Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488
Rev. Colomb. Entomol. vol.41 no.2 Bogotá July/Dec. 2015
Sección Médica / Medical
Resistance of Aedes aegypti (Díptera: Culicidae) to temephos in Paraná State, Brazil
Resistencia de Aedes aegypti (Díptera: Culicidae) a temefos en el estado de Paraná, Brasil
JONNY E. DUQUE L.1, ALLAN M. SILVA2, ELAINE C. S. FANTINATTI3 and MARIO A. NAVARRO-SILVA4
1Ph. D. Centro de Investigaciones en Enfermedades Tropicales (CINTROP). Grupo de Investigación en Enfermedades Infecciosas y Metabólicas (GINEM).Facultad de Salud, Escuela de Medicina, Departamento de Ciencias Básicas, Universidad Industrial de Santander, Bucaramanga, Colombia. jonedulu@uis.edu.co.
2 Ph. D. Laboratorio Central, Secretaria de Estado da Saúde do Paraná, Brasil.
3 M. Sc.
4 Ph. D. Laboratorio de Entomologia Médica e Veterinária, Universidade Federal do Paraná, Departamento de Zoologia. Pós-gradua 9 ao em Entomologia. PO Box 19020, 81531-980 Curitiba, Paraná, Brasil. mnavarro@ufpr. br. Corresponding autor.
Abstract: The number of dengue cases has increased drastically the state of Paraná since 1995 without a consistent explanation. In order to contribute to this subject, the susceptibility of A. aegypti to the insecticide temephos was evaluated in several municipalities in Paraná, along with seasonal variation of oviposition of Aedes spp. in Maringá and Jacarezinho. Comparison of mortality and resistance rates RR95 (CD = 0.0060 mg/L) of the Rockefeller temephos strain (Rock) on individuals from these locales showed that vector populations from Foz do Iguaju (67.5 ± 7.7; RR95 = 3.9), Paranavaí (58.1 ± 14; RR95 = 3.4), Maringá (57.8 ± 5.2; RR95 = 6.9), Ibipora (68.75 ± 3.9; rR95 = 6.6), and Jacarezinho (73.3 ± 7.5; RR95 = 3) were resistant; while those from Cambé (81.56 ± 10; RR95 = 2.6) had an incipient change in susceptibility. The vector populations in Maringá and Ibipora displayed average resistances (RR95 > 5 and < 10), while the others showed low resistance (RR95 < 5). In Maringá and Jacarezinho, oviposition fluctuation was lower in winter.
Key words: Chemical control. Dengue. Susceptibility. Vector.
Resumen: El dengue se incrementó drásticamente desde 1995 en el Estado de Paraná sin una explicación consistente. En un intento de contribuir con información a este problema, fue analizada la susceptibilidad de A. aegypti al insecticida temefos en varios municipios del Estado de Paraná, así como la variación estacional de la ovoposición de Aedes spp. en Maringá y Jacarezinho. La comparación del porcentaje de mortalidad y tasa de resistencia RR95 de CD = 0,0060 mg/L a temefos de la cepa Rockefeller con los individuos de las localidades seleccionadas mostró como resistentes a las poblaciones del vector de Foz de Iguazú (67,5 ± 7,7; RR95 = 3,9), Paranavaí (58,1 ± 14; RR95 = 3,4), Maringá (57,8 ± 5,2; RR95 = 6,9), Ibipora (68,75 ± 3,9; RR95 = 6,6) y Jacarezinho (73,3 ± 7,5; RR95 = 3) y una incipiente alteración de la susceptibilidad en la población de Cambé (81,56 ± 10; RR95 = 2,6). Las poblaciones del vector de Maringá e Ibipora presentaron resistencia media (RR95 > 5 e < 10) y las demás, baja resistencia (RR95 < 5). En Maringá e Ibipora la fluctuación de ovoposición fue menor en invierno.
Palabras clave: Control químico. Dengue. Susceptibilidad. Vector.
Introduction
The World Health Organization estimates that more than 96 million people are infected by dengue virus every year, 550 thousand of which are hospitalized and about 20 thousand die as consequence of the disease (Funasa 2002; Guzman et al. 2010; Bhatt et al. 2013). In this context, Brazil has a large portion of these cases due to its continental dimensions, climate diversity and socio-environmental conditions that hinder symmetrical procedures actually effective in controlling the vector, Aedes aegypti (Linnaeus, 1762).
