Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488
Rev. Colomb. Entomol. vol.41 no.2 Bogotá July/Dec. 2015
Activity schedule and foraging in Protopolybia sedula (Hymenoptera, Vespidae)
Horario de actividad y forrajeo en Protopolybia sedula (Hymenoptera, Vespidae)
MATEUS DETONI1,2, MARIA DO CARMO MATTOS1,3, MARIANA MONTEIRO DE CASTRO1,4, BRUNO CORRÉA BARBOSA1,5 and FÁBIO PREZOTO1,6
1 Laboratòrio de Ecologia Comportamental e Bioacùstica - LABEC, Universidade Federal de Juiz de Fora. Campus Universitario, Bairro Martelos, CEP 36036-900, Juiz de Fora, Minas Gerais, Brazil.
2 Graduate Student. matedetoni@hotmail.com.
3 Graduate Student. marry.carmo@hotmail.com.
4 Ph. D. Student. marimc.jf@gmail.com.
5 M. Sc. Student. barbosa.bc@outlook.com.
6 Ph. D. Professor. fabio.prezoto@ufjf.edu.br. Corresponding author.
Abstract: Protopolybia sedula is a social swarming wasp, widely spread throughout many countries in the Americas, including most of Brazil. Despite its distribution, studies of its behavioral ecology are scarce. This study aimed to describe its foraging activity and relation to climatic variables in the city of Juiz de Fora in southeastern Brazil. Three colonies were under observation between 07:00 and 18:00 during April 2012, January 2013, and March 2013. Every 30 minutes, the number of foragers leaving and returning to the colony was registered along with air temperature and relative humidity. Activity began around 07:30, increased between 10:30 and 14:30, and ended around 18:30. A mean of 52.7 exits and 54 returns were measured every 30 minutes. The daily mean values were 1,107 ± 510.6 exits and 1,135 ± 854.8 returns. Only one colony showed a significant correlation between forager exits and temperature (rs = 0.8055; P < 0.0001) and between exits and relative humidity (rs = -0.7441; P = 0.0001). This paper shows that climatic variables are likely to have little control on the foraging rhythm of P. sedula when compared to other species, suggesting the interaction of other external and internal factors as stimuli of species foraging behavior.
Key words: Social wasps. Foraging behavior. Climatic variables.
Resumen: Protopolybia sedula es una avispa social enjambradora, ampliamente distribuida por varios países de las Américas, inclusive gran parte de Brasil. No obstante su distribución, son escasos los estudios sobre su ecología comportamental. Este estudio tuvo el fin de describir el horario de forrajeo y su relación con factores climáticos en Juiz de Fora, de Brasil. Tres colonias estuvieron bajo observación, entre las 00: 07 a las 18:00 horas, durante abril de 2012, enero de 2013 y marzo de 2013. Cada 30 minutos se registró el número de avispas forrajeadoras que salían o retornaban, así como temperatura y humedad del aire. La actividad forrajeadora se iniciaba cerca de las 07:30 con intensificación entre las 10:30 y 14:30 terminando cerca de las 18:00. Se registró una media de 52,7 salidas y 54 retornos cada 30 minutos. La media diaria fue de 1.107 ± 510,6 salidas y 1.135 ± 854,8 retornos. Sólo una colonia mostró relación significativa entre las salidas y la temperatura (rs = 0,8055; P < 0,0001) y entre salidas y humedad (rs = -0,7441; P = 0,0001). Este trabajo demuestra que las variables climáticas ejercen poco control sobre el ritmo de forrajeo de P. sedula en comparación con otras especies, sugiriendo la interacción de otros factores extrínsecos e intrínsecos estimulando esta actividad.
Palabras clave: Avispa social. Comportamiento de forrajeo. Variables climáticas.
