Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488
Rev. Colomb. Entomol. vol.42 no.1 Bogotá Jan.,/June 2016
SECCIÓN CONTROL / CONTROL
ARTÍCULOS DE INVESTIGACIÓN / RESEARCH PAPER
Entomopathogenic nematodes in the control of cassava root mealybug Dysmicoccus sp. (Hemiptera: Pseudococcidae)
Nematodos entomopatogénicos para el control de la cochinilla de la raíz de la yuca, Dysmicoccus sp. (Hemiptera: Pseudococcidae)
Bruna A. GuideI; Elaine A. SoaresII; Camila R. B. ItimuraIII; Viviane S. AlvesIV
IDoctoranda en Agronomía. Universidade Estadual de Londrina. Cx. Postal 10.011, CEP: 86057-970, Londrina, Paraná, Brasil. bruhguide@gmail.com. Corresponding author
IIGraduada en Biología. Universidade Estadual do Norte do Paraná. CEP: 86300-000, Cornélio Procópio, Paraná, Brasil. elainesoarescp@hotmail.com
IIIM. Sc. Agronomia. Universidade Estadual do Norte do Paraná, CEP: 86360-000, Bandeirantes, Paraná, Brasil. camilabuenp@gmail.com
IVProf. Dr. Universidade Estadual do Norte do Paraná. CEP: 86300-000, Cornélio Procópio, Paraná, Brasil. vivialves@uenp.edu.br
ABSTRACT
The objective of this work was to evaluate the efficiency of entomopathogenic nematodes in the control of cassava root mealybug Dysmicoccus sp. under laboratory and greenhouse conditions. Cochineals were reared in "Cabotiá" pumpkin in climatic chambers at 27 ± 1 °C, with relative humidity (RH) of 70 ± 10%, and without photoperiod. The selection test was carried out with 15 isolates, and the ones which caused the greater percentage of insect mortality were used in concentration tests (0, 5, 10, 20, 50 Infective Juveniles/cm2), in sand column displacement, in the in vivo production of Galleria mellonella larvae, and in pathogenicity tests in greenhouse. The isolates NEPET11 (Heterorhabditis sp.) and RSC05 (H. amazonensis) showed the greatest virulence to cochineals in the selection trial, with mortality percentages of 93% and 90%, respectively, and did not differ between each other. In the concentration test, the isolate NEPET11 showed the greatest insect mortality in lower concentrations. With regard to the displacement test, both isolates showed 100% insect mortality, with no significant difference. In the G. mellonella larval production trial of NEPET11 and RSC05 isolates, approximately 7.0 x 104 and 7.2 x 104 infective juveniles/g larvae were produced, respectively, with no significant difference between treatments. Tests in greenhouse pots did not produced significant results.
Key words: Microbial control. Heterorhabditis. Steinernema. Cassava pests.
RESUMEN
El objetivo de este trabajo fue evaluar la eficiencia de los nematodos entomopatógenos en el control de la cochinilla de la raíz de yuca Dysmicoccus sp. en condiciones de laboratorio e invernadero. Las cochinillas fueron criadas sobre zapallos "Cabotiá" en cámara climática a 27 ± 1 °C, HR: 70 ± 10% y sin fotofase. Fue realizado una prueba de selección con 15 aislamientos y los que causaron mayor porcentaje de mortalidad en los insectos fueron utilizados en pruebas de concentraciones (0, 5, 10, 20, 50 juveniles infectivos/cm2), desplazamiento en columna de arena, producción in vivo en larvas de Galleria mellonella (L.) (Lepidoptera: Pyralidae) y prueba de patogenicidad en invernadero. Los aislados NEPET11 (Heterorhabditis sp.) y RSC05 (H. amazonensis) fueron los que presentaron mayor virulencia sobre las cochinillas en el ensayo de selección con porcentaje de mortalidad del 93% y el 90%, respectivamente, no sin diferencia estadística entre ellos. En la prueba de concentraciones, el aislado NEPET11 presentó mayor mortalidad en los insectos en menores concentraciones ensayadas. En relación con la prueba de desplazamiento, ambos aislados presentaron 100% de mortalidad de los insectos, sin diferencia significativa entre ellos. En el ensayo de producción en larvas de G. mellonella de los aislados NEPET11 y RSC05, la producción final fue aproximadamente 7.0 x 104 y 7.2 x 104 juveniles infectivos/g de larvas, respectivamente, sin diferencia significativa entre los tratamientos. La prueba en macetas en invernadero tampoco presentó resultados significativos.