In Brazil, the 48.3% of all dengue cases occurs in the main cities in the northeast region. The southeast region is responsible for 37.2%, the Mid-West region 7.6%, the North region, 5.7% and the South region 1.2% of the cases. Most are reported during the hot months, occurring mainly in two periods during the first semester: in the Southeast, Midwest and South regions during the first three months and in the Northeast region during the following three months, indicating an established seasonality (Camara et al. 2007).
Many strategies are applied to control the increment of dengue cases in Brazil, ranging from environmental management and community educational programs to local vector control with chemical and biological products.
However, the main method applied to control populations of A. aegypti is the application of the organophosphate temephos with historical records since 1967 (Ministério da Saúde 1968).
The "status" of susceptibility to insecticides is periodically evaluated in larval populations of A. aegypti as indicated by the National Program for Dengue Control ("Programa Nacional de Controle da Dengue", PNCD) and the National Network for the Resistance Monitoring of A. aegypti to insecticides ("Rede Nacional de Monitoramento da Resistencia de A. aegypti", MoReNAa) (WHO 1992; Funasa 1999; Braga and Valle 2007). As a result of this insecticide monitoring, different studies determined susceptibility and, in some instances, warned of the appearance of resistant individuals, as reported for the following Brazilian states: Macoris et al. (1999), Campos and Andrade (2001) and Macoris et al. (2003) in Sao Paulo, Lima et al. (2003) in Rio de Janeiro, Braga et al. (2004) in Alagoas, Sergipe and Rio de Janeiro, Carvalho et al. (2004) in the Federal District, Duque et al. (2004) and Prophiro et al. (2011) in Paraná, Lima et al. (2006) and Lima et al. (2011) in Ceará and Beserra et al. (2007) in Paraíba.
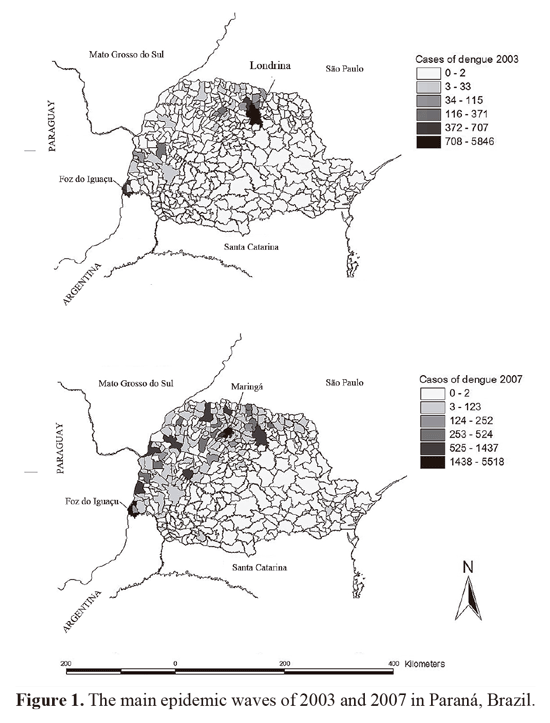
As advocated by MoReNAa, tests to detect loss of susceptibility to temephos should be conducted preferably in strategic places, especially in sentinel-municipalities with historical persistence of the disease. In the South region, Paraná is the single state with reported autochthonous cases where three municipalities have a continuous history of occurrences since 1995: Foz do Iguazu, Maringá e Londrina (MS/ SVS 2005, 2007). However, there are no published historical records regarding the susceptibility levels in other municipalities with dengue cases.
An important aspect to be clarified is whether the progressive increase in dengue cases in new areas of the state of Paraná is related to chemical resistance to the insecticide temephos (Fig. 1). In this sense, it is necessary to start with the establishment of historical records on the susceptibility of temephos. In this way, could be addressed new approaches of the ecological, genetic and operational elements that contribute to understand at development of resistance in this region (Georghiou and Taylor 1986; Bisset 2002). Basically, it is necessary to initiate studies to establish a baseline of the current status of resistance in the State for further investigations and more precise studies on its biological and evolutionary interrelations. To do so, sampling must be extended to more strategic places, such as localities close to national and international borders and also increase the number of municipalities, including areas with high commercial and tourist flow that connect Paraná to Mato Grosso do Sul, Sâo Paulo, Paraguay and Argentina. Consequently, it is expected that the development of resistance can be detected early in areas of occurrence of dengue, allowing effective and economic decisions regarding control procedures.