Introduction
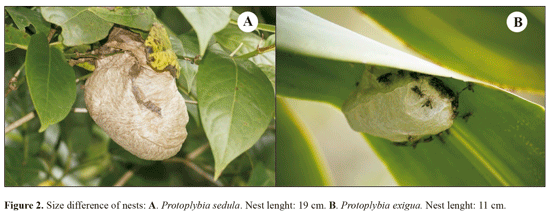
The swarm-founding social wasp Protopolybia sedula (de Sausurre, 1854) is a widely spread species, distributed throughout various countries in the Americas such as Colombia, Venezuela, Guyana, Suriname, Ecuador, Peru, Paraguay, Argentina and most of Brazil (from Maranhao to Santa Catarina state). The nests are small (about 14 cm tall and 11 cm wide), sessile, covered by an envelope and usually found in shady areas under leaves of trees and bushes (Wenzel 1998).
Protopolybia Ducke, 1905 species are recorded in studies that measure diversity in anthropic areas (Lima et al. 2000; Alvarenga et al. 2010). However, studies about its foraging behavior are scarce (Ribeiro Junior et al. 2006). Foraging activity in social wasps results in the collection of different materials: water for cooling the colony, carbohydrates for feeding adults and larvae, vegetal fiber as construction material and protein for nourishing the larvae (Hunt 2007; Prezoto et al. 2008; Elisei et al. 2010; Clemente et al. 2012; Barbosa et al. 2014). Lepidoptera caterpillars represent up 90% of the main prey captured by social wasps (Gobbi and Machado 1985, 1986; Prezoto et al. 1994; Prezoto et al. 2005, 2006). Caterpillars are common agricultural pests, making wasps valuable allies by providing ecological services of predating them (Prezoto 1999; Prezoto et al. 2008; Elisei et al. 2010).
Environmental factors such as intensity of light, wind speed, relative humidity of air and temperature, significantly affects wasps’ foraging behavior, whether stimulating or inhibiting it (Bonabeau 1998; Richter 2000). Studies on various social wasps species’ foraging behavior have shown that this activity is strongly related to temperature and relative humidity of air, and usually varies among the seasons of the year and different phases of the colonial cycle (Lima and Prezoto 2003; Elisei et al. 2005; Ribeiro-Junior et al. 2006; Castro et al. 2011).
This paper describes the foraging activity for P. sedula and its relation with climatic variables.
Material and methods
The study was carried out in the municipality of Juiz de Fora (21°48’21"S 43°22’09"W, 781 m altitude), Minas Gerais state, southeast region of Brazil. Observations were made in three P sedula colonies (C1, C2 and C3), from 07:00 h to 18:00 h during a single day each, totaling 33 hours of data during the months of April of 2012 and January and March of 2013, according to the methodology suggested by Prezoto et al. (1994).
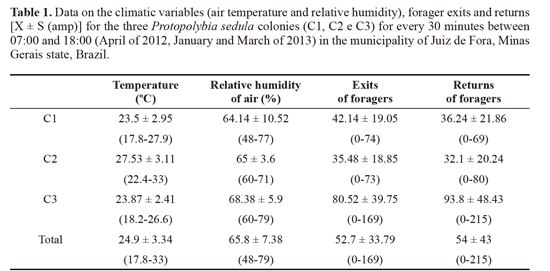
We registered the number of wasps that left and returned to the colony for foraging every 30 minutes; air temperature (°C) and relative humidity (%) was also measured in these intervals by means of a digital thermohygrometer set near the colony. In order to relate the climatic variables and the foraging activity, data on the air temperature and relative humidity was correlated with the number of foragers leaving the colony by using Spearman’s coefficient of correlation. Calculations were done with the freeware software BioEstat 5.3.