Palabras clave: Control microbiano. Heterorhabditis. Steinernema. Plagas de yuca.
Introduction
Cassava culture (Manihot esculenta Crantz) is of major importance for tropical regions in the world, particularly in developing countries, where it plays major role in the feeding of more than 500 million people. This is due to the high productivity of carbohydrates per area, and for being a culture that does not need technological resources for its production (Cock 1982; Takahashi and Gonçalo 2005; FAO 2013).
In Brazil, it is grown mainly in the north, northeast and south regions, and plays important role in human and animal feeding. Moreover, it is used as raw material for several industrial products, placing the country as the forth greatest cassava producer, with approximately 20 million tons/year (Otsubo et al. 2002; Seab 2012; FAO 2013). However, studies have proved significant reduction in root production, which is related to the scarce knowledge concerning insect pests that occur in the culture, and to scarce alternatives of management and control (Pietrowski et al. 2010; Oliveira and Paula-Moraes 2011).
Among important insect pests of this culture, cassava root mealybug Dysmicoccus sp. (Hemiptera: Pseudococcidae), which is found in the center-south and south regions of the country (Pietrowski et al. 2010), stands out for being a sap sucking insect of tuberous roots. Thus, it reduces storage accumulation and delays the plant's development, causing direct damage to productivity (Takahashi and Gonçalo 2005; Oliveira et al. 2005; Pietrowski et al. 2010).
Both, study and control of this insect are difficult due to its cryptic habits, as it is sheltered and protected under the soil, preventing the action of most of its natural enemies (Souza and Ribeiro 2003; Alves et al. 2009a). Besides, it is important to emphasize that there are no records of efficient products for cassava culture, allowing significant losses in areas of great occurrence of these pests (Pietrowski et al. 2010).
On the other hand, cochineals might be easy target for entomopathogenic nematodes (EPNs) (Rhabditida: Heterorhabditidae and Steinernematidae), which are naturally found in the soil, and which can also adapt themselves to this environment when they are released in directed applications. Thus, they are suggested for the control of insects that spend at least one stage of life cycle in the soil (Grewal et al. 2001), such as cochineals. Consequently, EPNs might be a promising alternative for the control of these pests (Stuart et al. 1997; Lewis et al. 2006; Alves et al. 2009a).
In studies carried out with coffee cultures (Andaló et al. 2004; Alves et al. 2009a, b), it was observed that some isolates of entomopathogenic nematodes proved to be virulent to coffee-root-cochineal Dysmicoccus texensis (Tynsley) (Hemiptera: Pseudococcidae). Since Dysmicoccus sp. has been identified as a close species to D. texensis, it is possible that these nematodes are an alternative for Dysmicoccus sp. control. Thus, the objective of this work was to evaluate the potential of the use of EPNs as control agents of cassava root mealybug, Dysmicoccus sp.
Material and methods
Experiments were carried out in the Laboratory of Entomology and Microbial Control (LECOM) and in a greenhouse of the State University of North Paraná (UENP) - Cornélio Procópio campus, in 2013.
Dysmicoccus sp. rearing. Insects were obtained from infected cassava plants in commercial areas, in the cities of Nova Londrina and Diamante do Norte, Paraná. Insects were collected in field and further sent to the Laboratory of Entomology and Microbial Control of UENP, where rearing was established. For this, "Cabotiá" pumpkins (hybrid from the species Cucurbita maxima Duchesne x Cucurbita moschata Duchesne) were used as substrate, preferably those with shriveled peel, with stem, and without lesions. Pumpkins were previously washed with water and soap, and were soaked in sodium hypochlorite solution (1%) for about ten minutes for sterilization. After that, they were put to dry on paper towel. Later on, collected insects were removed from infected plants and placed in the pumpkins with the aid of fine brushes. Once infected, the pumpkins were placed in medium-sized plastic trays and kept in climatic chamber at 27 ± 1 ºC, RH 70 ± 10%, and without photoperiod (Guerreiro et al. 2003; Alves, et al. 2009a, b).