This study is intended to analyze the degree of susceptibility to temephos of populations of Aedes aegypti in Paraná State and assess oviposition fluctuations of Aedes spp. in different seasons in Maringá and Jacarezinho.
Material and methods
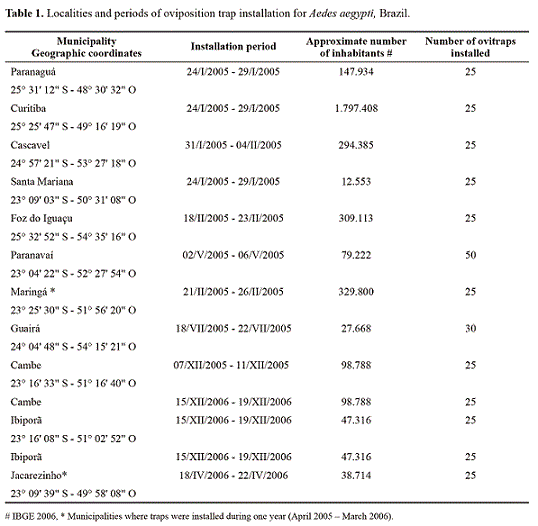
Sampling. In partnership with the "Secretaria Estadual de Saúde de Paraná", oviposition traps (ovitraps) were set up with a 500 mL hay solution at 10% (Funasa 2002). In each ovitrap, a "eucatex" palette was placed for the oviposition of A. aegypti females in two ways: the first in a known period of high egg-density (summer 2004-2005) to perform the susceptibility tests and, the second, during 12 months for the municipalities of Maringá and Jacarezinho, respectively considered localities of high and low infestation of A. aegypti. The last two localities were only selected in order to determine oviposition fluctuation in localities with a different history of dengue cases (Table 1).
Three or four ovitraps were placed randomly per area for 5 days in urban area peridomiciles (in residential neighborhoods and downtown) of the following municipalities with confirmed records of presence of dengue and/or Aedes spp. (Secretaria Estadual de Saúde): Curitiba, Paranaguá, Foz do Iguazu, Cascavel, Paranavaí, Maringá, Ibiporá, Cambé, Santa Mariana, Jacarezinho and Guaira. In general, 25 ovitraps were distributed per locality, except Paranavaí with 50 and Guaira with 30. This methodology of installation of the ovitraps ensures higher diversity in populations of the mosquitoes present in each sampling for analysis of resistance (SIsFaD - SESA/PR 2006). In Maringá and Jacarezinho, where oviposition fluctuation was also analyzed, ovitraps were placed for 12 months in the same conditions as the other
localities. The installation of the traps in these two localities was recommended by the "Secretaria Estadual de Saúde" due to the high number of dengue cases in the region. It is important to clarify that the PNCD’s criterion for installing traps by number of homes was not rigorously followed due to operational problems in the project. The eggs obtained in the second set of traps were not distinguished at the species level; which were meant to evaluate the oviposition activity of the two species.
The ovitrap palettes with positive results were analyzed at Laboratorio de Entomologia Médica e Veterinária at Universidade Federal do Paraná (LEMV-UFPR). Eggs were later quantified under a dissecting microscope, individually placed in 770 mL cups of water with triturate cat food (Purina® Cat Chow®) (0.36 g/ cup) in order to induce hatching. The palettes were removed after 24 hours of immersion, in order to avoid decrease in water quality. The larvae were kept in these cups with approximately 1g of food per day until they reached the pupa stage and, in this phase, they were transferred to cages for the separation of the adults and identified as for sex and species in order to obtain strains of A. aegypti F1 or F2 generations. Specimens of A. (Stegomyia) albopictus (Skuse), 1894 obtained from positive palettes were not used for the analysis of susceptibility to insecticides.
Bioassays. The insecticide temephos 90% technical grade (lot 002/2005) was obtained from Fersol Mairinque Sao Paulo. With the purpose of verifying the quality of temephos, a calibration of the chemical product was carried out with the susceptible reference lineage A. aegypti Rockefeller. Bioassays followed the protocol recommended by the World Health Organization (1981) and the resistance-monitoring program in Brazil (MoRenAa and PNCD).
