Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Colombiana de Entomología
versión impresa ISSN 0120-0488
Rev. Colomb. Entomol. vol.41 no.2 Bogotá jul./dic. 2015
Intestinal proteases of Moneilema armatum (Coleoptera: Cerambycidae) fed with Opuntia cladodes
Proteasas intestinales de Moneilema armatum (Coleoptera: Cerambycidae) alimentado con cladodios de Opuntia
JORGE ARIEL TORRES-CASTILLO1, CÉSAR LEOBARDO AGUIRRE-MANCILLA2, ADRIANA GUTIÉRREZ-DÍEZ3, SUGEY RAMONA SINAGAWA-GARCÍA3, REYNA IVONNE TORRES-ACOSTA4, EDUARDO ALEJANDRO GARCÍA-ZAMBRANO3, VÍCTOR AGUIRRE-ARZOLA3 and FRANCISCO ZAVALA-GARCÍA3
1Universidad Autónoma de Tamaulipas - Instituto de Ecología Aplicada. División del Golfo No. 356. Colonia Libertad. CP. 87019. Cd. Victoria, Tamaulipas, México, jorgearieltorres@hotmail.com. Tel: (52) 834-31-81800 ext. 1601. Corresponding autor.
2 Tecnológico Nacional de México, Instituto Tecnológico de Roque. Km. 8 Carretera Celaya-Juventino Rosas, Celaya, Guanajuato, México. C.P. 38110.
3 Universidad Autónoma de Nuevo León-Facultad de Agronomía-Laboratorio de Biotecnología, Francisco Villa s/n Col. Ex Hacienda El Canadá, Escobedo, Nuevo León, México. C. P 66050.
4Estudiante de Doctorado, Departamento de Parasitología, Universidad Autónoma Agraria Antonio Narro, Buenavista, Saltillo, Coahuila, México.
Abstract:This work provides an overview of proteolytic activity in the intestine of Moneilema armatum (Coleoptera: Cerambycidae) adults and inhibition of such enzymes by proteins obtained from the cladodes of Opuntia ficus-indica. Active intestinal proteases were detected at a neutral and slightly alkaline pH through zymography and spectrophotometric assays using synthetic substrates. These enzymes were susceptible to a specific serine protease inhibitor (PMSF). It was also demonstrated that trypsin-, chymotrypsin-, and elastase-like activities were present. Although the cladodes of O. ficus-indica possess endogenous inhibitors of serine proteases, it appears that not all proteases of M. armatum were affected, particularly those with elastase-like activity. The results indicated that M. armatum exhibits a variety of serine protease activities, and some of these proteolytic activities were insensitive to endogenous O. ficus-indica protease inhibitors.
Key words: Digestive proteases. Protease inhibitor. Zymography.
Resumen:Este trabajo provee una visión general de la actividad proteolítica en el intestino de adultos de Moneilema armatum (Coleoptera: Cerambycidae) y la inhibición de dichas enzimas por proteínas obtenidas de los cladodios de Opuntia ficus-indica . Las proteasas intestinales activas fueron detectadas de pH neutral a ligeramente alcalino a través de zimografía y ensayos espectrofotométricos usando sustratos sintéticos. Estas enzimas fueron susceptibles a un inhibidor específico para serin proteasas (PMSF) y también se demostró que las actividades proteolíticas presentes correspondieron a actividades tipo tripsina, quimotripsina y elastasa. Aunque los cladodios de O. ficus-indica poseen inhibidores de serin proteasas endógenos, parece ser que no todas las proteasas de M. armatum fueron afectadas, particularmente la actividad de tipo elastasa. Estos resultados indican que M. armatum exhibe una variedad de actividades de serin proteasas y que algunas de estas actividades podrían haberse vuelto insensibles a la presencia de dichos inhibidores endógenos.
Palabras clave: Proteasas digestivas. Inhibidor de proteasas. Zimografía.
Introduction
Phytophagous insects and plants have evolved together over millions of years, with plants synthesizing toxic compounds to protect themselves and the insects developing detoxification mechanisms and processes to inactivate the plant defenses (Mello and Silva-Filho 2002). A diverse group of defensive proteins, whose accumulation is induced by jasmonic acid signaling, play a critical role in plant defense against chewing insects, throughout direct action on the digestive process and absorption of nutrients, which highlights the indigestion as an effective strategy in the defense against herbivores (Felton 2005).
On the other hand, the knowledge of the digestive processes of phytophagous insects is crucial to understand the biochemical elements involved in their success to attack plants (Mello and Silva-Filho 2002). Some of the mechanisms that plants have evolved include the production of numerous compounds, such as lectins, toxins and enzyme inhibitors (Zheng and Dicke 2008). In general, studies on the understanding the plant-phytophagous insect interaction have been focused on plants with economic importance and model plants (Fery and Schalk 1991; Thaler et al. 1996; Stotz et al. 2000; Hermsmeier et al. 2001; Mello and Silva-Filho 2002; Mithofer et al. 2005; Lawrence et al. 2008; Zheng and Dicke 2008). However, the study of the mechanisms involved in the interaction of other insect species and non-model plant species must be conducted to address basic questions and also as opportunities to develop potential biotechnological applications to control insect pests.
