Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488
Rev. Colomb. Entomol. vol.41 no.2 Bogotá July/Dec. 2015
Polymers nanofibers as vehicles for the release of the synthetic sex pheromone of Grapholita molesta (Lepidoptera, Tortricidae)
Nanofibras poliméricas como vehículos de eliminación de feromona sexual sintética de Grapholita molesta (Lepidoptera, Tortricidae)
RICARDO BISOTTO-DE-OLIVEIRA1,2, ROSANA M. MORAIS3, ISABEL ROGGIA2, SANDRA J. N. SILVA2, JOSUÉ SANT’ANA1 and CLÁUDIO N. PEREIRA2
1 Ph. D. PPG-Fitotecnia, Departamento de Fitossanidade, Faculdade de Agronomia Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brazil. ricardo.bisotto@ufrgs.br, josue.santana@ufrgs.br. Corresponding author.
2 Tecnano Pesquisas e Servias Ltda. Porto Alegre - RS - Brazil. com.br, sandrajussara@tecnano.com.br, claudio@tecnano.com.br.
3 Ph. D. FEPAGRO, Centro de Pesquisa em Florestas - Rio Grande do Sul, RS, Brazil.rosana-morais@fepagro.rs.gov.br.
Abstract: The use of pheromones is a promising alternative in insect management and control. Recently, the incorporation and release of active ingredients from nanofibers has been of interest for agricultural purposes due to their higher surface area to volume ratios. The objective of this study was to produce nanofibers incorporating synthetic sex pheromones from the oriental fruit moth, Grapholita molesta (OFM), using different polymers. The nanofibers with pheromones were produced by electrospinning and were evaluated for: i) homogeneous distribution of OFM pheromone in nanofibers by electroantennography (EAG) tests; ii) dose-responses of OFM males to acetate cellulose nanofibers; iii) EAG responses of OFM males to different polymer nanofibers exposed under controled conditions for up to 5 weeks; iv) attractiveness of G. molesta to nanofibers containing synthetic OFM in field conditions; v) quantification of incorporated pheromone in PCL and PVAc nanofibers using gas chromatography and vi) morphology using scanning electron microscopy. The best field results were achieved in smoother fibers with higher impregnation. Pheromone incorporating nanofiber vehicles were tested that achieved the controlled release of the pheromone for up to three weeks in nanoscale and microscale. The polymer, solvent, and dosage incorporated in the nanofibers were important for controlled delivery. Understanding these factors is important for the development of better pheromone dispensers.
Key words: Polymer. Electrospinning. Nanofibers. Electroantennography. Pheromone.
Resumen: El uso de feromonas es una alternativa promisora en el manejo y el control de plagas. Recientemente, la incorporación y eliminación de ingredientes activos de las nanofibras han despertado el interés para objetivos agrícolas debido a su elevada área en comparación a la superficie de proporción de volumen. El objetivo de este estudio fue producir nanofibras incorporando la feromona sexual sintética de la mariposa oriental Grapholita molesta (OFM) utilizando diferentes polímeros. Las nanofibras con feromona producidas por la técnica de electrohilado fueran evaluadas por: i) distribución homogénea de feromona de OFM en nanofibras por testes de electroantenografía (EAG); ii) dosis-respuestas de machos de OFM a nanofibras de acetato de celulosa; iii) respuestas EAG de machos de OFM a diferentes nanofibras de polímeros expuestos a condiciones controladas por hasta 5 semanas; iv) atracción de G. molesta a nanofibras conteniendo feromona sintética en condiciones de campo; v) cualificación de feromonas incorporadas de nanofibras de PCL y PVAc por cromatografía de gas y; vi) su morfología utilizando microscopio electrónico de exploración. Los mejores resultados de campo fueron conseguidos en fibras más blandas con mayor impregnación. Diferentes vehículos de nanofibras incorporando feromona fueron analizadas que consiguieron eliminación controlada de feromona hasta tres semanas, en nano y micro escalas. El polímero, solvente y dosis incorporadas en las nanofibras son importantes para la liberación controlada. Entender estos factores es importante para desarrollar mejores distribuidores de feromonas.
Palabras clave: Polímero. Electrohilado. Nanofibras. Electroantenografía. Feromona.
Introduction
The use of pheromones is a promising alternative in the management and control of agricultural pests. These substances have the advantage of high specificity and its use does not harm the environment, compared to traditional methods based on the use of insecticides (Vilela and Mafra-Neto 2001). These attractive compounds can be used in agricultural systems in order to monitor or control insects. The control can be obtained by collecting mass of males, as well as confounding by sexual mating disruption or when the male confusion is caused due to the excess chemical stimulus in the field, preventing mating (Degen et al. 2005; Kovanci et al. 2005; Charmillot et al. 1997).
In Brazil, the semiochemicals market has increased considerably, bringing the use of pheromones in many cultures as an alternative to control pests important species, such as pink worm Pectinophora gossypiella (Saunders, 1843) (Lepidoptera, Gelechiidae), tomato borer Tuta absoluta (Meyrick, 1917) (Lepidoptera, Gelechiidae), cotton boll weevils Antonomus grandis Boheman, 1843 (Coleoptera, Curculionidae), sand fly Lutzomyia longipalpis Lutz and Neiva, 1912 (Diptera, Psychodidae), sugar cane borer Diatraea saccharalis (Fabricius, 1794) (Lepidoptera, Pyralidae) among others (ISCA 2013).