A diagnostic concentration (DC) was applied to qualitatively detect the presence of individuals resistant to the DC 0.0060 mg/L (determined at our laboratory, LEMV-UFPR), corresponding twices the CL99 (0.0030 mg/L) of the Rockefeller strain (WHO 1981; 1992; Lima et al. 2003). As a lower susceptibility by the free populations in comparison to Rockefeller’s was identified, a series of multiple concentrations was performed as a quantitative analysis of resistance. Eight concentrations of the insecticide, including the diagnostic concentration, were established and used in all experiments together with 100 larvae distributed in four replicates per concentration, in addition to the control treatment with ethanol. The tests were repeated four times on different days and the experimental design was according to Robertson and Preisler (1992). All tests were performed concomitantly to the ones with Rock strain and under controlled conditions of temperature (25 °C ± 1), humidity (70% ± 10) and photoperiod (12:12).
Criteria and statistical analysis. Mortality responses to diagnostic concentration over 98% were considered susceptible; concentrations between 98-80% indicated moderate resistance and below 80%, resistance (Davidson and Zahar 1973). The resistance ratio (RR50 and RR95), as a quantitative indicator, was calculated comparing lethal concentrations (LC50, LC95) with Rock strain as follows: LC50 and 95 of locality/LC50 and 95 of Rock strain. Resistance levels are defined as low when lower than 5 times, medium when between 5 and 10, and high when higher than 10 (Mazzari and Georghiou 1995). In this study, we considered only RR 95 for the classification of resistance. The software Probit GW-Basic (Finney 1971) was used to determine the lethal concentrations lC 50, 95, A2 test, slope and confidence interval (CI). In order to detect statistical differences in oviposition and in the number of positive ovitraps installed in the municipalities of Maringá and Jacarezinho over a one-year period, a variance analysis for data with normal distribution (ANOVA) was applied. When significant differences were detected, a posteriori test Tukey HSD (Honest significant difference) was applied.
Results
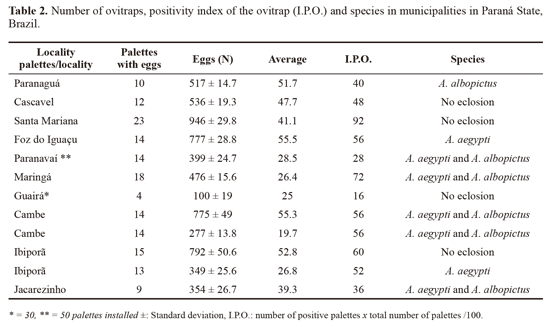
Oviposition traps installed for the susceptibility tests. Of the 355 ovitraps collected in Paraná, 160 were positive for immature specimens with a total of 6298 eggs, where Santa Mariana was the municipality with the highest ratio of oviposition and Guaira, the lowest. In Curitiba no positive ovitraps were detected. After eggs hatching, the species obtained were A. aegypti and A. albopictus (Table 2).
Oviposition activity during one year in Maringa and Jacarezinho. Maringa showed more positive palettes and eggs (N palettes = 119, N eggs = 7831) than Jacarezinho (N palettes = 113, N eggs = 4528), even though such values did not yield significant statistical differences for either eggs or positive ovitraps (F = 1.104, P = 0.304 and F = 0.03, P = 0.857), respectively. However, when considering the same variables in relation to the seasons (spring, summer, fall and winter), significant differences were observed (F = 4.48, P = 0.014). The Tukey HSD test confirmed that oviposition activity between the two municipalities decreased in winter (June, July and August), although the presence of Aedes was reported during the whole period of observation (Fig. 2).
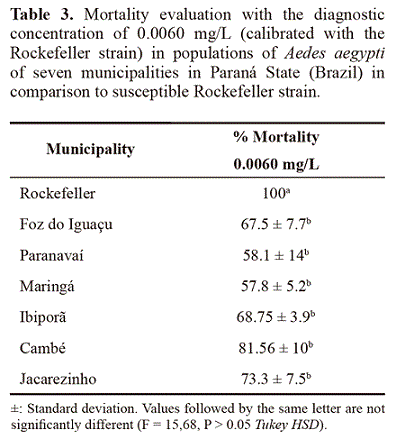
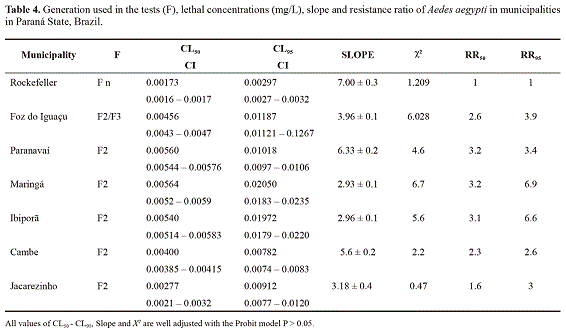
Bioassays evaluating diagnostic concentration, multiple concentrations and resistance rates. The comparison of A. aegypti Rock with populations from the municipalities evaluated showed that all those tested with DC (0.0060 mg/L) indicated resistance (Table 3). The resistance rates determined from the curve of multiple concentrations from all analyzed municipalities (RR95) confirms that the populations in Maringa and Ibipora have medium resistance and the remaining, low resistance. It was also observed that the X2 test adjusts well to the Probit model and the slope of all analyzed localities were lower compared to the susceptible strain (Rockefeller), confirming a difference in the response to the insecticide (Table 4).