Several types of enzymes have been found as functional elements of the digestive process, which includes the proteases. Proteases, also referred to as peptidases, break peptide bonds, allowing the degradation and subsequent absorption of dietary protein. In the case of insects, the most common digestive proteases have been classified based on their specificity, catalytic mechanism, optimum pH and sensitivity to chemical inhibitors, defining several groups that include serine proteases, cysteine proteases, aspartic proteases and metalloproteases which are widely distributed amongst insects (Terra 1990; Terra and Ferreira 1994). Several studies have shown the possible selection and adaptation of insect digestive proteases in response to the type of diet, achieving a high degree of specialization; these observations suggest that the insect enzymes could become insensitive to the defensive mechanisms of the plant tissues on which the insects are feeding (Jongsma and Bolter 1997; Bown et al. 2004; Moon et al. 2004). Because proteases are involved in the digestive process, which is vital for the assimilation of nutrients, they are considered targets of many plant defensive components, including the proteases inhibitors, low molecular weight proteins resistant to degradation and also other factors that could interfere with the proteolytic activity (Ryan 1990; Lawrence and Koundal 2002).
There are cases in which the interaction between the plant and insect is evident, but the molecular principles and biochemical characteristics of both systems are often unknown, this is the case of the insects that attack the members of the Cactaceae family. A typical example is cactus longhorn beetle, Moneilema armatum LeConte, 1853 (Coleoptera: Cerambycidae). The life cycle of M. armatum can be summarized as follows: adult insects feed on cactus plants in the summer and then oviposit in the damaged plant tissue; the hatched larvae remain and feed on the plant during the winter and then pupate to emerge as adults in late spring (Miller 2008). The beetles often congregate during the reproductive stage in groups of 20 or more individuals, and the damage they cause to the plants can be considerable even at lower densities. This interaction is an example of specialization in which a phytophagous insect has adapted to feed on a plant resource that is mainly restricted to arid zones. Through evolution, this species of insect probably was adapted to colonize some Opuntia species and therefore has been able to overcome some of the plant’s defensive mechanisms. The aim of this work was to initiate the description of the proteolytic active components in the intestine of M. armatum and the effect of the protein fractions obtained from Opuntia ficus-indica (L.) Mill. on the respective proteolytic activity.
Material and methods
Collection of biological material. Adult specimens of M. armatum were collected feeding on wild cacti ( Opuntia spp.) in the town of Salinas Victoria, Nuevo Leon, Mexico, on April 2013 and taken to the Biotechnology Laboratory of the Faculty of Agronomy of the Autonomous University of Nuevo Leon, in General Escobedo, Nuevo Leon, Mexico. The intestines were removed and stored at -70 °C until use. The O. ficus-indica cladodes used for inhibitory protein fraction were approximately one month old when collected from a commercial crop located in Salinas Victoria, Nuevo Leon; the cladodes were frozen and stored at -70 °C until use.
Protein extraction from M. armatum. The intestines were ground in a cold mortar, and 3 ml of distilled water (at pH 3, 4 °C) per gram of tissue were added. The mix was kept in an ice bath for 15 min, with periodic stirring and then was centrifuged at 10,000 x g at 4 °C for 10 min. The supernatant was recovered and precipitated using cold acetone at 1:3 ratio. The sample was centrifuged at 10,000 x g at 4 °C for 5 min, and the pellet was recovered and dissolved in 1 ml of distilled water. This was injected into an open-column gel filtration system using Sephadex G-25 (Sigma-Aldrich, MO, USA) to separate the low molecular weight components and the protein fraction. The protein fractions were collected based on their absorbance at 220 nm and concentrated using acetone precipitation as indicated above. The final pellet was kept at -70 °C until use. This procedure was done several times to generate enough aliquots of enzymes and they were kept at -70 °C. For each assay a new aliquot was thawed and dissolved in 300 pl of water before using.
Protein extraction from O. ficus-indica cladodes. The cladodes samples were thawed and homogenized using water (1:4, w/v) in a blender, and then centrifuged at 8,000 x g at 4 °C for 10 min. A 50 ml aliquot of the supernatant was precipitated with acetone as previously described. The pellet was dissolved in 10 ml of distilled water and centrifuged at 10,000 x g at 4 °C for 10 min, and the clarified supernatant was recovered. The clarified fraction was subjected to ultrafiltration using regenerated cellulose membranes with a molecular weight cutoff of 30,000 (Millipore, MA, USA) to obtain a protein fraction with low content of mucilage, and this was used as the source of Opuntia cladode proteins. Previously, serine proteases inhibitor activity was detected in the low molecular weight fractions.
Hydrolysis of synthetic substrates by the proteases from M. armatum. As part of the screening of the intestinal proteolytic activities of M. armatum , tests for hydrolysis were performed to determine the predominant catalytic mechanism in the intestinal extracts. The potential to hydrolyze synthetic substrates coupled to p -nitroanilide (Sigma-Aldrich, MO, USA) was investigated, with modifications of the protocol described by Erlanger et al (1961). Proteolytic activity was related to the increase in absorbance after incubation time; control proteolytic activity was expressed as proteolytic activity units (PAU) and defined as the increase in absorbance of 0.01 units at 405 nm related to mg of protein after 15 min incubation at 37 °C. Three pH conditions were examined: pH 6 (36.85 ml of 0.1 M citric acid with 63.15 ml of 0.2 M Na 2 HPO 4 ), pH 7 (17.65 ml of 0.1 M citric acid with 82.35 ml of 0.2 M Na2HPO4) and pH 8 (0.1 M Tris-HCl). Three synthetic substrates were assessed: N-benzoyl-DL-arginine-p-nitroanilide hydrochloride (BAp-NA) for trypsin-like serine proteases; N-succinyl-Ala-Ala-Ala-p-nitroanilide (SAAApNa) for elastase-like serine proteases; and Ala-Ala-Phe-p-nitroanilide (AAPpNa) for chymotrypsin-like serine proteases. The blank reaction was prepared with 675 pl buffer, 75 pl 0.01 M substrate solution and 300 pl acetic acid at 30%. The proteolytic reaction was prepared with 665 pl buffer, 75 pl 0.01 M substrate, and 10 pl enzyme, followed by incubation at 37 °C for 15 min. The reaction was stopped by the addition of 300 pl acetic acid at 30%, and the absorbance was measured at 405 nm to obtain units of absorbance (UA). The PAU was calculated according to the following formula:
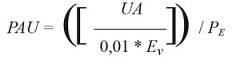
Where UA corresponds to the absorbance units of reaction, Ev equals to the volume (ml) of the enzyme, and [Pe ] corresponds to the protein concentration of the enzymatic extract (mg/ml).