Numerous forms of dispersal have been developed for capturing Grapholita molesta (Busk, 1916) (Lepidoptera, Tortricidae), especially pastes, polymer sachets and microencapsulated formulations (ISCA 2013). Recently, the incorporation and release of active ingredients from nanofibers has been of great interest for pharmaceutical and agricultural purposes. Due to their higher surface area to volume ratio, nanofibers have been studied for applications such as drug delivery carriers (Ramakrishna 2005). There is increasing interest in better dispersal of agricultural inputs in crops aiming to increase productivity and lower costs. Electrospinning (ES) is one of the most interesting new methods for drug delivery (Liao et al. 2008; Xu et al. 2008; Chakraborty et al. 2009; Tamaro et al. 2009; Cui et al. 2006) and the nanofibers produced can also bring benefits to the agriculture area.
The novel application of nanotechnology, specifically polymeric nanofiber as vehicles for semiochemicals dispensing in agriculture was based on a pioneering study conducted in Germany by Hellmann et al. (2009). In this study, the authors used the electrospinning technique for producing cellulose acetate nanofibers containing the grape berry moth Lobesia botrana (Denis & Schiffermuller, 1775) (Lepidoptera, Tortricidae) synthetic sex pheromone. The authors examined the possibility of incorporating the pheromone of this species within the nanofibers for their use in mating disruption in grapevine orchards.
Subsequently, Lindner et al. (2011) realized laboratory tests to characterize the nanofibers containing the L. botrana pheromone. Hummel et al. (2011) performed bioassays in the wind tunnel, also quantifying the mass loss of nanofibers with the aid of analytical balances and by conducting thermogravimetric analysis (TGA). According to Hummel et al. (2011), in the future, these nanofibers vehicles containing pheromone could be deposited directly on the plants, with the aid of portable electrospinning machine, which would allow the coverage of orchard large areas, while increasing the efficiency of the sexual confusion technique. Thus, in theory, it would be possible to produce nanofibers containing an emulsion encapsulating pheromone molecules. These small scale capsules dispersed in continuous polymer phase produced by electrospininng method would have a large area relative to the total, lowering cost and increasing efficiency of the mating disruption strategy.
To date, information about the use of nanofibers as vehicles for dispersing insect pheromones are scarce and largely based on the results obtained by Hellmann et al. (2009), Hummel et al. (2011) and Lindner et al. (2011), which served as parameters in the selection and polymer concentrations pheromones to be investigated. The OFM synthetic pheromone was chosen as a model compound for the study of a nanofibers vehicle because of its known efficiency in the pest management when used in other formulations. Thus, the objectives of this study were: i) evaluate the distribution of OFM pheromone in nanofibers through electroantennographic bioassays; ii) estimate the dose-responses of the oFm males to acetate cellulose nanofibers; iii) measure the EAG responses of OFM males to nanofibers exposed at controled conditions for until 5 weeks; iv) evaluate the attractiveness of adult G. molesta to nanofibers containing synthetic sex pheromone in field conditions; v) quantify the pheromone in PVAc and PCL nanofibers by gas chromatography and; vi) determine the morphology of the nanofibers produced by scanning electron microscopy (SEM).
Materials and methods
Insects rearing. The G. molesta individuals used in the bioassays were obtained from rearing in BIOECOLAB-UFRGS, Department of Crop Protection, Faculty of Agronomy, Federal University of Rio Grande do Sul, kept under controlled conditions (25 ± 1 °C, 60 ± 10% relative humidity, and a 16-8 L-D hour photoperiod). The larvae were artificial diet fed, following the methodology of Arioli et al. (2007) and the adults were fed with water honey solution (15% honey and 5% methylparaben). Only male insects were used in the bioassays.
Electroantennography (EAG). Insects used in the bioassays had the antennas sectioned at the pedicel and were connected to a bifurcated silver electrode. The antenna’s apical and basal ends were attached between the registration and the neutral electrode, respectively. Both were immersed in Spectra 360® salt-free gels to enhance the electrical conductivity of the sample. The analogical signal response, measured in millivolts, was captured, amplified and processed with a data acquisition controller (IDAC -4 Syntech®) and subsequently recorded by software (EAG2000, Syntech).
All treatments were individually inserted into Pasteur pipettes. The front end of the pipette was placed into a hole in a metal tube (1cm diameter x 18 cm in length) oriented toward the antenna, having an approximated 1 cm distance. The antennas were subjected to air pulses generated by a flow controller (CS -02, Syntech®) in a volume of 2.5 mL/ 0.5 s with different treatments. It has been stipulated time of one minute between successive stimuli so that the antenna regained their capacity to perceive odorant. The electrophysiological responses of each treatment were recorded in millivolts and the EAG average responses were compared by ANOVA (Tukey test; a = 0.05) using the BioEstat 5.0 statistics program (Ayres et al. 2007). The antennas were used only once in each repetition. For each EAG response test, 15 virgin males aged between three to seven days were used.