Discussion
The diagnostic concentration established in the laboratory bioassays with A. aegypti Rock accused out that all localities compared to that strain were less susceptible, indicating the existence of a resistance process. However, variations in the intensity of susceptibility were detected as follows: Foz do Iguazu, Paranavaí, Maringá, Ibipora and Jacarezinho as the most resistant and Cambé with an incipient modification in susceptibility, according to the criterion of Davidson and Zahar (1973). This implies that the populations of A. aegypti from the sampled municipalities are under a distinguished selection pressure, leading to varied degrees of susceptibility when compared to a totally susceptible lineage (WHO 1992). The criterion of Mazzari and Georghio (1995) indicate that the analyzed municipalities may be considered of medium and low resistance. These resistance rates compared to other studies, such as in Rio de Janeiro by Lima et al. (2003), in Alagoas, Sergipe and Rio de Janeiro by Braga et al. (2004), in Ceará by Lima et al. (2006) and in Paraíba by Beserra et al. (2007) are lower, but similar to values found in municipalities in Sao Paulo State by Campos and Andrade (2001) and Macoris et al. (2003). Similarly, the RR values found in the study conducted in Curitiba (Paraná) by Duque et al. (2004) are also considered to be low when compared to the results found in this study. It is important to state that due to the low resistance levels found in this study, it cannot be determined whether the historical dengue epidemics in Paraná are linked to the resistance to temephos. However, a change in insecticide should be considered as was indicated for Montella et al. (2007) when RR > 3.
The slope of all the localities analyzed was lower compared to the susceptible strain (Rock), confirming a difference in the response to the insecticide and showing a loss of susceptibility as a consequence to the movement of higher concentrations of insecticide in the wild strain. The analysis indicates for Paraná the need of a systematic review of the traditional methods (insecticides) applied to the control of A. aegypti populations in order to determine whether they should be replaced by other chemical or biological products as a preventive method against the resistance increase, without compromising the vector’s control.
In southern Brazil (States of Paraná, Santa Catarina and Rio Grande do Sul), the number of reported cases of dengue amounts to only 1.2% of the whole country. This is a direct consequence of seasonal features which influence the vector’s dynamics with a reduction in the natural population due to the area presents low temperatures (Camara et al. 2007). Such population reductions in the coldest periods lead to a reduction in chemical control activities with a consequent decrease in selective pressure, the opposite of localities with higher temperatures, where resistance ratios are usually higher.
On the other hand, considering the tropical characteristics of northern Paraná and the vector’s adaptive capabilities, infestation ratios in this area could become similar to levels in places where the vector persists. This would suggest an augment of insecticide applications, consequently pushing resistance ratios to higher levels.
In the ovitraps installed in Maringá and Jacarezinho from April 2005 to March 2006, oviposition occurred practically without interruption, even in the periods of low temperatures reported during winter station. Thus, individual Aedes that survive the cold season may continue the biological and genetic processes that develop resistance. This fact permits further investigation in order to confirm whether seasonality truly influences resistance ratios.
In the future, a factor that may balance resistance ratios in Paraná with other States is gene flow, hereditary factors connected to dispersion and interbreeding among populations, as observed by Rawlins (1998). This must be specifically evaluated with genes that provide insecticide resistance in the South region. Even though some studies suggest huge differences among populations of A. aegypti from several regions of Brazil, and indicate low gene flow (Costa-Ribeiro et al. 2007), there is little elucidation on this matter in localities close to Paraná. For instance, Paduan et al. (2006) considers the possibility of the existence of a gene flow related to active migration of the mosquitoes’ populations. It is possible that this phenomenon helps to the spread of resistance in the border areas of the state of Paraná. That research includes Paraná’s individuals, which are closely related to the ones from Campinas in Sao Paulo State.