Detection of trypsin inhibitors, chymotrypsin inhibitors and elastase inhibitors in the cladodes. The determination of the inhibitory activity was based on the hydrolysis of substrates coupled to p-nitroanilide, as described above (Erlanger et al. 1961) using pH 7 (17.65 ml of 0.1 M citric acid with 82.35 ml of 0.2 M Na2HPO4) and pH 8 (0.1 M Tris-HCl). Inhibitory activities were evaluated against their respective protease (bovine trypsin, bovine chymotrypsin and porcine elastase) (Sigma-Aldrich, MO, USA). Control proteolytic reactions were prepared using standard enzymes in a reaction mixture containing 665 pl of buffer, 10 pl of enzyme, incubated for 15 min followed by the addition of 75 pl 0.01 M of the corresponding substrate. Reaction with the substrate was developed by 15 min and stopped with addition of 300 pl of acetic acid. The absorbance was measured at 405 nm to obtain the UA. The inhibition reaction was prepared in a similar manner to the proteolytic reaction but including 50 pl of inhibitor sample to 615 pl buffer, with 10 pl enzyme, incubated for 15 min and followed by addition of 75 pl of substrate and then incubated for 15 min, finally the reaction was stopped by adding 300 pl of acetic acid. The absorbance was measured at 405 nm. The inhibitory activity was detected by measuring the decrease in absorbance at 405 nm in proteolytic reactions in the presence of inhibitor fraction, and expressed as units of inhibitor (UI) which were defined as the reduction in 0.01 units of absorbance per 15 min/mg of protein added and calculated with the following equation:
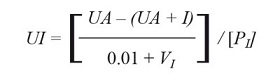
Where, UA are the units of absorbance in the absence of inhibitor, (UA + I) refers to the absorbance in the presence of inhibitor, VI is the volume (ml) of the inhibitor and PI is the protein concentration of the inhibitor extract (mg/ml).
Electrophoresis and proteases zymography. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to Laemmli (1970) using 10% polyacrylamide gels and 0.2% porcine skin gelatin copolymerized for protease detection. Samples for electrophoresis and zymography were dissolved in 2X Laemmli Sample Buffer (65.8 mM Tris-HCl, pH 6.8, 26.3% (w/v glycerol, 2.1% SDS and 0.01% bromophenol blue) (BioRad, CA, USA). Pre-stained SDS-PAGE standards, broad range from BioRad were used as molecular weight markers. The electrophoresis conditions were 80 V for 20 min, followed by 120 V for 40 min. After electrophoresis, the gels were rinsed with distilled water to remove the excess of SDS. The protease detection was performed by incubating the gels for 1 h at 37 °C at pH 5 (48.5 ml 0.1 M citric acid with 51.50 ml 0.2 M Na2HPO4), pH 6 (36.85 ml 0.1 M citric acid with 63.15 ml 0.2 M Na2HPO4), pH 7 (17.65 ml 0.1 M citric acid with 82.35 ml 0.2 M Na 2 HPO 4 ) and pH 8 (0.1 M Tris-HCl) depending of the pH assay. After incubation, the gels were stained with 0.05% Coomassie blue, with clear bands on a dark-blue background indicating the presence of proteases. The activity of the serine proteases was corroborated with a specific chemical inhibitor, PMSF (phenylmethanesulfonyl fluoride), at 10 mM in DMSO (dimethyl sulphoxide). The proteins were submitted to zymography as described above in the presence and absence of PMSF, using 2 pl of 10 mM solution with 5 pg of proteins, when no PMSF was used, only DMSO was added.
Inhibitory effect comparison between M. armatum and two different insects. Proteolytic activity for the three catalytic types was determined as indicated for the hydrolysis of synthetic substrates for the enzymes of M. armatum and absorbance of samples was adjusted to 0.5 UA as proteolytic control reaction before submitting the samples to the inhibition assay. To compare the inhibitory activity present in the cladode, a series of inhibitory assays were performed using synthetic substrates as mentioned above. Intestinal crude extracts from Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) and Prostephanus truncatus (Horn, 1878) (Coleoptera: Bostrychidae) larvae and M. armatum beetles were used as protease source. Extraction of proteins for M. armatum was done as indicated above. In the case of S. frugiperda the intestine of a larva was extracted by maceration with cold water (150 mg/ml, w:v). The same procedure was applied for P truncatus larvae extraction, 0.500 pg of the whole third instar larvae were macerated and extracted with 5 ml of water. Crude sample for both insects was centrifuged at 10 000 x g at 4 °C for 10 min. The clear supernatant was recovered and precipitated by using cold acetone at 1:3 ratio. The sample was centrifuged at 10 000 x g at 4 °C for 5 min, and the pellet was recovered and kept at -70 °C until use. Pellets were dissolved in 1 ml of distilled water before using. Procedure was done three times for the assays. Two micrograms of Opuntia proteins were added to the inhibition reaction mixture. Inhibition percentage was calculated as the decrease in total absorbance when comparing the proteolytic reactions in presence and absence of inhibitor. Reactions were conducted at pH 7 for trypsin and pH 8 for chymotrypsin and elastase activities, considering the absorbance of control reaction without inhibitor as the 100% of activity and minor absorbance as inhibition.