Entomopathogenic nematodes isolates. Nematodes were obtained from the inoculation of isolates provided by Brazilian institutes, which were partners during the development of the present study [Embrapa Wheat (Passo Fundo - RS), the Federal University of Lavras (Lavras- MG), and the Biological Institute (Instituto Biológico) (Campinas - SP)]. For isolates multiplication, it was used Galleria mellonella (L.) larvae (Lepidoptera: Pyralidae), which were reared in laboratory, at room temperature, on the modified artificial diet of Parra (1998). When necessary, isolates were multiplied in Galleria mellonella last-instar larvae, according to the methodology described by Molina and Lopes (2001). After larvae infection confirmation, these larvae were transferred to dry chamber and kept in climatic chamber at 23 ± 1 ºC, without photoperiod, for five days. Afterwards, larvae were transferred to White traps (White 1927) for the collection of infective juveniles (IJs). Traps were kept under the same conditions mentioned above. IJs in aqueous suspensions (distilled water + IJs) were daily collected and stored in plastic recipients, which were kept in climatic chamber at 16 ± 1 ºC, without photoperiod, for a maximum period of seven days after production, in order to be further used in bioassays.
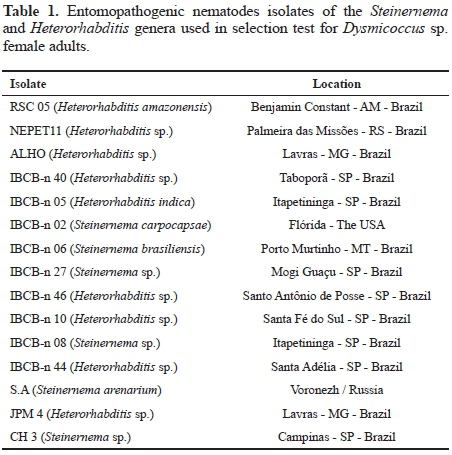
Selection of entomopathogenic nematodes isolates. For the selection trial, 15 entomopathogenic nematodes were evaluated (Table 1). Each treatment was replicated five times, and each plot corresponded to a plastic cup containing 70 g sterile sand and a 3 cm2 piece of "Cabotiá" pumpkin, using paraffin at the bottom, where ten insects were placed (adult females). Insects were covered with sand. Afterwards, isolates were inoculated (100 IJs/cm2 + distilled water, totaling 10 mL aqueous suspension) (Alves et al. 2009a). Cups were closed with plastic lids with holes and kept in climatic chamber at 25 ± 1 ºC, 70 ± 10% RH, without photoperiod. An additional treatment (control) was carried out, which received distilled water. Evaluation was carried out five days after inoculation. Dead insects were counted and the confirmation was carried out by means of stereoscopic microscope dissection. Data were subjected to analysis of variance (ANOVA), and means were compared by the Scott-Knott test (P < 0.05), using the statistics software SISVAR 5.4 (Ferreira 2011).
Concentrations test. The two isolates which proved to be more virulent to Dysmicoccus sp. were selected: Heterorhabditis amazonensis (RSC05) Andaló, Nguyen & Moino Jr., and Heterorhabditis sp. (NEPET11). Both isolates were tested in five different concentrations: 0 (control), 5, 10, 20, 50 IJs/cm2, and the same methodology of the isolates selection test was used. Five days later, evaluation was carried out, and results were subjected to regression analysis by the computer program Excel 2010, for the determination of the regression curve and of the equation of regression for the evaluated interval.
Vertical displacement in sand column. Nematodes used in this process were the same as those used in concentrations test. The experiment was carried out under laboratory conditions, with five replications. Each plot consisted of a 5 cm height and 5 cm diameter PVC tube, which was placed on the base of a 9 cm diameter Petri dish. A 3 cm2 piece of "Cabotiá" pumpkin with paraffin at the bottom was placed at the bottom of the Petri dish, where ten Dysmicoccus sp. female adults were placed. The tube was then filled with sterilized sand to the top (approximately 80 g), and then nematodes suspension was applied at the concentration of 100 IJs/cm2 on the surface area of the tube, with the addition of distilled water, totaling 20 mL suspension. In the control, only distilled water was applied. PVC tubes were covered with the cap of the Petri dish and kept in climatic chamber at 25 ± 1 ºC, RH of 70 ± 10%, and without photoperiod. Evaluations were carried out five days later. Dead insects were counted and the confirmation was carried out by means of stereoscopic microscope dissection. Mortality data were subjected to analysis of variance and to the Scott-Knott mean test (P < 0.05) by using the computer program Sisvar (Ferreira 2011), in order to compare the means.