Production of electrospinning nanofibers containing the OFM synthetic sex pheromone. The polymer nanofibers were produced at Tecnano Research and Services Ltd., using a custom made electrospinning machine with a 60 kV HV supply. The syringe pump consisted of 3 ml syringe and a rotary aluminum collector was adjusted to about 60 rpm. In this process, polymer dispersions were prepared dissolved in specific solvents, with and without the synthetic sex pheromone of G. molesta (P9000 - 90 Bedoukian OFM Technical Pheromone). The polymers used were cellulose acetate (CA), polyvinyl acetate (PVAc), polycaprolactone (PCL), polyvinyl pyrrolidone (PVP) and styrene-butadiene-styrene (SBS) copolymer, and its blends. The solvents used were dichloromethane (DCM), dimethylformamide (DMF), ethanol (EtOH), tetrahydrofuran (THF) and water, either alone or in solvent blends. The polymer, or polymer blend, was dissolved using the solvent mix in order to obtain a final polymer concentration ranging from 0.02 to 10% (w/v) of OFM synthetic pheromone that was fed into the ES machine with a syringe pump at a rate of 0.05 ml/min using a 3 ml syringe with a blunted needle. A voltage of 1.81 kV/cm was applied between the needle and the earthed collector. The nanofiber films were deposited onto the glass slides and directly onto the metal stub for microscope analysis. The produced nanofibers were isolated and placed at -10 °C until the time of the bioassays.
Bioassays were conducted in laboratory (EAG) and in field conditions. Initially, based on Hellmann’s work (2009), cellulose acetate nanofibers were produced and tested to verify the possibility of incorporating the G. molesta synthetic sex pheromone in these nanoscale matrixes.
Bioassays
i) Distribution of OFM pheromone in cellulose acetate nanofibers through electroantennographic tests. Cellulose acetate nanofibers having dimensions of 100 cm2 were produced containing about 10% OFM pheromone based on polymer concentration. To check the homogeneity of the distribution of the pheromone within the matrixes, each nanofiber was divided into 25 quadrants measuring 4 cm2 and identified for use in electroantennographic bioassays. The G. molesta male antennal stimuli from each of the quadrants removed from nanofibers was evaluated by the bioactivity test and subjected to ANOVA determinations by Tukey test (a = 0.05).
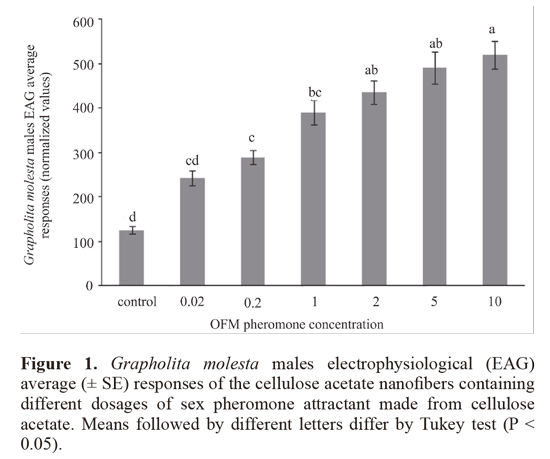
ii) Dose-responses of the OFM males to cellulose acetate nanofibers. EAG responses of G. molesta males were evaluated after stimuli of CA nanofibers containing 0.02, 0.2, 1, 2, 5 and 10% (w/v) synthetic sex pheromone. The EAG dose-response effect was observed after stimuli of the antennae by 2 cm2 cut pieces nanofibers having different concentrations and offered separately from the lowest to the highest dose. The bioassays data were subjected to ANOVA determinations by Tukey test (a = 0.05).
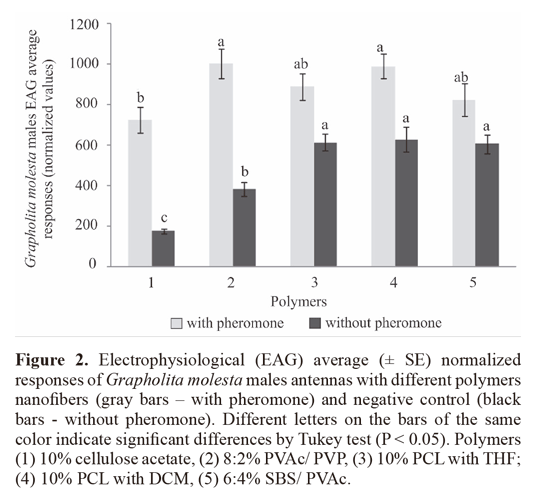
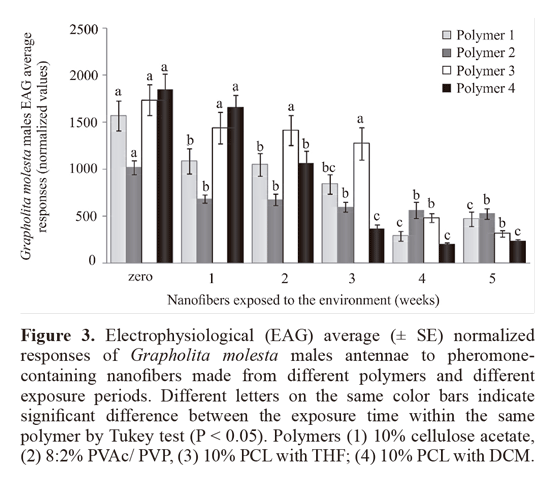
iii) EAG responses of OFM males to different polymer nanofibers exposed at controled conditions for until 5 weeks. The different polymers nanofibers containing 0.2% OFM pheromone and without pheromone were exposed in a controlled environment (25 °C , 14 h photoperiod , 70% RH ) for up to five weeks nanofibers: 1) Cellulose acetate; 2) PVAc/ PVP; 3) PCL + THF; 4) PCL + DCM. The electrophysiological responses of G. molesta males were compared in the same polymer nanofibers containing pheromone between different exposure times and control (not exposed). The bioassays data were subjected to ANOVA determinations by Tukey test (a = 0.05).