Statistical analysis. Completely randomized ANOVA was done to analyze and determine differences in the interaction of enzymes with the pH values, proteolytic activities for each substrate and inhibition of intestinal proteolytic activities. Mean comparisons were done by Tukey test for all the experiments. Statistical analyses were conducted with SAS v9.0.
Protein quantification. The absorbance of the insect intestinal extract was measured at 220 nm, and the protein concentration was determined by comparing the absorbance obtained with a standard curve of bovine serum albumin (mg/ml) (Aitken and Learmonth 1996; Torres-Castillo et al. 2013).
Results
Intestinal proteolytic profile of M. armatum. The proteolytic enzymes detected in this research correspond to naturally accumulated enzymes because the beetles were directly collected while they were feeding on Opuntia cladodes (Fig. 1A). Zymography was performed to determine the number and profile of the M. armatum intestinal proteases and, according to the pH analyses (Fig. 1B), the higher activity was found at neutral and slightly alkaline pH values;however, a constant activity also was detected for the high molecular weight bands at pH values ranging from 5 to 8. The most notable enzymes at a wide pH range were bands corresponding to relative molecular weight of 117.5, 95.2, 86.4 and 70.5 kDa. The more intense bands at pH 7 and 8 had relative molecular weights of 55.3, 41.5 and 26.2 kDa. The activities detected by zymography correspond to serine proteases with apparently more intense activity at pH 8. These enzymes were also inhibited with PMSF (Fig. 2), an irreversible chemical inhibitor specific for serine proteases, thus confirming their catalytic mechanism.
Serine proteases detection from M. armatum. The biochemical identification of the serine proteases present in the digestive tract of M. armatum was performed, and their hydrolytic capacity was evaluated using specific substrates. Trypsin-like activity, chymotrypsin-like activity and elastase-like activity were detected, and each one was analyzed at different pH values. A decrease in the activity when the reactions were incubated at pH 6 was found, indicating that the activity was higher between pH 7 and 8 for the three catalytic types (Table 1). When the activities of protease (trypsinlike, chymotrypsin-like and elastase like) were compared at different pH, no significant differences were found; however, trypsin-like proteases shown the higher activity.
Serine proteases inhibitory activities present in O. ficus indica cladodes. The detection of inhibitory activity against standard serine proteases in O. ficus-indica cladodes was focused on the presence of inhibitors against trypsin, chymotrypsin and elastase catalytic types. The presence of inhibitors for the three catalytic types was confirmed, with the most abundant inhibitory activity being against chymotrypsin with 3641.73 Ul/mg P, followed by trypsin with 2442 UI/ mg P, both of them were not statistically different. However, elastase-like activity was much lower (431. 2 Ul/mg P) and statistically different.
Effect of O. ficus-indica proteins on proteases of M. armatum. Hydrolysis of the specific substrates in the presence of protein fraction obtained from O. ficus-indica cladodes was performed to determine whether the inhibitory activity from the cladodes could inhibit the proteases of the intestine of M. armatum. The inhibition of the trypsin-like (545.6 Ul/mg P) and chymotrypsin-like (645.3 Ul/mg P) activities was confirmed; however, the elastase-like activity was less inhibited with approximately 38.1 Ul/mg P.
Comparison of inhibition in three insect species. Under the assay condition, activity of the three catalytic serine protease types was detected, and only the P truncatus elastase activity was low, probably due to affinity with the substrate used or to the low accumulation of such protease type. The more inhibited activity was the trypsin-like protease activity in the three species, with around 17% of inhibition in M. armatum, 60% and 65% of inhibition for S. frugiperda and P truncatus respectively when cladode proteins were added to reaction mixtures. Chymotrypsin activity was inhibited around 9.5% in M. armatum, 5% of inhibition was detected in S. frugiperda and practically no inhibition was detected in P truncatus species. Elastase-like activity was practically no inhibited in the three species with values minor to 0.5% (Table 2).
Discussion
Coleopteran digestive proteases have been detected and associated with certain well-known families, where the type of digestive proteases seem not to be closely related to the current type of diet but, rather, are related to the taxonomic order as a result of adaptation to the diet throughout the evolutionary history (Mochizuki 1998; Terra and Cristofoletti 1996). For Coleoptera, serine, cysteine and metalloproteases have been reported as predominating proteases types (Terra 1990; Terra and Ferreira 1994; Terra and Cristofoletti 1996; Mochizuki 1998; Johnson and Rabosky 2000; Castro-Guillen et al. 2012). For this work, results showed mainly neutral and slightly alkaline proteolytic activity in M. armatum intestine, which is typically associated with serine proteases.
