de Janeiro, including reference to an exotic etiological agent. Neotropical Entomology 40 (3): 393-397. [ Links ]
FERRAZ, A. C. P.; DALLAVECCHIA, D. L.; SILVA, D. C.; CARVALHO, R. P; SILVA FILHO, R. G.; AGUIAR-COELHO, V. M. 2012. Alternative diets for Chrysomya putoria, an Old World screwworm fly. Journal of Insect Science 12 (43): 1-11. [ Links ]
FERREIRA, M. J. M. 1983. Sinantropia de Calliphoridae (Diptera) em Goiánia, Goiás. Revista Brasileira de Biologia 43: 199-210. [ Links ]
GOMES, L.; GOMES, G.; DESUÓ, I. C. 2009. A preliminary study of insect fauna on pig carcasses located in sugarcane in winter in southeastern Brazil. Medical and Veterinary Entomology 23 (2): 155-159. [ Links ]
GRACZYK, T.; KNIGHT, R.; TAMANG, L. 2005. Mechanical transmission of human protozoan parasites by insects. Clinical Microbiology Reviews 1 (18): 128-132. [ Links ]
GREENBERG, B. 1971. Flies and disease, vol. I: Ecology, classification and biotic associations. Princeton University Press, New York. 865 p. [ Links ]
GREENBERG, B.; SZYSKA, M. L. 1984. Immature stages and biology of 15 species of Peruvian Calliphoridae, Diptera. The Entomological Society of America 77: 488-517. [ Links ]
GREEN, P. W. C.; SIMOND, M. S. J.; BLANEY, W. M. 2003. Diet nutriment and rearing density effect the growth of black blowfly larvae Phormia regina (Diptera: Calliophoridae). European Journal of Entomology 100: 39-43. [ Links ]
GUIMARAES, J. H.; PAPAVERO, N. 1999. Myiasis in man and animals in the Neotropical Region: Bibliographic database. Editora Pléiade/Fapesp, Sao Paulo. 308 p. [ Links ]
HALL, D. G. 1948.The blowflies of North America.Entomological Society of America, Lanham, MD. 447 p. [ Links ]
LAAKE, E. W.; GUSHING, E. C.; PARISH, H. E. 1936. Biology of the primary screwworm fly, Cochliomyia americana, and a comparison of its stages with those of C. macellaria. U.S. Department Agriculture Technical Bulletin 500: 1-24. [ Links ]
MARKENCO, M. I. 1985. Development of Chrysomya albiceps (Wiedman) (Diptera: Calliphoridae). Entomologicheskoe Obozrenie 1: 79-84. [ Links ]
MARQUEZ, A. T.; MATTOS, M. S.; NASCIMENTO, S. B. 2007. Miíases associadas com alguns fatores sócio-económicos em cinco áreas urbanas do Estado do Rio de Janeiro. Revista da Sociedade Brasileira de Medicina Tropical 40 (2): 175-180. [ Links ]
MELLO, R. P. 2003. Chave para identificado das formas adultas das espécies da família Calliphoridae (Diptera: Brachycera, Cyclorrhapha) encontradas no Brasil. Revista Entomología y Vectores 10 (2): 255-268. [ Links ]
MELLO, R. S.; QUEIROZ, M. M. C.; AGUIAR-COELHO, V. M. 2007. Population fluctuations of calliphorid species (Diptera, Calliphoridae) in the Biological Reserve of Tinguá, state of Rio de Janeiro, Brazil. Iheringia Série Zoologia 97 (4): 48-485. [ Links ]
MENDONQA, P. M.; QUEIROZ, M. M. C.; DE-ALMEIDA, J. M, J. M. 2009. Rearing Chrysomya megacephala on artificial diets composed of varying concentrations of albumin. Brazilian Archives of Biology and Technology 52 (2): 421-426. [ Links ]
MOYA-BORJA, G. E. 2003. Erradicado ou manejo integrado das miíases neotropicais das Américas. Pesquisa Veterinária Brasileira 23: 131-138. [ Links ]
OLIVEIRA-COSTA, J.; MELLO-PATIU, C. A. 2004. Application of forensic entomology to estimate of the postmortem interval (PMI) in homicide investigations by the Rio de Janeiro Police Department in Brazil. Journal of Forensic Medicine and Toxicology 5 (1): 40-44. [ Links ]
