Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488
Rev. Colomb. Entomol. vol.41 no.2 Bogotá July/Dec. 2015
A method for measuring the concentration of CO2 released by entomopathogenic nematodes
Método para medir la concentración de CO2 liberado por nematodos entomopatógenos
PAULO HENRIQUE DE S. SABINO1, ALCIDES MOINO JR.1, VANESSA ANDALÓ2, LIDIANY M. Z. LIMA3 and FERNANDA S. SALES1
1 Department of Entomology, Federal University of Lavras, C. P 3037, 37200-000 Lavras, MG, Brazil. phsabino09@gmail.com. Corresponding author.
2 Federal University of Uberlandia, Department of Agricultural Sciences, C. P 593, 38500-000 Monte Carmelo, MG, Brazil.
3 Department of Chemistry, Federal University of Lavras, C. P 3037, 372000-000 Lavras, MG, Brazil.
Abstract: This study aimed to standardize a method for measuring the concentration of CO2 released by infective juveniles (IJ's), in order to assess their metabolic activity. The nematodes used in this experiment were Heterorhabditis amazonensis JPM4 and Steinernema carpocapsae All. A standardized gas chromatography (GC) method was used for CO2 analysis. There was a linear increase in CO2 concentration associated with increased numbers of IJs. Additionally, the CO2 concentration obtained for S. carpocapsae was higher than that obtained for H. amazonensis. The standardized GC method was adequate to measure the concentration of CO2 released by IJ's.
Key words: Metabolism. Standardization. Steinernematidae. Heterorhabditidae.
Resumen: En el presente trabajo se estandarizó un método para estimar la concentración de CO2 liberada por los juveniles infectantes (JIs) de nematodos, con el objeto de medir su actividad metabòlica. Los nematodos utilizados fueron Heterorhabditis amazonensis JPM4 y Steinernema carpocapsae All. Para el análisis de CO2 fue utilizado el método de cromatografía de gases. Hubo un aumento linear en la concentración de CO2 relacionado con el de la concentración de los JIs. Igualmente se encontró mayor la concentración de CO2 en S. carpocapsae en relación con H. amazonensis. La estandarización de la metodología fue adecuada para medir la concentración de CO2 liberada por los JIs.
Palabras clave: Metabolismo. Estandarización. Steinernematidae. Heterorhabditidae.
Introduction
Entomopathogenic nematodes (EPNs) are biological control agents of various insect pests due to benefits offered as rapid action on the host, specificities and abilities to adapt to new environments, besides can act synergistically with other entomopathogenic agents (Ferraz 1998). The cycle of parasitism begins with third-stage infective juveniles (IJs), which carry symbiotic bacteria internally. The IJs penetrate the host through natural openings such as the mouth, anus or spiracles or even through the cuticle, as exemplified by some species of the genus Heterorhabditis. Once inside the host, the IJs release their symbiotic bacteria (Poinar 1990). These bacteria multiply rapidly, causing septicemia and death of the host within 24 to 48 h (Grewal 2001).
Nematodes are aerobic organisms that can enhance their survival under conditions of low oxygen availability by inducing a state of dormancy (anaerobiosis) (Glazer 2002). The ability of nematodes to survive in aerobic and anaerobic conditions is highly variable among species and among different life stages within a species (Föll et al. 1999).
The amount of CO2 released by the respiration of microorganisms is one of the most traditional and commonly used methods to measure the metabolic activity of soil microbial populations (Moreira and Siqueira 2006). Respiration reflects the microbiological activity of organisms and can be measured by quantifying the released CO 2 , which results from the activity of aerobic microorganisms (Ramos-Rodrigues et al. 1999). Therefore, both EPNs and mutualistic bacteria have high respiration rates (Smigielski 1994).
In this context, the present study aimed to standardize a method to measure the concentration of CO2 released by the IJs of two species of nematodes to assess their metabolic activity.
Materials and methods
Multiplication of EPNs. The nematodes used were Steinernema carpocapsae All (isolated from a soil sample in North Carolina, USA) and Heterorhabditis amazonensis JPM 4 (isolated from a soil sample in Lavras, Minas Gerais, Brazil), maintained in aqueous suspension (500 IJs/ml) at 16 ± 1 °C at the Laboratory of Insect Pathology, Federal University of Lavras, Minas Gerais, Brazil. The original concentration of 500 IJ/ml was used to multiply the entomopathogenic nematodes. The final concentrations of nematodes to use in the bioassay were obtained from this multiplication. The rearing of the Galleria mellonella specimens followed the methodology adapted by Dutky et al. (1964) using an artificial diet modified by Parra (1998).