By using specific substrates and a specific chemical inhibitor, the presence of serine proteases in M. armatum was confirmed, including trypsin-, chymotrypsin- and elastase-like activities, consistent with some reports that associate serine proteases with certain coleopteran families, including the Cerambycidae family. In the case of the cerambycid larvae Osphranteria coerulescens Redt, the proteolytic activity was detected in a broad range of pH but it showed a peak at pH 8, although authors remarked that most of activity is retained between pH 7 and 9. On the other hand, also serine protease (trypsin, chymotrypsin and elastase - like) activities were detected by using chromogenic substrates in Morimus funereus Mulsant, also a cerambycid larvae (Loncar et al. 2009; Loncar et al. 2010; Sharifi et al. 2012). The presence of intestinal serine proteases in Oemona hirta Fabricius (Cerambycidae), also was supported by proteolytic assays using a general substrate and specific chemical inhibitors, which showed that only serine protease inhibitors had effect on total proteolytic activity; the predominant endopeptidase activity was attributed to trypsin and chymotrypsin-like proteases while, exopeptidase was related to leucine aminopeptidase. Catalytic types were demonstrated by using synthetic specific substrates, indicating higher activities at alkaline pH from 8 to 11 for trypsin and chymotrypsin, respectively (Shaw and Christeller 2009). In other cerambycid, Cerambix cerdo L., the proteolytic activity also was detected at alkaline pH, and seemed to be stimulated depending on the quantity and quality of the diet; which, could be considered as part of the metabolic changes, that the insect use for adaptation when preferred food source is not available (Bozic et al. 2001). Although most reports on Cerambycidae recorded serine proteases as predominant; recently, cysteine proteases have also been detected in Stromatium fulvum Villers, a cerambycid that feeds on dying plant material and wood, natural food with relative low protein contents. Detection was done using synthetic substrates and chemical specific inhibitors, although dependence on pH values also was an indicative. Results revealed that most of the activity was associated to serine proteases (trypsin, chymotrypsin and elastase) at alkaline pH, a minor activity peak at pH 5 related to cysteine proteases (Cathepsin B and chathepsin L) and also a minor activity of aminopeptidase was detected. Results reflect a wide diversity of digestive proteases that Cerambycidae could present, which theoretically would prevent disruption or increase efficiency of the digestive process (Zibaee 2014). Serine proteases have been reported in a wide range of coleopteran families, which shows their functional relevance and their implications as important digestion elements. Several ideas have arisen and have suggested that serine proteases were the basal digestion elements in primitive coleopterans, but as a result of the continuous exposition to diets rich in plant serine protease inhibitors possibly the proteolytic battery was gradually enriched by other catalytic mechanisms. However, it would appear that in Cerambycidae, this character was conserved (Terra and Cristofoletti 1996; Johnson and Rabosky 2000).
These enzymes could vary in concentration or catalytic types throughout the whole intestine, as reported for a cerambycid larvae in which leucyl aminopeptidase-, elastase-and trypsin-like activities were predominant and their relative abundance was similar, in contrast, to a low abundant chymotrypsin activity that also was detected, a fact that shows the heterogeneity of proteases pattern that potentially could be present in the intestine (Bozic et al. 2003; Loncar et al. 2009; Sharifi et al. 2012). Although the use of alkaline proteolytic enzymes (i.e., serine proteases) is considered a primitive character in Coleoptera and that cysteine, metallo-and aspartic proteases have arisen and selected through evolution, Cerambycidae has likely maintained or returned to an ancestral state by utilizing serine proteases, which may be considered as a feature of this taxonomic family (Johnson and Rabosky 2000; Crook et al. 2009).
Insects studied here were obtained from the field, were those feeding under natural conditions. Consequently, the detected activities corresponded to naturally occurring enzymes in the digestive tract of this cerambycid species, which were analyzed with the zymography technique and with hydrolysis of synthetic substrates, obtaining similar results to those reported for proteases detection in other cerambycid species (Loncar et al. 2009; Sharifi et al. 2012).
Adaptation of the digestive process corresponds to the type of diet and it is a reflection of evolution of each species; therefore, specialist insects that have co-evolved with certain plant species are thought to have developed strategies to cope with the defense mechanisms of those plants, including the expression of several proteases to avoid the negative effects of plants proteinase inhibitors (Mello and Silva-Filho 2002; Felton 2005). In the case of Opuntia species, some studies focused on defensive proteins have confirmed the presence of protease inhibitors in Opuntia streptacantha and O. joconostle seeds, and also their presence in cladodes has been detected, suggesting that these proteins or related ones migth be present in the whole plant body. Their potential to inhibit proteases from several insects has been also demonstrated, but their role as a plant defense mechanism still remains to be studied. In previous reports, only trypsin inhibitory activity was studied; however, in this work, also chymotrypsin inhibitory activity and elastase inhibitory activity are reported at least as part of the crude extract, which increases the diversity of serine protease inhibitors for Opuntia (Torres-Castillo et al. 2007; Torres-Castillo et al . 2009; Aguirrezabala-Cámpano et al . 2013). So, it resulted interesting to evaluate if the proteases detected in M. armatum could be targeted by endogenous inhibitors present in Opuntia cladodes. Firstly, detection of inhibitory activity against standard proteases was done and it was confirmed the presence of trypsin-like and chymotrypsin-like inhibitory activities as the more abundant and only a few inhibitory activity against elastase-like. On the other hand, when the detection of the three types of proteolytic enzymes in M. armatum was confirmed, it was considered the confrontation of the digestive proteases with cladode proteins to evaluate if some inhibition could be detected, this showed a noticeable inhibition of trypsin and chymotrypsin-like activities, and only a reduced inhibition of the elastase activity; suggesting that probably the elastase-like activity due to the low inhibition could be employed as part of the digestive proteases set to overcome the inhibitory effect of Opuntia proteins on the insect digestive process, thus allowing to this beetle species to exploit Opuntia cladodes as a food source. Plant proteases inhibitors are considered as elements involved in plant defense mechanisms, where they function as a selective factor, which could lead to the insects to develop adaptation strategies ranging from increasing of protease secretion up to the modification of the proteolytic set with different catalytic types (Applebaum 1964; Ryan 1990; Johnson and Rabosky 2000; Jongsma and Beekwilder 2011). It is also suggested that some adaptive responses include the production of insensitive proteases, proteases with different catalytic mechanisms or combinations of different proteases, a fact that could be present in M. armatum ; however, more studies are necessary to make a functional assignment of inhibitors and determine whether there was selective pressure leading to the emergence of insensitive proteases in M. armatum .