Results and discussion
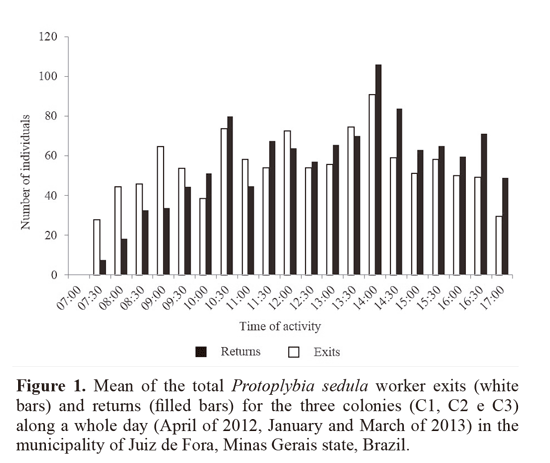
Activity begun around 07:30 and ended around 18:00; forager exits intensified between 10:30 and 14:30, the hottest hours of day (Fig. 1). These agree with the results of Ribeiro Junior et al. (2006) and Rocha and Giannotti (2007), who recorded the activity of Protopolybia exigua (Saussurre, 1854) ranging from around 07:00 to around 18:00. This rhythm is typical for neotropical social wasps during hot and humid seasons (spring and summer), a time of the year in which the foraging activity is intense (Elisei et al. 2013).
A mean of 52.7 exits and 54 returns for every 30 minutes was registered (Table 1). For the daily mean, 1107 ± 510.6 exits and 1135 ± 854.8 forager returns were measured. This was higher than the number recorded by Lopez et al. (2013) for Polybia emaciata Lucas, 1854, in Colombia, with 262 exits and 270 returns. The study made by Rocha and Giannotti (2007) on P. exigua in Bahia state, Brazil, found a mean of 44.4 exits and 37.6 returns per hour. This difference can be explained as follows: colonies and individuals of P. sedula are smaller in comparison to other swarming species such as Apoica Lepeletier, 1836, Polybia Lepeletier, 1836 and Synoeca de Saussurre, 1852; compared to P exigua, even though individuals are similar in size, the colonies of P sedula are bigger, explaining an intense foraging flux (Fig. 2A-B).
Activity intensification during the hottest hours of the day (from 10:30 to 15:00, Fig. 1) was observed in other swarming social wasps studied at the same locality; Polybia platycephala Richards, 1951 (Lima and Prezoto 2003), Synoeca cyanea (Fabricius, 1775) (Elisei et al. 2005), P. exigua (Saussurre, 1854) (Ribeiro Junior et al. 2006) and also for P emaciata (López et al. 2013) in Colombia.
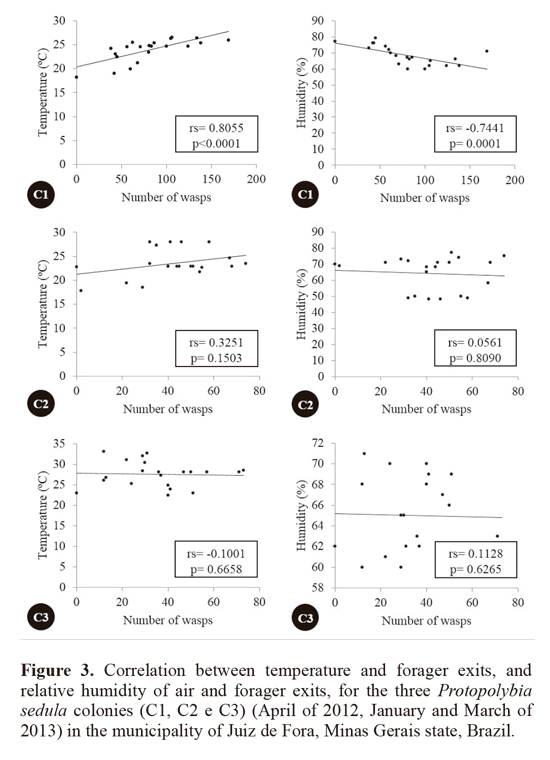
Comparison between forager exits, air temperature and relative humidity showed various results. Colony C1 was the only one to show significant values for the correlation between forager exits, air temperature (rs = 0.8055; P < 0.0001) and relative humidity of air (rs = -0.7441; P = 0.0001). Correlation results for both temperature and exits, and humidity and exits were not significant for colonies C2 and C3 (Fig. 3).