Nevertheless, based on Costa-Ribeiro et al. (2007) and Paduan et al. (2006), we hypothesize that a modification of the susceptibility of the vector’s populations can be linked to an inherent factor in the region, that is, the resistance is initiated by intrinsic factors of operational control. Also, it should be considered that the similarity of susceptibility with Sao Paulo State might be explained by its geographic proximity, as well as the genetic patterns of A. aegypti. This can only be answered by assessing the main road routes that link Sao Paulo, Mato Grosso do Sul, Paraguay and Argentina to Paraná in regards to the detection of genes for insecticide resistance.
The resistance ratios of A. aegypti populations analyzed in Paraná are lower than those reported in other Brazilian States. Further studies must be conducted to understand whether the resistance pattern found is a consequence of internal operational factors or factors related to the vector’s dynamics and dispersion.
Acknowledgements
Jonny E Duque L thanks CNPq for the scholarship granted during his Ph.D from 2004 to 2008. We wish to thank Secretaria Estadual de Saúde of Paraná and the research groups in Entomology for providing material for this study. To the researcher Maria de Lourdes da Graga Macoris of Superintendencia de controle de Endemias - SUCEN, Marília (SP) and Dr. Denise Valle and Dr. José Bento Pereira Lima of Instituto de Biologia do Exército at Instituto Oswaldo Cruz (RJ) for their helpful contribution in the bioassays. We are especially grateful to Denise Vale and José Bento Pereira Lima for the supply of temephos and the elucidation of doubts encountered during the course of this work.
Literature cited
BHATT, S.; GETHING, P. W.; BRADY, O. J.; MESSINA, J. P.; FARLOW, A. W.; MOYES, C. L.; DRAKE, J. M.; BROWNSTEIS, J. S.; HOEN, A. G.; SANKOH, O.; MYERS, M. F.; GEORGE, D. B.; JAENISCH, T.; WINT, G. R. W.; SIMMONS, C. P.; SCOTT, T. W.; FARRAR, J. J.; HAY, S. I. 2013. The global distribution and burden of dengue. Nature 496 (7446): 504-507. [ Links ]
BESERRA, E. B.; FERNANDES, C. R. M.; QUEIROGA, M. F. V; CASTRO, F. P. JR. 2007. Resistencia de populates de Aedes aegypti (L.) (Diptera: Culicidae) ao organofosforado temefós na Paraíba. Neotropical Entomology 36 (2): 303-307. [ Links ]
BRAGA, I. A.; LIMA, J. B. P; SOARES, S. S.; VALLE, D. 2004. Aedes aegypti resistance to temephos during 2001 in several municipalities in states of Rio de Janeiro, Sergipe, and Alagoas, Brazil. Memórias do Instituto Oswaldo Cruz 99 (2): 199-203. [ Links ]
BRAGA, I. A.; VALLE D. 2007. Aedes aegypti: vigilancia, monitoramento da resistencia e alternativas de controle no Brasil. Epidemiologia e Servidos de Saúde 16 (4): 295-302. [ Links ]
BISSET, J. A. 2002. Uso correcto de insecticidas: control de la resistencia. Revista Cubana de Medicina Tropical 54 (3): 202219. [ Links ]
CÁMARA, P. F.; THEOPHILO, G. R. L.; SANTOS, T. G.; PEREIRA, G. S. R. F.; CÁMARA, P. D. C.; MATOS, R. R. 2007. Estudo retrospectivo (histórico) da dengue no Brasil: características regionais e dinamicas. Revista da Sociedade Brasileira de Medicina Tropical 40 (2): 192-196. [ Links ]
CAMPOS, J.; ANDRADE, C. F. S. 2001. Susceptibilidade larval de duas populates de Aedes aegypti a inseticida químicos. Revista de Saúde Pública 35 (3): 232-236. [ Links ]