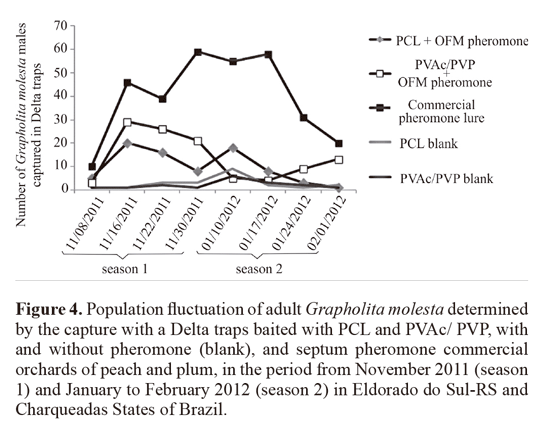
iv) Attractiveness of G. molesta to PVAc/PVP blend and PCL nanofibers containing synthetic sex pheromone in field conditions. The nanofibers containing the OFM sex pheromone attractiveness was evaluated in peach and plum orchards located in the district of Charqueadas and Eldorado do Sul, (RS - Brazil). Delta traps were baited with PVAc/ PVP blend and PCL nanofibers, produced with 2 different solvents, measuring 4 cm2, both made in the electrospinning with containing 10% OFM synthetic sex pheromone additivated with antioxidant and UV protector. Delta traps were also for means of comparison, baited with 2 different controls: nanofibers without pheromone, as negative controls; and commercial bait (Isca Lure Grafolita®), as positive control in order to evaluate the presence of the oriental fruit moth inner the orchards. The nanofibers were placed inside screened sachets and hanged on the inside top of the traps. These were installed in the plants branches at an approximate height of 1.70 m above the ground and spaced 30 meters apart. The five treatments were arranged in randomized blocks within areas of approximately 0.5 hectares, and had eight repetitions. There were two periods of installation (11/01/11 and 01/03/12), from which the attractive field remained for 30 days, and weekly number of registered individuals of G. molesta captured. The bioassays data were subjected to Kruskal-Wallis test (Student-Newman; a = 0.05) using the BioEstat 5.0 statistics program (Ayres et al. 2007).
v) Quantify the pheromone in PVAc and PCL nanofibers by gas chromatography. Oriental fruit moth (OFM) pheromone (Bedoukian Research, Danbury, CT) was used for most of the lab and field tests in this research. This pheromone was chosen because synthetic OFM pheromone is readily available. Synthetic OFM pheromone is a mixture of (Z)-8-dodecen-1-yl acetate, (E)-8-dodecen-1-yl acetate, and (Z)-8-dodecen-1-ol, in a 93:6:1 ratio. The amount of pheromone in the samples was calculated by gas chromatography (GC) Dani model Master A, equipped with a flame photo-ionization detector (PID) and a megabore capillary column (DB-1. 30 m x 0.25 mm, 0.53 mm diameter, Agilent Technologies). The initial temperature of the injector was 250 °C and the detector was heated to 200 °C. After the injection of the sample, the temperature of the oven was maintained at 135 °C for 2 minutes and then heated to 200 °C with a margin of 10° C.min-1. The samples were analised in triplicate and the quantity of volume injected was 1 pL.
vi) Morphology of the nanofibers produced by scanning electron microscopy (SEM). Images from sEm of the electrospun samples of PCL and PVAc nanofibers were performed on a JEOL - JSM 6060 (JEOL Ltd.) electron microscope. The piece of aluminum foil containing the fibers was mounted on an aluminum stub and sputter-coated with 15 nm of gold for analysis. Three SEM photographs from every sample were used for analysis. The analysis was performed by measuring the diameter in every photograph 30 times. The software used for the measurements and statistical analysis was ImageJ 1.47x.
The all data were submitted to normality and homoscedasticity tests.
Results
i) Distribution of OFM pheromone in cellulose acetate nanofibers through electroantennographic bioassays. There was no significant difference (P > 0.05) between the electrophysiological responses of G. molesta antennae male within the different portions coming from the different quadrants of the nanofibers. This result indicates that the synthetic sex pheromone of G. molesta was evenly distributed in all parts of the cellulose acetate nanofiber. That is an important find since the electrospinning is a random mat of nanofibers and validate the fact that we are using cut pieces of an entire mat.
ii) Dose-responses of the OFM males to cellulose acetate nanofibers. The electrophysiological responses of G. molesta males subjected to stimuli with nanofibers containing different percentages of pheromone were statistically significant (F = 24.27, df = 6, P < 0.0001) (Fig. 1). The sizes of electrophysiological responses were similar among treatments nanofibers containing 0.02, 0.2 and 1% and between treatments containing 2, 5 and 10% pheromone. This result demonstrates the presence of pheromone in the nanofiber, whereas the antennae of G. molesta males demonstrated bioactivity to stimuli, increasing the size of EAG response as a function of the concentration of pheromone in the nanofiber.
iii) EAG responses of OFM males to different polymer nanofibers exposed at controled conditions for until 5 weeks. The EAG responses of G. molesta males showed a significant difference only between 10% CA polymer in contrast with (8:2% PVAc/PVP polymers) and 10% PCL from DCM polymer, while the other had no difference (F = 3.05, df = 4, P > 0.05) (Fig. 2). When compared to their respective controls, the treatments containing pheromone produced significantly greater EAG responses, except for treatment 5 (6:4% SBS/ PVAc).