PAES, M. J.; BRITO, L. G.; MOYA-BORJA, G. E.; DAEMON, E. 2005. Comportamento reprodutivo e longevidade de casais isolados e agrupados de Lucilia cuprina, sob condijoes controladas. Revista Brasileira de Parasitologia Veterinária 14 (1): 21-25. [ Links ]
PARRA, J. P.; PANIZZI, A. R.; HADAD, M. N. 2009. Índices nutricionais para medir consumo e utilizado de alimentos por insetos. In: Panizzi, A. R; Parra, J. R. P. (Eds.). Bioecologia e nutrido de insetos: base para o manejo integrado de pragas. (1st ed.). Brasilia, EMBRAPA/CNPq. 1164 p. [ Links ]
PIRES, S. M.; CÁRCAMO, M. C.; ZIMMER, C. R.; RIBEIRO, P. B. 2009. Influencia da dieta no desenvolvimento e investimento reprodutivo de Chrysomya megacephala (Fabricius, 1794) (Diptera: Calliphoridae). Arquivos do Instituto Biológico 76 (1): 41-47. [ Links ]
QUEIROZ, M. M. C.; MILWARD-DE-AZEVEDO, E. M. V. 1991. Técnicas de criado e alguns aspectos da biologia de Chrysomya albiceps (Wiedemann) (Diptera, Calliphoridae), em condijoes de laboratório. Revista Brasileira de Zoologia 8: 75-84. [ Links ]
REIS, P. R.; HADDAD, M. L. 1997. Distribuido de Weibull como Modelo de Sobrevivencia de Iphiseiodes zuluagai Denmark e Muma (Acari: Phytoseiidae). Anais da Sociedade Entomológica do Brasil 26(3): 441-444. [ Links ]
RIBEIRO, M. J. R.; DIAS, M. F. D.; TESHIMA, E.; BARBONI, A. R. 2011. Insalubridade ambiental e aspectos sociais associados a patógenos intestinais isolados de dípteros. Revista Engenharia Sanitária e Ambiental 6 (1): 83-90. [ Links ]
SILVA, C.; MOYA-BORJA, G. E.; AZAMBUJA, P. 2008. Use of polyester pad as a new physical substrate for rearing Cochliomyia hominivorax Coquerel (Diptera: Calliphoridae) larvae. Neotropical Entomology 37 (3): 349-351. [ Links ]
SILVA, D. C.; AGUIAR-COELHO, V. M.; CUNHA-E-SILVA, S. L.; CARVALHO, R. P.; MOYA-BORJA, G. E. 2012. Desenvolvimento pósembrionário de Cochliomyia macellaria (Fabricius) (Diptera: Calliphoridae), criada em duas dietas naturais, sob condifoes controladas. Revista Biotemas 25 (4): 131-137. [ Links ]
TAYLOR, D. B.; MANGAN, R. L. 1987. Comparison of gelled and meat diets for rearing screwworm, Cochliomyia hominivorax (Diptera: Calliphoridae), larvae. Journal of Economic Entomology 80 (2): 427-432. [ Links ]
THYSSEN, P. J.; MORETTI, T. C.; UETA, M. T.; RIBEIRO, O. B. 2004. O papel de insetos (Blattodea, Diptera e Hymenoptera) como possíveis vetores mecánicos de helmintos em ambiente domiciliar e peridomiciliar. Cadernos de Saúde Pública 20 (4): 1096-1102. [ Links ]
TOMPKINS, P.; BIRD, C. 1988. A vida secreta das plantas. Expressao e Cultura, Porto Alegre. 324 p. [ Links ]
VAMOSI, S. M. 2005. Interactive effects of larval host and competition on adult fitness: an experimental test with seed beetles (Coleoptera: Bruchidae). Functional Ecology 19 (5): 859-864. [ Links ]
WALL, R.; WEARMOUTH, V. J.; SMITH, K. E. 2002. Reproductive allocation by the blow fly Lucilia sericata in response to protein limitation. Physiological Entomology 27: 267-274. [ Links ]
ZUCOLOTO, F. S. 2000. Nutrifao e alimentafao. In: Malavasi, A.; Zucchi, R. A.(Eds.). Moscas-das-frutas de importancia económica no Brasil: conhecimento básico e aplicado. Holos Editora, Ribeirao Preto. 327 p. [ Links ]
Received: 16-Sep-2013
Accepted: 16-Feb-2015
SILVA, D. C.; AGUIAR, V. M.; CUNHA E. SILVA, S. L.; CARVALHO, R. P; SILVA, A.; MOYA-BORJA, G. E. 2015. Adult longevity and reproductive capacity in Cochliomyia macellaria (Diptera: Calliphoridae) reared on an alternative diet. Revista Colombiana de Entomología 41 (1): 126-131. Enero-Junio 2015. ISSN 0120-0488.