Studies relating forage and environmental variables have been conducted with many species. López et al. (2013) and Lima and Prezoto (2003) studied P emaciata e P platycephala and both studies verified that the increase in the foraging activity is related to temperature increases and humidity decreases, mainly during the hot and humid season (spring and summer). Similar results were found by Ribeiro Junior et al. (2006) regarding temperature for another species of Protopolybia. In the same locality, Elisei et al. (2005) verified that the intensification of forager exits in S. cyanea occurs in the hottest hours of the day.
Absence of correlation between forager exits and climatic variables observed for colonies C2 and C3 is evidence that, during certain phases of the biological cycle, the foraging demand does not depend on climatic variables, but on the colony’s biological needs. Castro et al. (2011) suggested that the lack of correlation between humidity and forager exits in certain colony phases for the wasp Mischocyttarus cassununga (von Ihering, 1903) might be due to the colony’s nutritional needs.
The presence of an envelope protecting P. sedula nests might generate a certain level of homogenization of the internal environment in terms of temperature and humidity, increasing the importance of the colony`s internal stimuli when regarding forager exits.
Conclusions
This work presents the first foraging rhythm study for P. sedula, showing that climatic variables possibly have low control on the foraging rhythm when compared to other social wasps species and suggesting the existence of an interaction between external and internal factors as stimuli for this activity in the species.
Acknowledgements
The authors thank Carlos Eduardo Sarmiento Monroy for collaboration and the Funda 9áo de Amparo á Pesquisa de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordena 9áo de Aperfei 9oamento de Pessoal de Nivel Superior (CAPES) for financial support.
Literature cited
ALVARENGA, R. B.; CASTRO, M. M.; SANTOS-PREZOTO, H. H.; PREZOTO, F. 2010. Nesting of social wasps (Hymenoptera, Vespidae) in urban gardens in Southeastern Brazil. Sociobiology 55: 445-452. [ Links ]
BARBOSA, B. C.; PASCHOALINI, M. F.; PREZOTO, F. 2014. Temporal activity patterns and foraging behavior by social wasps (Hymenoptera, Polistinae) on fruits of Mangifera indica L. (Anacardiaceae). Sociobiology 61: 239-242. [ Links ]
BONABEAU, E. 1998. Social insect colonies as complex adaptive systems. Ecosystems 1:437-443. [ Links ]
CASTRO, M. M.; GUIMARÁES, D. L.; PREZOTO, F. 2011. Influence of environmental factors on the foraging activity of Mischocyttarus cassununga (Hymenoptera, Vespidae). Sociobiology 58: 133-141. [ Links ]
CLEMENTE, M. A.; LANGE, D.; DEL-CLARO, K.; PREZOTO, F.; CAMPOS, N.R.; BARBOSA, B. C. 2012. Flower-visiting social wasps and plants interaction: Network pattern and environmental complexity. Psyche 2012: 1-10. [ Links ]
ELISEI, T.; RIBEIRO-JUNIOR, C.; GUIMARÁES, D. L.; PREZOTO, F. 2005. Foraging activity and nesting of swarmfounding wasp Synoeca cyanea (Fabricius, 1775) (Hymenoptera, Vespidae, Epiponini). Sociobiology 46 (2): 317-327. [ Links ]
ELISEI, T.; NUNES, J. V.; RIBEIRO-JUNIOR, C.; FERNANDES JUNIOR, A. J.; PREZOTO, F. 2010. Uso da vespa social Polistes versicolor no controle de desfolhadores de eucalipto. Pesquisa Agropecuária Brasileña 45: 958-964. [ Links ]