The formulation with the polymer 10% PCL with THF (Polymer 3) showed the greater retention of the pheromone producing electrophysiological responses equal to the new fiber until the third week of exposure, after being significantly equal to the control matrix. Thus, this formulation was one of those chosen for the preparation of nanofibers that were used in the experiments under field conditions. The formulation 10% PCL with DCM (Polymer 4) triggered electrophysiological responses similar to the new fiber only in the first week, decreased in relation to this, the other exposure times. With respect to 8:2% PVAc/ PVP (Polymer 2), although all treatments exhibited have lower response than the new fiber, these were stable and did not differ between itself from the first to the fifth week (Fig. 3). Furthermore, this was the only polymer which triggered electrophysiological responses were significantly higher after the fifth week of exposure compared to its control (no addition of pheromone in the fiber).
For field conditions bioassays, only the nanofibers that showed the best characteristics of homogeneity, retention and gradual release of pheromone, under laboratory conditions, were selected (PVAc and PCL), while the others made from cellulose acetate and SBS/ PVAc were eliminated for these experiments.
iv) Attractiveness of adult G. molesta to PVAc and PCL nanofibers containing synthetic OFM sex pheromone in field conditions. The Delta traps baited with nanofibers made of 8:2% PVAc/ PVP and 10% PCL with THF captured 13.75 ± 0.38 males and 9.78 ± 0.32 on average, respectively, did not differ from each other (P > 0.05). These averages were significantly higher than their respective controls (2.12 and 2.75 males on average), however, were lower than the average obtained with the commercially available bait (Isca Lure Grafolita®) (39.75 ± 0.51) (P < 0.05, H = 26.75, df = 4) (Fig. 4).
v) Quantify the pheromone in PVAc and PCL nanofibers. Pheromone content in the tested nanofibers. The calibration curve showed a good repetition of the method (R2 = 0.99). After validating the GC measurement for measuring the pheromone content in nanofibers, the following nanofibers were evaluated: PVAc/ PVP, PCL with THF and PCL with DCM.
The three nanofibers samples with impregnated dodecenyl acetate were evaluated by GC analysis (Table 2). The PCL nanofibers produced using the solvent THF had the higher pheromone content. The different percentage of pheromone based on polymer mass found using the same polymer blend suggests that the solvent and co-solvent selection had importance to the encapsulation efficiency.
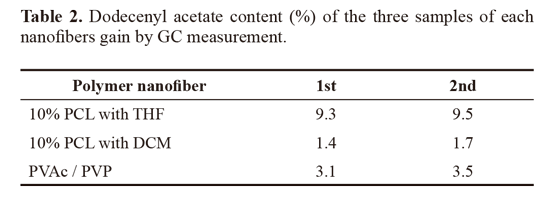
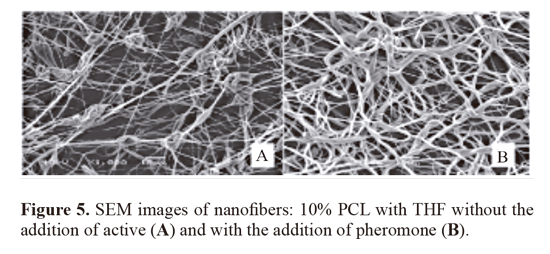
vi) Morphology of the nanofibers produced by scanning electron microscopy (SEM) and through this morphology. Following are presented the images obtained for the samples analyzed in scanning electron microscopy. The figure 5 shows that the electronic microscope images of PCL nanofibers produced using the THF solvent had the format of beads-on-strings (A), while smoother fibers (B) were obtained with the pheromone addiction. The nanofibers containing the active (0.835 ± 0.4 mM), had higher mean diameter greater than the control (0.370 ± 0.2 mM) (Table 1). The increase in the average diameter of the nanofibers was statistically significant P < 0.05. Figures 5 and 6 show photomicrographs of fibers from polycaprolactone dissolved in organic solvent mixture. The same features were seen in figure 6 for the fibers made using the solvent DCM. In that second case, there were more beads than the first. The nanofibers containing the active (0.681 ±0.3 mM), showed larger diameter compared to control (0.442 ± 0.1 mM). The increase of average diameter of nanofibers showed at table 1 was statistically significant (P < 0.05).
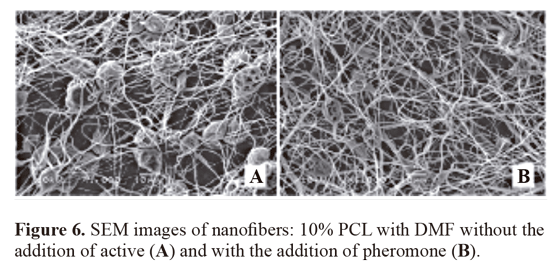
On the other side, the nanofibers produced with PVAc/ PVP were smoother (Fig. 7). The fibers containing the active (1.217 ± 0.5 mM) had greater average diameter compared to control (1.630 ± 0.7 mM). This increase in diameter was also statistically significant (P < 0.05) (Table 1).