The EPNs were multiplied in the last-instar specimens of G. mellonella following the methodology of Kaya and Stock (1997) and maintained in an aqueous suspension at 16 ± 1 °C up to 1 week before using the EPNs in the experiment. Various concentrations of the IJs were prepared in 96-well polystyrene plates used in serological tests. To this end, 0,1 ml of the IJ suspension per well was added to a total of ten wells, thus obtaining the desired amount of IJs in 1 ml of suspension.
Bioassay to measure the release of CO2. The bioassay was performed with five replicates of the following six treatments: control (distilled water), 500, 750, 1,000, 1,250 and 1,500 IJs/ml. One milliliter of nematode suspension and 1 ml of distilled water were added to each 9-ml tube vacuum used for blood collection (Vacuette®). Prior to the CO 2 analyses, the IJs were stored in the tubes for 48 h in a climate-controlled chamber at 27 ± 1 °C and a relative humidity (RH) of 70 ± 10%. The bioassay was conducted under aerobic conditions because oxygen entered the tubes when they were opened to add nematodes. However, this has no effect on the results because the amount is negligible for the analyses.
The CO2 analysis was performed in the Center for Analysis and Chemical Prospecting (Central de Análises e Prospecto Química - CAPQ), Department of Chemistry, UFLA. For the CO 2 analysis, the gas chromatography was performed using a GC-2010 apparatus with a thermal conductivity detector (TCD) at 250 °C with a + 50 mV polarity, an injector temperature of 220 °C and He carrier gas at a linear velocity of 50 cm/s. The initial temperature of the column (Rt-QPLOT - 30 m x 0.32 mm ID x 10 pm) was 50 °C for a period of 3.5 min, followed by a heating ramp of 50 °C/min up to 150 °C and maintenance at that temperature for 2 min. The injection was performed in a 1:20 split mode. The total analysis time was 7.5 min. The quantification was performed via external standardization. The data were subjected to an analysis of variance and a regression analysis using the SISVAR software (Ferreira 2011).
Results and discussion
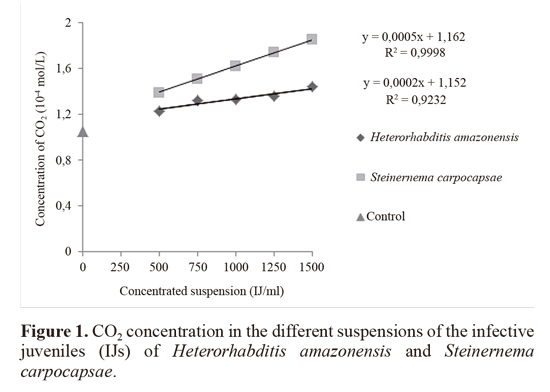
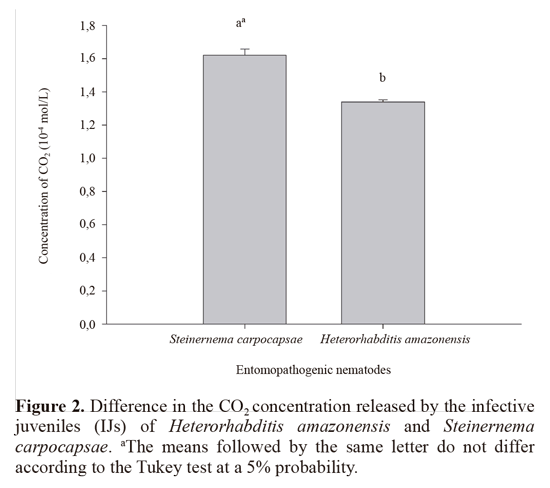
According to the results, there was a linear increase in the CO2 concentration with increasing concentrations of the IJ suspensions for the two species of nematodes (Fig. 1). It was also observed that the IJs of S. carpocapsae resulted in a higher CO2 concentration (1.62 x 10-4 mol/L) compared with the IJs of H. amazonensis (1.34 x 10-4 mol/L) (Fig. 2).