ELISEI, T.; NUNES, J. V; RIBEIRO-JUNIOR, C.; FERNANDES-JUNIOR, A. J.; PREZOTO, F. 2013. What is the ideal weather for social wasp Polistes versicolor (Olivier) go to forage?. EntomoBrasilis 6: 214-216. [ Links ]
GOBBI, N.; MACHADO, V L. L. 1985. Material capturado e utilizado na alimentajao de Polybia (Myraptera) paulista Ihering, 1896 (Hymenoptera - Vespidae). Anais da Sociedade Entomológica do Brasil 14 (2): 189-195. [ Links ]
GOBBI, N.; MACHADO, V L. L. 1986. Material capturado e utilizado na alimentajao de Polybia (Trichothotrax) ignobilis (Haliday, 1836) (Hymenoptera - Vespidae). Anais da Sociedade Entomológica do Brasil 15 (suplemento): 118-124. [ Links ]
HUNT, J. H. 2007. The evolution of social wasps. Oxford University Press, New York. 259 p. [ Links ]
LIMA, M. A. P.; LIMA, J. R.; PREZOTO, F. 2000. Levantamento dos géneros de vespas sociais (Hymenoptera, Vespidae), flutuajao das colonias e hábitos de nidificado no campus da UFJF, Juiz de Fora, MG. Revista Brasileira de Zoociéncias 2: 69-80. [ Links ]
LIMA, M. A. P.; PREZOTO, F. 2003. Foraging activity rhythm in the Neotropical swarm-founding wasp Polybia platycephala sylvestris (Hymenoptera: Vespidae) in different seasons of the year. Sociobiology 42 (3): 745-752. [ Links ]
LÓPEZ-G., Y; HERNÁNDEZ-D., J.; CARABALLO, P. 2013. Actividad de forrajeo de la avispa social Polybia emaciata (Hymenoptera: Vespidae: Polistinae). Revista Colombiana de Entomología 39 (2): 250-255. [ Links ]
PREZOTO, F.; GIANOTTI, E.; MACHADO, V. L. L. 1994. Atividade forrageadora e material coletado pela vespa social Polistes simillimus Zíkan, 1951 (Hymenoptera, Vespidae). Insecta 3: 11-19. [ Links ]
PREZOTO, F. A. 1999. Importancia das vespas como agentes no controle biológico de pragas. Biotecnologia Ciéncia & Desenvolvimento 2 (9): 24-26. [ Links ]
PREZOTO, F.; LIMA, M. A. P.; MACHADO, V L. L. 2005. Surveys of prey captured and used by Polybia platicephala (Richards) (Hymenoptera: Vespidae, Epiponini). Neotropical Entomology 34: 849-851. [ Links ]
PREZOTO, F.; SANTOS-PREZOTO, H. H.; MACHADO, V L. L.; ZANUNCIO, J. C. 2006. Prey captured and used in Polistes versicolor (Oilivier). Neotropical Entomology 35 (5): 707-709. [ Links ]
PREZOTO, F.; CORTES, S. A. O.; MELO, A. C. 2008. Vespas: de vilas a parceiras. Ciencia Hoje 48: 70-73. [ Links ]
RIBEIRO-;JUNIOR, C.; GUIMARÀES, D. L.; ELISEI, T.; PREZOTO, F. 2006. Foraging activity rhythm of the Neotropical swarm-founding wasp Protopolybia exigua (Hymenoptera, Vespidae, Epiponini) in different seasons of the year. Sociobiology 47 (1): 115-123. [ Links ]
RICHTER, M. R. 2000. Social wasp (Hymenoptera, Vespidae) foraging behavior. Anais da Sociedade Entomológica do Brasil 45: 121-150. [ Links ]
ROCHA, A. A.; GIANNOTTI, E. 2007. Foraging activity of Protopolybia exigua (Hymenoptera, Vespidae) in different phases of the colony cycle, at an area in the region of the Mèdio Sao Francisco River, Bahia, Brazil. Sociobiology 50: 813-831. [ Links ]
WENZEL, J. W. 1998. A generic key to the nests of hornets, yellow jackets, and paper wasps worldwide (Vespidae: Vespinae, Polistinae). American Museum Novitates 3224: 1-39. [ Links ]
Received: 8-Dec-2014
Accepted: 3-Jun-2015
Suggested citation:
DETONI, M.; MATTOS, M. do C.; CASTRO, M. M. de; BARBOSA, B. C.; PREZOTO, F. 2015. Activity schedule and foraging in Protopolybia sedula (Hymenoptera, Vespidae). Revista Colombiana de Entomologia 41 (2): 245-248. Julio -Diciembre 2015. ISSN 0120-0488.