Discussion
The present study was based upon the few papers using nanofiber as pheromone vehicles from the literature. The nanofibers polymer selection was firstly based on study carried out by Hellmann et al. (2009), where cellulose acetate film were tested in vitro and found to be an excellent vehicle for the transport of European grape berry moth pheromone Lobesia botrana due to features such as the ability to incorporate pheromone molecules and its rate of release for weeks straight. In the present study, the efficacy of incorporation and release of pheromone from nanofiber mats and its even distribution inside the matrixes was evaluated by the bioassays proposed. Pheromones impregnation dosages that caused major electrophysiological responses of G. molesta males concentrations were 2 to 10%.
Because of the short duration of the cellulose acetate nanofiber in the initial tests, new polymers and its blends were also tested. Because of the chemical similarity between PVAc and PCL, both esters, with the major G. molesta component pheromone, dodecenyl acetate, those polymers were chosen. Styrene-butadiene-styrene was selected because rubber septa are known dispenser for pheromones and that the longer carbon chain of the principal component-dodecenyl acetate- is non polar. Polyvinyl pyrrolidone was preferred for blending with PVAc to add polar group and an amphyphilic character for increase the solubilization of the dodecenol molecule, other pheromone component. Cellulose acetate polymer was less efficient than PVAc and PCL the bioassays performed in this study, when assessed in vivo by electrophysiological responses of insects, in respect to pheromonal retention, and also for environment exposed durability. So these polymers were selected for the following testing.
The polyvinyl acetate blend (PVAc/ PVP), despite a drop in electrophysiological responses when the fibers were exposed to environment, the response remained throughout the five weeks at the same rate of release. The blend PVAc/ PVP is extensively studied in the pharmaceutical industry due to the efficiency of drug release, are ideal for this reason the healing drug delivery, requiring a release and prolonged linear (Jannesari et al. 2011). Such behavior is regarded as one of the properties assigned to this amphiphilic blend. Since the (Z)-8-dodecenyl acetate molecule has polar and non-polar groups, the amphiphilic character match each of these groups, and this could be one of the reasons of the controlled release achieved.
The polymer PCL showed the longest pheromone retention period even after exposure to environment, which is an important feature for agricultural purposes. This polymer is biodegradable polyester, aliphatic, what is also a desirable feature when applications to agriculture are in concern. Aliphatic polyesters have been employed as slow-release agents in agriculture for a long time (Sinclair 1973). They degrade into natural materials harmless to the environment while slowly releasing the encapsulated herbicides or pesticides. Because of these properties, two different formulations were tested, having two different solvent mixes, DCM or THF blended with DMF solvent. These two formulations have different impregnating efficiency when GC measured, as will be discussed below.
Analyzing the electronic microscope images, the PCL nanofibers were all beads-on-strings, although the fibers became smoother with the pheromone addiction. In the images 6A and 6B, it is observed that change. The reduction of the beads and reduction of fiber diameter are two morphologic characteristics that result from the increased polarity of the electrospinning solution (Tungprapa et al. 2007). The solvent used was tetrahydrofuran, an aprotic solvent with dielectric constant (e) 7.58 to 20 oC. The nanofibers containing the active (0.835 ± 0.4 mM), had higher mean diameter greater than the control (0.370 ± 0.2 mM). The increase in the average diameter of the nanofibers was statistically significant P < 0.05. The nanofibers in the form of bead-in-strings were becoming flat with the incorporation of the active ingredient (Fig. 5).
The same features were seen in figure 6 for the fiber made from polycaprolactone dissolved in DCM, although with more beads than the based on THF. The solvent dichloromethane is an aprotic solvent with a higher dielectric constant (e) 9.08 to 20 °C. The nanofibers containing the active (0.681 ± 0.3 mM), showed larger diameter compared to control (0.442 ± 0.1 mM) (Table 1). The increase of average diameter of nanofibers was statistically significant (P < 0.05).
In the third case, the nanofibers produced with PVAc/ PVP were smoother. The fibers containing the active (1.217 ± 0.5 mM) had greater average diameter compared to control (1.630 ± 0.7 mM). This increase in diameter was also statistically significant (P < 0.05). Smoother, or uniform, fibers with fewer beads are desirable, since they indicate that they are more resistant and more compatible (Doshi and Reneker 1995). One way to reduce the amount of beads is to increase solution conductivity (Ramakrishna et al. 2005), which is obtained by selection of more polar solvents by the addition of salts to the solution, or by adding an active ingredient with polarity, which seems to have been the case under discussion. The smoother fiber should have occurred due to the high dielectric constant of the solution, higher than in the previous cases cited solvents (dielectric constant of 80 and 24, at 20 oC, for the cases of water and ethanol).
In the PVAc/ PVP fibers, there was also interweaving of the fibers, which is desirable because the increased resistance of the resulting film, but it is noted fusion of the fibers, which may indicate a case of wet-spinning, when the solvent is not evaporated completely in route to the collector metal (Tang et al. 2009). That signifies that the solvent has boiling point higher than other solvents used and not evaporated completely until reaching the metal collector. This high boiling point of the solvent used is the reason for the case of wet-spinning. In this case, increasing the distance collector or a change in concentration of each solvent can optimize the solution. This was not done because it standardized the distance between the needle and collector for 3 samples and their respective controls.