CARVALHO, M. S. L.; CALDAS, E. D.; DEGALLIER, N.; VILARINHOS, P. T. R.; SOUZA, L. C. K.; YOSHIZAWA, M. A. C.; KNOX, M. B.; OLIVEIRA, C. 2004. Susceptibility of Aedes aegypti larvae to the insecticide temephos in the Federal District, Brazil. Revista de Saúde Pública 38 (5): 623-629. [ Links ]
COSTA-RIBEIRO, M. C. V.; LOURENQO-DE-OLIVEIRA, R.; FAILLOUX, A. B. 2007. Low gene flow of Aedes aegypti between dengue-endemic and dengue-free areas in Southeastern and Southern Brazil. The American Journal of Tropical Medicine and Hygiene 77 (2): 303-309. [ Links ]
DAVIDSOn, G.; ZAHAR, D. A. 1973. The practical implications of resistance of malaria vectors to insecticides. Bulletin of the World Health Organization 49: 475-483. [ Links ]
DUQUE, J. E. L.; MARTINS, F. M.; ANJOS, F. A.; KUWABARA, E. F.; NAVARRO-SILVA, M. A. 2004. Susceptibilidade de Aedes aegypti aos inseticidas temephos e cipermetrina, Brasil. Revista de Saúde Pública 38 (6): 842-843. [ Links ]
FINNEY, D. J. 1971. Probit Analysis. Cambridge University Press, 3rd. Edition. [ Links ]
FUNASA. 1999. Reuniao técnica para discutir status de resistencia de Aedes aegypti e definir estratégias a serem implantadas para monitoramento da resistencia no Brasil. Fundagao Nacional de Saúde, Ministério da Saúde, Brasilia. [ Links ]
FUNASA. 2002. Programa Nacional de Controle da Dengue. Brasília. Fundagao Nacional de Saúde, Ministério da Saúde, Brasília. [ Links ]
GEORGHIOU, G. P.; TAYLOR, C. E. 1986. Pesticide Resistance: Strategies and Tactics for Management. Ed. National Research Council. National Academy Press, Washington DC. 157. 169 p. [ Links ]
GUZMAN, M. G.; HAL STEAD, S.B.; ARTSOB, H.; BUCHY, P.; FARRAR, J.; GUBLER, D. J.; HUNSPERGER, E.; KROEGER,A. ; MARGOLIS, H. S.; MARTÍNEZ, E.; NATHAN, M. B.; PELEGRINO, J. L.; SIMMONS, C.; YOKSAN, S.; PEELING, R. W. 2010. Dengue: a continuing global threat. Nature Reviews Microbiology S7-S16 | doi:10.1038/nrmicro2460. [ Links ]
INSTITUTO BRASILEIRO DE GEOGRAFIA E ESTATÍSTICA -IBGE. 2006. Censo demográfico 2006. Rio de Janeiro. [ Links ]
LIMA, J. B. P.; PEREIRA DA CUNHA, M.; SILVA-JR, R. C.S. ; GALARDO, A. K. R.; SOARES, S. S.; BRAGA, I. A.; RAMOS, R. P.; VALLE, D. 2003. Resistance of Aedes aegypti to organophosphates in several municipalities in the state of Rio de Janeiro and Espirito Santo, Brazil. The American Journal of Tropical Medicine and Hygiene 68 (3): 329-333. [ Links ]
LIMA, E. P; OLIVEIRA, A. M. F.; LIMA, J. W. O.; RAMOS, A. N. J.; CAVALCANTI, L. P G.; PONTES, R. J. S. 2006. Resistencia do Aedes aegypti ao temefós em municípios do Estado do Ceará. Revista de la Sociedad Brasileña de Medicina Tropical 39 (3): 259-263. [ Links ]
LIMA, E. P; PAIVA, M. H. S.; ARAUJO, A. P; SILVA, E. V G.; SILVA, U. M.; OLIVEIRA, L. N.; SANTANA, A. E. G.; BARBOSA, C. N.; NETO, C. C. P.; GOULART, M. O.; WILDING, C. S.; AYRES, C. F. J.; SANTOS, M. A. M. 2011. Insecticide resistance in Aedes aegypti populations form Ceará, Brasil. Parasites and Vectors 4 (5):1-12. [ Links ]
MACORIS, M. L. G.; ANDRIGHETTI, M. T. M.; TAKAKU, L.; GLASSER, C. M.; GARBELOTO, V C.; CIRINO, V C. B. 1999. Alteragoes de resposta de susceptibilidade de Aedes aegypti a inseticidas organofosforados em municipios do estado de Sao Paulo, Brasil. Revista de Saúde Pública 33 (5): 521-2. [ Links ]