In the case of the insects used for comparison, there is information about their intestinal protease battery and also from other functional and biochemical properties. The presence of trypsin and chymotrypsin enzymes in the gut of S. frugiperda and the differential expression influenced by the diet was reported (Oliveira et al. 2013), which was associated to insensitive trypsin expression when insect were fed with an inhibitor-enriched diet, indicating that resistance mechanism was related to insensitive protease expression. For P truncatus, the presence and biochemical characterization of intestinal serine proteases (trypsin, chymotrypsin and elastase) has been reported (Aguirre et al. 2009; Castro-Guillen et al. 2012). A wide diversity of serine proteases was reported in P truncatus, where trypsin-like activity had five proteases, chymotrypsin-like activity had nine proteases and the elastase-like was represented by four proteases, indicating a complex proteolytic system. These proteases shown different sensitivity toward several inhibitors, where a chymotrypsin and elastases were signaled as insensitive to plant protease inhibitors (Castro-Guillen et al. 2012); and similarly in this work, elastase from M. armatum where no inhibited by plant proteinase inhibitors from Opuntia . The fact of having a diverse proteolytic system for digestion, increase the possibilities to cope with proteinase inhibitor present in the natural diet as suggested by other works (Castro-Guillen et al . 2012), as seen in the present study, several catalytic types could be related to resistance to natural occurring inhibitors in the diet.
Recently, another case of an insect that attacks Opuntia plants was reported (the case of Cactophagus spinolae), where cysteine and serine proteases were detected; in the latter group, trypsin, chymotrypsin and elastase-like proteases were detected by zymography and by synthetic substrate hydrolysis. When protease activities and a crude protein extract from Opuntia cladode were challenged, trypsin and chymotrypsin-like activities were most sensitive, while elastase-like activity was only slightly inhibited and no inhibition was detected toward cysteine proteases, but using zymography an increase in proteolysis was observed in the latter case. It is proposed that cysteine proteases could be used as an alternative way to cope with the presence of serine protease inhibitors in the natural diet (Aguirre-Mancilla et al. 2014), whereas in this work, the presence of an insensitive elastase-like protease activity seemed to be the mechanism to manage natural inhibition.
In this study, when inhibition of proteolytic extracts of M. armatum, S. frugiperda and P. truncatus were compared, inhibition patterns showed clear differences in the case of trypsin-like inhibitory activity where the M. armatum trypsinlike activity was the less inhibited, while 10% of reduction was detected for chymotrypsin-like inhibitory in M. armatum; finally, the elastase-like inhibitory activities practically were no detected, which could reflects that Opuntia inhibitors have differences in the inhibition patterns so, these inhibitors could be challenged against a broad range of insects to evaluate their potential as candidate in control pests, but that will require additional experiments. It is thought that protease inhibitors are widely dispersed amongst plants (Ryan 1990); however, their types, isoforms, concentration and specificities will vary depending of the plant species and effects also will be related to the evaluated proteases (Jongsma and Bolter 1997). When proteases of phytophagous insects are assayed for inhibition, it could be found some adaptations to inhibitors present in the natural diet, whereas inhibitors obtained from other plants could show variable effects, since partially inhibition to total abolition of protease activity. This could be associated with differences at the complex of protease - protease inhibitor interaction, and those differences would be helpful to determinate if one inhibitor will affect or not to a target protease (Lawrence and Koundal 2002).
In the case of the present comparison, some differences in the inhibition percentage were observed; nevertheless, more detailed studies are needed with purified proteases and protease inhibitors to elucidate interactions, kinetic parameters, molar ratios and complex stability; which will reinforce the results of this work. This is the first report on the proteases of M. armatum and the effects of cladode endogenous proteins of O. ficus-indica, which constitutes an interesting topic for interaction between non model organisms; however, to holistically understand the impact of M. armatum feeding on Opuntia and how these plants can overcome insect attacks more research is necessary.
Acknowledgements
CONACYT-Mexico is acknowledged for their financial support of the project CB-2009-133186, to the Biotechnology Laboratory, Facultad de Agronomía of the Universidad Autónoma de Nuevo León for the technical facilities.