Yang et al. (2004) investigated the effects of solvents, including DCM, EtOH, DMF and mixtures of solvents, the morphology and diameter of PVP nanofibers obtained by ES. In solvent systems simple to 4% by weight of PVP, the nanofibers electrospining from ethanol were smooth, whereas the nanofibers from DMF and DCM exhibited beads-on-strings structure. In systems solvent mixtures, a mixture of ethanol/ DMF, with a mass ratio of 50/50 was obtained as a good solvent for PVP producing nanofibers with diameters as small as 20 nm. In a solvent mixture such as PVP concentration was increased from 4 to 8% by weight, the fiber diameter increased from 20 to 50 nm, no significant change in size distribution.
It is well known that physicochemical properties such as dielectric constant, boiling point, surface tension and viscosity of the polymer solution are important factors to define the morphological characteristics of the nanofibers generated. The polymer concentration and molecular weight of the polymer is also related to the latter property. Thin and uniform nanofibers were produced by electrospinning by authors from PVP solutions in solvents with a dielectric constant significantly high, low surface tension and low viscosity (Chuangchote et al. 2009).
The field exposure experiment demonstrated that although the PVAc/ PVP and tested polyester 10% PCL with THF had statistically superior capture than their respective controls, it was lower than that obtained with the commercial rubber septum. The results also showed that higher pheromone content is related to higher capture. The reason may be that the mass of the commercial septum is much higher than the nanofiber.
Thus, these results indicate that adjustments are needed to maximize the ability to capture the field with the use of these polymers, as well as further investigation of other polymers capable of use in the manufacture of nanofibers used as dispersing pheromones.
The results of chromatographic analysis showed a greater pheromone impregnation of PCL with THF nanofibers (Table 1). These nanofibers had the greater pheromone persistence in the environment. The pheromone content was also higher than that found in PVAc/ PVP blend. The validation of the technique for pheromone dosage in nanofibers was obtained after the field trials that was the reason we could not use fibers with similar content of pheromone. For future studies the pheromone content should be normalized in order to determine what are the predominant factors involved in the controlled release of pheromone.
The results of table 1 and SEM images shows that there is a relationship between the appearance of the nanofibers, the pheromone content in each fiber and the controlled delivery of the nanofibers. Other studies should be done to assess the relationship between the morphology of the fibers, the polymer utilized and the pheromone release. The polymer, solvent and dosage incorporated in the nanofibers are important to the controlled delivery. Understand these factors is important in order to develop better pheromone dispensers.
Acknowledgements
The authors are grateful for the financial support provided by Brazil’s National Council for Scientific and Technological Development (CNPq), FAPERGS (Fundagao de Amparo à Pesquisa do Estado do Rio Grande do Sul) and FINEP (Financiadora de Estudo e Projetos).
Literature cited
ARIOLI, C. J.; MOLINARI, F.; BOTTON, M.; GARCIA, M. S. 2007. Técnica de criagao de Grapholita molesta (Busck, 1916) (Lepidoptera: Tortricidae) em laboratòrio utilizando dieta artificial para a produgao de insetos visando estudos de comportamento e controle. Boletim de Pesquisa e Desenvolvimento, Bento Gongalves, Embrapa Uva e Vinho, 14 p. [ Links ]
AYRES, M.; AYRES, JR. M.; AYRES, D. L.; DOS-SANTOS, A. S. 2007. BioEstat 5.0 - Aplicagoes estatísticas nas áreas das ciencias biológicas e médicas. Belém, Sociedade Civil Mamirauá, 364p. [ Links ]
CHAKRABORTY, S.; LIAO, I. C.; ADLER, A.; LEONG, K. W. 2009. Electrohydrodynamics: A facile technique to fabricate drug delivery systems. Advanced Drug Delivery Review 61: 1043-1054. [ Links ]
CHARMILLOT, P. J.; PASQUIER, D.; DORSAZ, L.; KEIMER, C. H.; HERMINJARD, P. H.; OLIVIER, R.; ZUBER, M. 1997. Lutte par confusion contre carpocapse Cydia pomonella L. en Suisse en 1996 au moyen des diffuseurs Isomate-C Plus. Revue Suisse de Viticulture, Arboriculture e Horticulture 29: 91-96. [ Links ]
CHUANGCHOTE, S.; SAGAWA, T.; YOSHIKAWA, S. 2009. Electrospinning of poly(vinyl pyrrolidone): Effects of solvents on electrospinnability for the fabrication of poly(p-phenylene vinylene) and TiO2 nanofibers. Journal of Applied Polymer Science 114: 2777-2791. [ Links ]