Qiu and Bedding (1999) investigated the effects of aerobic and anaerobic conditions on physiological changes related to the infectivity and survival of the IJs of S. carpocapsae. Under aerobic conditions, the survival rate and infectivity declined to 55% during the eighth week of storage. The IJs became inactive 16 h after incubation in completely anaerobic conditions but could be revived when returned to aerobic conditions for another 7 days. Moreover, a rapid decline in energy reserves, such as trehalose and glycogen, occurs over time under anaerobic conditions, demonstrating that the IJs rely on carbohydrate as an energy source under these conditions; however, the lipid content did not change significantly. The lipid content decreased when the nematodes returned to aerobic conditions, which indicates that lipid catabolism does not occur under anaerobic conditions but only occurs under aerobic conditions (Patel et al. 1997).
The release of CO2 is related to the metabolic activity of organisms; it was found that it is possible to quantify this process via gas chromatography. The proposed method allows the concentration of CO2 released by IJs to be measured in a practical way, which enables the standardization of this method, thus assisting in various studies and enabling a better understanding of the metabolic activities of EPNs.
Acknowledgements
The authors thank the Minas Gerais Research Foundation for financial support.
Literature cited
DUTKY, S. R.; THOMPSON, J. V.; CANTWE, G. E. 1964. A technique for the mass propagation of the DD-136 nematode. Journal of Insect Pathology 6 (4): 417-422. [ Links ]
FERRAZ, L. C. C. B. 1998. Nematóides entomopatogenicos. pp. 541-569. En: Alves, S. B. (Ed.). Controle microbiano de insetos. FEALQ. Piracicaba. 1163 p. [ Links ]
FERREIRA, D. F. 2011. Sisvar: A computer statistical analysis system. Ciencia e Agrotecnologia 35, 1039-1042. [ Links ]
FOLL, R. L.; PLEYERS, A.; LEWANDOUSKI, G. J.; WEMTER, C.; HEGERMANN, V.; PAUL, E. R. J. 1999. Anaerobiosis in the nematode Caenorhabditis elegans. Comparative Biochemistry and Physiology 124: 269-280. [ Links ]
KAYA, H. K.; STOCK, S. P. 1997. Techniques in insect nematology. pp. 281-324. En: Lacey, L. A. (Ed.). Manual of tecniques in insect pathology. California, USA. 281 p. [ Links ]
MOREIRA, F. M. S.; SIQUEIRA, J. O. 2006. Metabolismo e processos microbianos. pp. 163-201. En: Moreira, F. M. S.; Siqueira, J. O. (Ed.). Microbiologia e Bioquímica do solo segunda edijao atualizada e ampliada. Lavras. 729 p. [ Links ]
PARRA, J. R. P. 1998. Criajao de insetos para estudos com patógenos. pp. 1015-1037. En: Alves, S. B. (Ed.). Controle microbiano de insetos. FEALQ. Piracicaba. 1163 p. [ Links ]
PATEL, M. N.; STOLINSKI, M.; WRIGHT, E. D. J. 1997. Neutral lipids and the assessment of infectivity in entomopathogenic nematodes: observations on four Steinernema species. Parasitology 114: 489-496. [ Links ]
POINAR JR, G. O. 1990. Biology and taxonamy of Steinernematidae and Heterorhabditidae. pp. 23-59. En: Gaugler, R. (Ed.). Entomopathogenic nematology. CAB International. Wallingford. 388 p. [ Links ]
QIU, L.; BEDDING, R. 1999. A rapid method for the estimation of mean dry weight and lipid content of the infective juveniles of entomopathogenic nematodes using image analysis. Nematology 1: 655-660. [ Links ]
RAMOS-RODRÍGUEZ, O.; CAMPBELL, J. F.; LEWIS, E. E.; SHAPIRO-ILAN, D. I.; RAMASWAMY, E. S. B. 1999. Dynamics of carbon dioxide release from insects infected with entomopathogenic nematodes. Journal of Invertebrate Pathology 94: 64-69. [ Links ]
SMIGIELSKI, A. J.; AKHURST, R. J.; BOEMARE, E. N. E. 1994. Phase variation in Xenorhabdus nematophilus and Photorhabdus luminescens: Differences in respiratory activity and membrane energization. Applied and Environmental Microbiology 60: 120-125. [ Links ]
Received: 29-Jun-2014
Accepted: 25-Sep-2015
Suggested citation:
SABINO, P. H. DE S.; MOINO JR., A; ANDALÔ, V; LIMA. L. M. Z.; SALES, F. S. 2015. A method for measuring the concentration of CO2 released by entomopathogenic nematodes. Revista Colombiana de Entomologia 41 (2): 280-282. Julio -Diciembre 2015. ISSN 0120-0488.