Literature cited
AGUIRRE, C.; CASTRO-GUILLEN, J. L.; CONTRERAS, L.; MENDIOLA-OLAYA, E.; DE LA VARA, L. G.; BLANCO-LABRA, A. 2009. Partial characterization of a chymotrypsin-like protease in the larger grain borer ( Prostephanus truncatus (Horn) in relation to activity of Hyptis suaveolens (L.) trypsin inhibitor. Journal of Stored Products Research 45: 133-138. [ Links ]
AGUIRRE-MANCILLA, C. L.; ÁLVAREZ-AGUIRRE, L. A.; MONDRAGÓN-JACOBO, C.; GUTIÉRREZ-DÍEZ, A.; RAYA-PÉREZ, J. C.; TORRES-CASTILLO, J. A. 2014. Gut enzymes from cactus weevil ( Cactophagus spinolae , Gyllenhal Coleoptera: Curculionidae) fed natural diet. Southwestern Entomologist 39: 477-490. [ Links ]
AGUIRREZABALA-CÁMPANO, M. T.; TORRES-ACOSTA, R. I.; BLANCO-LABRA, A.; MEDIOLA-OLAYA, M. E.; SINAGAWA-GARCÍA, S. R.; GUTIÉRREZ-DÍEZ, A.; TORRES-CASTILLO, J. A. 2013. Trypsin inhibitors in xoconostle seeds ( Opuntia joconostle Weber.). Journal of Plant Biochemistry and Biotechnology 22: 261-268. [ Links ]
AITKEN, A.; LEARMONTH, M. 1996. Protein Determination by UV Absorption. pp. 3-6. In: Walker, J. M. (Ed.). The Protein Protocols Handbook. Humana Press. New York. U.S.A. 809 p. [ Links ]
APPLEBAUM, S. W. 1964. Physiological aspects of host specificity in the Bruchidae-I. General considerations of developmental compatibility. Journal of Insect Physiology 10: 783-788. [ Links ]
BOWN, D. P.; WILKINSON, H. S.; GATEHOUSE, J. A. 2004. Regulation of expression of genes encoding digestive proteases in the gut of a polyphagous lepidopteran larva in response to dietary protease inhibitors. Physiological Entomology 29: 278290. [ Links ]
BOZIC, N.; VUJCIC, Z.; MRDAKOVIC, M.; IVANOVIC, J.; NENADOVIC, V. 2001. Midgut proteinase activities of Cerambyx cerdo (Coleoptera, Cerambycidae) larvae fed on different diets. Acta Entomologica Serbica 6: 147-150. [ Links ]
BOZIC, N.; VUJCIC, Z.; NENADOVIC, V; IVANOVIC, J. 2003. Partial purification and characterization of midgut leucylaminopeptidase of Morimus funereus (Coleoptera: Cerambycidae) larvae. Comparative Biochemistry and Physiology Part B 134: 231-241. [ Links ]
CASTRO-GUILLÉN, J. L.; MENDIOLA-OLAYA, E.; GARCÍA-GASCA, T.; BLANCO-LABRA, A. 2012. Partial characterization of serine peptidases in larvae of Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae), reveals insensitive peptidases to some plant peptidase inhibitors. Journal of Stored Products Research 50: 28-35. [ Links ]
CROOK, D. J.; PRABHAKAR, S.; OPPERT, B. 2009. Protein digestion in larvae of the red oak borer Enaphalodes rufulus . Physiological Entomology 34: 152-157. [ Links ]
DE OLIVEIRA, C. F. R.; DE PAULA SOUZA, T.; PARRA, J. R. P.; MARANGONI, S.; DE CASTRO SILVA-FILHO, M.; MACEDO, M. L. R. 2013. Insensitive trypsins are differentially transcribed during Spodoptera frugiperda adaptation against plant protease inhibitors. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 165: 19-25. [ Links ]
ERLANGER, B. F.; KOKOWSKY, N.; COHEN, W. 1961. The preparation and properties of two new chromogenic substrates of trypsin. Archives of Biochemistry and Biophysics 95: 271-278. [ Links ]
FELTON, G. W. 2005. Indigestion is a plant's best defense. Proceedings of the National Academy of Science 102: 18771-18772. [ Links ]
FERY, R. L.; SCHALK, J. M. 1991. Resistance in pepper ( Capsicum annuum L.) to western flower thrips Frankliniella occidentalis (Pergande). HortScience 26: 1073-1074. [ Links ]
HERMSMEIER, D.; SCHITTKO, U.; BALDWIN, I. T. 2001. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata . I. Large-scale changes in the accumulation of growth- and defense-related plant mRNAs. Plant Physiology 125: 683-700. [ Links ]
JOHNSON, K. S.; RABOSKY, D. 2000. Phylogenetic distribution of cysteine proteinases in beetles: evidence for an evolutionary shift to an alkaline digestive strategy in Cerambycidae. Comparative Biochemistry and Physiology Part B 126: 609619. [ Links ]
JONGSMA, M. A.; BEEKWILDER, J. 2011. Co-evolution of insect proteases and plant protease inhibitors. Current Protein and Peptide Science 12: 437-447. [ Links ]
JONGSMA, M. A.; BOLTER, C. 1997. The adaptation of insects to plant proteinase inhibitors. Journal of Insect Physiology 43: 885-895. [ Links ]
LAEMMLI, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680-685. [ Links ]
LAWRENCE, P. K.; KOUNDAL, K. R. 2002. Plant protease inhibitors in control of phytophagous insects. Electronic Journal of Biotechnology 5: 1-17. [ Links ]
LAWRENCE, S. D.; NOVAK, N. G.; JU, C. J.; COOKE, J. E. K. 2008. Potato, Solanum tuberosum , defense against Colorado potato beetle, Leptinotarsa decemlineata (Say): Microarray gene expression profiling of potato by Colorado potato beetle regurgitant treatment of wounded leaves. Journal of Chemical Ecology 34: 1013-1025. [ Links ]