CUI, W.; LI, X.; ZHU, X.; YU, G.; ZHOU, S.; WENG, J. 2006. Investigation of drug release and matrix 25 degradation of electrospun poly(DL-lactide) fibers with paracetamol inoculation. Biomacromolecules 7: 1623-1629. [ Links ]
DEGEN, T. H.; CHEVALLIER, A.; FISCHER, S. 2005. Evolution de la lutte pheromonale contre les vers de la grappe. Revue Suisse de Viticulture, Arboriculture e Horticulture 37: 273-280. [ Links ]
DOSHI, J.; RENEKER, D. H. 1995. Electrospinning process and applications of electrospun fibers. Journal of Electrostatics 35: 151-156. [ Links ]
HELLMANN, C.; GREINER, A.; WENDORFF, J. H. 2009. Design of pheromone releasing nanofibers for plant protection. Polymers for Advances Technologies. Available in: www.interscience.wiley.com/journal/pat. 2009. (Review date: 19 December 2013). [ Links ]
HUMMEL, H. E.; HEIN, D. F.; BREUER, M.; LINDNER, I.; GREINER, A.; WENDORFF, J. H.; HELLMANN, C.; DERSCH, R.; KRATT, A.; KLEEBERG, H.; LEITHOLD, G. 2011. Organic nanofibers containing insect pheromone disruptants: a novel technical approach to controlled release dispensers with potential for process mechanization. Communications in Agricultural and Applied Biological Sciences 76: 809-17. [ Links ]
ISCA Tecnologias 2013. Apresenta informagoes sobre ferramentas e solugoes para manejo de pragas. Available in: http://www.isca.com.br/. (Review date: 19 December 2013). [ Links ]
JANNESARI, M.; VARSHOSAZ, J.; MORSHED, M.; ZAMANI, M. 2011. Composite poly(vinyl alcohol)/poly(vinyl acetate) electrospun nanofibrous mats as a novel wound dressing matrix for controlled release of drugs. International Journal of Nanomedicine 6: 993-1003. [ Links ]
KOVANCI, O. B.; WALGENBACH, J. E.; KENNEDY, G. G.; SCHAL. J. E. E. 2005. Effects of application rate and interval on the efficacy of sprayable pheromone for mating disruption of the Oriental fruit moth Grapholita molesta. Phytoparasitica 33 (4): 334-342. [ Links ]
LIAO, Y; ZHANG, L.; GAO, Y.; ZHU, Z. T.; FONG, H. 2008. Preparation, characterization, and encapsulation/release studies of a composite nanofiber mat electrospun from an emulsion containing poly (lactic-co-glycolic acid). Polymer (Guildf) 49 (24): 5294-5299. [ Links ]
LINDNER, I.; HEIN, D. F.; BREUER, M.; HUMMEL, H. E.; DEUKER, A.; VILCINSKAS, A.; LEITHOLD, G.; HELLMANN, C.; DERSCH, R.; WENDORFF, J. H.; GREINER, A. 2011. Organic electrospun nanofibers as vehicles toward intelligent pheromone dispensers: characterization bylaboratory investigation. Communications in agricultural and applied biological sciences 76: 819-29. [ Links ]
RAMAKRISHNA, S.; FUJIHARA, K.; TEO, W-E. 2005. An introduction to electrospinning and nanofibers. World Scientific Publishing Co. Pte Ltd. 382 p. [ Links ]
SINCLAIR, R. G. 1973. Slow release pesticide system: Polymers of lactic and glycolic acids as ecologically beneficial, cost effective encapsulating materials. Environmental Science and Technology 7 (10): 955-956. [ Links ]
TAMMARO, L.; RUSSO, G.; VITTORIA, V. 2009. Encapsulation of diclofenac molecules into poly(s-caprolactone) electrospun fibers for delivery protection. Journal of Nanomaterials 1-8. [ Links ]
TANG, Z.; QIU, C.; MCCUTCHEON, J. R.; YOON, K.; MA,H. ; FANG, D.; LEE, E.; KOPP, C.; HSIAO, B. S.; CHU, B. 2009. Design and fabrication of electrospun polyethersulfone nanofibrous scaffold for high-flux nanofiltration membranes. Journal of Polymer Science: Part B: Polymer Physics 47: 2288-2300. [ Links ]
TUNGPRAPA, S.; PUANGPARN, T.; WEERASOMBUT, M. 2007. Electrospun cellulose acetate fibers: effect of solvent system on morphology and fiber diameter. Cellulose 14 (6): 563-575. [ Links ]
VILELA, E. F. ; MAFRA-NETO, A. 2001. Registro de feromônios comerciais e legislação. pp. 151-159. In: Vilela, E. F.; Della Lucia, T. M. C. 2001. Feromônios de Insetos (Biologia, química e emprego no manejo de pragas). Segunda Edição. Editora Holos. Ribeirão Preto, SP, 206 p. [ Links ]
XU, X.; CHEN, X.; MA, P.; WANG, X.; JING, X. 2008. The release behavior of doxorubicin hydrochloride from medicated fibers prepared by emulsion-electrospinning. European Journal of Pharmaceutics and Biopharmaceutics 70: 165-170. [ Links ]
YANG, Q.; LI, Z.; HONG, Y; ZHAO, Y; QIU, S.; WANG, C.; WEI, Y 2004. Influence of solvents on the formation of ultrathin uniform poly(vinyl pyrrolidone) nanofibers with electrospinning. Journal of Polymer Science: Part B: Polymer Physics 42: 3721-3726. [ Links ]
Received: 31-Jan-2014
Accepted: 23-Jun-2015
Suggested citation:
BISOTTO-DE-OLIVEIRA, R.; MORAIS, R. M.; ROGGIA, I.; SILVA, S. J. N.; SANT'ANA, J.; PEREIRA, C. N. 2015. Polymers nanofibers as vehicles for the release of the synthetic sex pheromone of Grapholita molesta (Lepidoptera, Tortricidae). Revista Colombiana de Entomologia 41 (2): 262269. Julio - Diciembre 2015. ISSN 0120-0488.