MACORIS, M. L. G.; ANDRIGHETTI, M. T. M.; TAKAKU, L.; GLASSER, C. M.; GARBELOTO, V C.; BRACCO, J. E. 2003. Resistance of Aedes aegypti from the state of Sao Paulo, Brazil, to organophosphates insecticides. Memorias do Instituto Oswaldo Cruz 98 (5): 703-708. [ Links ]
MAZZARI, M. B.; GEORGHIO, G. P 1995. Characterization of resistance to organophosphate, carbamate, and pyrethroid insecticides in field populations of Aedes aegypti from Venezuela. Journal of the American Mosquito Control Association 11 (3): 315-322. [ Links ]
MINISTERIO DA SAÚDE. 1968. Endemias Rurais - Métodos de trabalho adotados pelo DNERu, Departamento Nacional de Endemias Rurais. [ Links ]
MINISTERIO DA SAÚDE / SERVIDO DE VIGILÁNCIA A SAÚDE - SVS. 2005. Dengue. [ Links ]
MINISTERIO DA SAÚDE / SECRETARIA DE VIGILÁNCIA EM SAÚDE - SVS.; 2007. Balando Dengue Janeiro a Julho de 2007 - URL: http://www.infectologia.org.br/anexos/MS-SVS_balan%C3%A7o%20dengue%20jan-jul%202007.pdf. [ Links ]
MINISTERIO DA SAÚDE / SECRETARIA DE VIGILÁÁNCIA EM SAÚDE - SVS.; 2007. Balando Dengue Janeiro a Julho de 2007 - URL: http://www.infectologia.org.br/anexos/MS-SVS_balan%C3%A7o%20dengue%20jan-jul%202007.pdf.
MONTELLA, I. S.; MARTINS, A. J.; VIANA-MEDEIROS, P. F.; LIMA, J. B. P.; BRAGA, I. A.; VALLE, D. 2007. Insecticide resistance mechanisms of Brazilian Aedes aegypti populations from 2001 to 2004. The American Journal of Tropical Medicine and Hygiene 77 (3): 467-477. [ Links ]
PADUAN, K. S.; ARAÚJO-JÚNIOR, J. P.; RIBOLLA, P. E. M. 2006. Genetic variability in geographical popolations of Aedes aegypti (Diptera, Culicidae) in Brazil elucidated by molecular markers. Genetics and Molecular Biology 29 (2): 391-395. [ Links ]
PROPHIRO, J. S.; SILVA, O. S.; DUQUE, J. E. L.; PICCOLI, C. F.; KANIS, L. A.; NAVARRO-SILVA, M. A. 2011. Aedes aegypti and Aedes albopictus (Diptera:Culicidae): coexistence and susceptibility to temephos, in munipalities with occurrence of dengue and differentiated characteristics of urbanization. Revista da Sociedade Brasileira de Medicina Tropical 44 (3): 300-305. [ Links ]
RAWLINS, S. C. 1998. Spatial distribution of insecticide resistance in Caribbean populations of Aedes aegypti and its significance. Pan American Journal of Public Health 4 (4): 243-251. [ Links ]
ROBERTSON, J. L.; PREISLER H. K. 1992. Pesticide bioassays with arthropods. CDC Press. United States of America. p. 127. [ Links ]
SISFAD - SESA/PR. 2006. Sistema de Informaçâo de Febre Amarela e Dengue. Ministério da Saúde, programa de computador utilizado pela FUNASA, em nivel nacional, para processar dados obtidos nas atividades de campo desenvolvidas pelos Agentes de Saúde. SESA/PR - Secretaria de Estado da Saúde do Paraná [ Links ].
WORLD HEALTH ORGANIZATION. 1981. Instructions for determining the susceptibility or resistance of mosquito larvae to insecticides). Geneva; 1981. (WHO/VBC/81.807). [ Links ]
WORLD HEALTH ORGANIZATION. 1992. Vector Resistance to pesticides. Fifteenth Report of The WHO Expert Committee on Vector Biology and Control. WHO Technical Report Series. 818: 1-62. [ Links ]
Received: 14-Apr-2014
Accepted: 23-Jul-2015
Suggested citation:
DUQUE L., J. E.; SILVA, A. M.; FANTINATTI, E. C. S.; NAVARRO-SILVA, M. A. 2015. Resistance of Aedes aegypti (Diptera: Culicidae) to temephos in Paraná State, Brazil. Revista Colombiana de Entomología 41 (2): 205-211. Julio -Diciembre 2015. ISSN 0120-0488.