LONCAR, N.; BOZIC, N.; NENADOVIC, V; IVANOVIC, J.; VUJCIC, Z. 2009. Characterization oftrypsin-like enzymes from the midgut of Morimus funereus (Coleoptera: Cerambycidae) larvae. Archives of Biological Science 61: 713-718. [ Links ]
LONCAR, N.; VUJCIC, Z.; BOZIC, N.; IVANOVIC, J.; NENADOVIC, V. 2010. Purification and properties of trypsinlike enzyme from the midgut of Morimus funereus (Coleoptera, Cerambycidae) larvae. Archives of Insect Biochemistry and Physiology 74: 232-246. [ Links ]
MELLO, M. O.; SILVA-FILHO, M. C. 2002. Plant-insect interactions: an evolutionary arms race between two distinct defense mechanisms. Brazilian Journal of Plant Physiology 14: 71-81. [ Links ]
MILLER, T. E. X. 2008. Bottom-up, top-down, and within-trophic level pressures on a cactus-feeding insect. Ecological Entomology 33: 261-268. [ Links ]
MITHOFER, A.; WANNER, G.; BOLAND, W. 2005. Effects of feeding Spodoptera littoralis on lima bean leaves. II. Continuous mechanical wounding resembling insect feeding is sufficient to elicit herbivory-related volatile emission. Plant Physiology 137: 1160-1168. [ Links ]
MOCHIZUKI, A. 1998. Characteristics of digestive proteases in the gut of some insect orders. Applied Entomology and Zoology 33: 401-407. [ Links ]
MOON, J.; SALZMAN, R. A.; AHN, J. E.; KOIWA, H.; ZHU-SALZMAN, K. 2004. Transcriptional regulation in cowpea bruchid guts during adaptation to a plant defence protease inhibitor. Insect Molecular Biology 13: 283-291. [ Links ]
RYAN, C. A. 1990. Protease inhibitors in plants: Genes for improving defenses against insects and pathogens. Annual Review of Phytopathology 28: 425-449. [ Links ]
SHARIFI, M.; GHOLAMZADEH CHITGAR, M.; GHADAMYARI, M.; AJAMHASANI, M. 2012. Identification and Characterization of midgut digestive proteases from the rosaceous branch borer, Osphranteria coerulescens Redtenbacher (Coleoptera: Cerambycidae). Romanian Journal of Biochemistry 49: 33-47. [ Links ]
SHAW, B. D.; CHRISTELLER, J. T. 2009. Characterization of the proteases in the midgut of the xylophagous larvae of Oemona hirta (Coleoptera: Cerambycidae). Insect Science 16: 381-386. [ Links ]
STOTZ, H. U.; PITTENDRIGH, B. R.; KROYMANN, J.; WENIGER, K.; FRITSCHE, J.; BAUKE, A.; MTTCHELL-OLDS, T. 2000. Induced plant defense responses against chewing insects. Ethylene signaling reduces resistance of Arabidopsis against Egyptian cotton worm however, not diamondback moth. Plant Physiology 124: 1007-1018. [ Links ]
TERRA, W. R. 1990. Evolution of digestive systems of insects. Annual Review of Entomology 35: 181-200. [ Links ]
TERRA, W. R.; FERREIRA, C. 1994. Insect digestive enzymes: properties, compartmentalization, and function. Comparative Biochemistry and Physiology Part B 109: 1-62. [ Links ]
TERRA, W. R.; CRISTOFOLETTI, P. T. 1996. Midgut proteinases in three divergent species of Coleoptera. Comparative Biochemistry and Physiology Part B 113: 725-730. [ Links ]
THALER, J. S.; STOUT, M. J.; KARBAN, R.; DUFFEY, S. S. 1996. Exogenous jasmonates simulate insect wounding in tomato plants ( Lycopersicon esculentum ) in the laboratory and field. Journal of Chemical Ecology 22: 1767-1781. [ Links ]
TORRES CASTILLO, J. A.; VARELA MARTÍNEZ, K.; BLANCO-LABRA, A.; MONDRAGÓN-JACOBO, C. 2007. Protease inhibitors present in Opuntia spp. VI International Congress on Cactus Pear and Cochineal 811: 293-298. [ Links ]
TORRES-CASTILLO, J. A.; JACOBO, C. M.; BLANCO-LABRA, A. 2009. Characterization of a highly stable trypsin-like proteinase inhibitor from the seeds of Opuntia streptacantha ( O. streptacantha Lemaire). Phytochemistry 70: 1374-1381. [ Links ]
TORRES-CASTILLO, J. A.; SINAGAWA-GARCÍA, S. R.; MARTÍNEZ-ÁVILA, G. C. G.; LÓPEZ-FLORES, A. B.; SÁNCHEZ-GONZÁLEZ, E. I.; AGUIRRE-ARZOLA, V E.; TORRES-ACOSTA, R. I.; OLIVARES-SÁENZ, E.; OSORIO-HERNÁNDEZ, E; GUTIÉRREZ-DÍEZ, A. 2013. Moringa oleífera: phytochemical detection, antioxidants, enzymes and antifugal properties. Phyton 82: 193-202. [ Links ]
ZHENG, S.; DlCKE, M. 2008. Ecological genomics of plant-insect interactions: From gene to community. Plant Physiology 146: 812-817. [ Links ]
ZIBAEE, A. 2014. Digestive proteolytic profile in Stromatium fulvum Villers (Coleoptera: Cerambycidae). Romanian Journal of Biochemistry 51: 17-30. [ Links ]
Received:17-Jun-2014
Accepted: 3-Aug-2015
Suggested citation:
TORRES-CASTILLO, J. A.; AGUIRRE-MANCILLA, C. L.; GUTIÉRREZ-DÍEZ, A.; SINAGAWA-GARCÍA, S. R.; TORRES-ACOSTA, R. I.; GARCÍA-ZAMBRANO, E. A.; AGUIRRE-ARZOLA, V ZAVALA-GARCÍA, F. 2015. Intestinal proteases of Moneilema armatum (Coleoptera: Cerambycidae) fed with Opuntia cladodes. Revista Colombiana de Entomología 41 (2): 249-256. Julio - Diciembre 2015. ISSN 01200488.