In vivo production of Heterorhabditis amazonensis (RSC05) and Heterorhabditis sp. (NEPET11) isolates in Galleria mellonella larvae. Isolates were multiplied according to the previously described methodology, and trials consisted of two treatments (two nematodes isolates: Heterorhabditis amazonensis - RSC05, and Heterorhabditis sp. - NEPET11). Each treatment had four replications, and each plot consisted of a 9 cm of diameter Petri dish. Two paper filters and ten G. mellonella larvae were placed in the Petri dish, and were weighed and selected by the size. Subsequently, with the aid of a micropipette, isolates were applied at concentration of 50 IJs/cm2, totaling approximately 2 mL suspension (Molina et al. 2004). Dishes were tapped and sealed with PVC film and kept in climatic chamber for 72 hours, at 24 ± 1 ºC, without photoperiod. After mortality confirmation, larvae were transferred to new Petri dishes containing only a clean and dry filter paper. They were kept for five days in climatic chamber at 24 ± 1 ºC, without photoperiod, in order to confirm the nematodes symptoms. Five day later, larvae were placed in White traps (one plot per trap). It was carried out daily collection of the IJs, which were properly quantified for the evaluation of production in distilled water suspension. Collection and quantification were repeated until the larvae production depletion. Data were subjected to analysis of variance and to the Scott-Knott mean test, by using the computer program Sisvar (Ferreira, 2011). Regression analysis was also carried out, using the computer program Excel, for the determination of the regression curve, and for the comparison of production between the two isolates during production.
Greenhouse test. Thirty stem cuttings of Caiuã cassava were previously planted in five liter pots, filled with soil and fertilizer, following the recommendations for the culture (Takahashi and Gonçalo 2005). When stem cuttings sprouted (with four to six leaves), infestation with cassava root mealybug was carried out, placing in each pot a 3cm2 piece of "Cabotiá" pumpkin infected with Dysmicoccus sp. nymphs and adults, in the stem and root for seven days. This procedure was repeated until the confirmation of the infestation by digging around the stem cutting, and by the presence of ants, which may be an indicative of the presence of mealybugs. After infestation confirmation, pots with plants were subjected to the treatments. Isolates Heterorhabditis amazonensis (RSC05) and Heterorhabditis sp. (NEPET11) were applied by means of direct inoculation of aqueous suspension in the soil, next to the plant stem and root, with the aid of a micropipette, at concentration of 100 IJs/cm2 on the surface area of the pot. The control treatment received only 20 mL distilled water. The experiment was carried out in randomized experimental design, with 10 replications. Evaluation occurred seven days after nematodes inoculation. Thus, plants were uprooted, and the total number of alive insects was counted in all root area (Alves et al. 2009a). Results were subjected to the Scott-Knott mean test (P < 0.05) by the statistical computer program Sisvar (Ferreira, 2011). Moreover, in treatments plots in which the isolates were inoculated, it was collected a sample of the soil (100 g), in order to test the persistence of EPNs by the live-bait methodology (Bedding and Akhurst 1975), using G. mellonella larvae.
Results and discussion
Selection of entomopathogenic nematodes isolates. It was observed that all the tested isolates showed pathogenicity to cassava root mealybug, with mortality values between 23.33 and 93.33%, differing from the control. It was also verified that isolates belonging to the Heterorhabditis genus were more virulent to insects when compared with isolates of the Steinernema genus, reaching mortality percentage up to 93.33%, at concentration of 100 IJs/cm2 (Table 2). Similarly, Alves et al. (2009a), when evaluating the action of ENPs isolates in different concentrations on Dysmicoccus texensis, verified that all the treatments were pathogenic to coffee-root cochineal, showing 100% mortality under laboratory conditions for the isolate CCA (Heterorhabditis sp.). The authors also observed that, in general, isolates belonging to the Heterorhabditis genus were more virulent to insects when compared to the Steinernema genus, which is in agreement with the data obtained in the present work. Also, Andaló et al. (2004) carried out selection test of nematodes and fungi isolates, aiming to control D. texensis, and observed that nematodes were more efficient than fungi, with mortality of up to 78% and 62%, respectively. However, the authors verified that the nematode isolates of Steinernema carpocapse Weiser was more efficient in cochineal, causing up to 78% mortality, which is different from the results obtained in this work, since the same isolate caused 61.66% mortality in cochineals. Moreover, Stuart et al. (1997), when evaluating the pathogenicity of different isolates on Dysmicoccus vacini, verified that isolates of the Heterorhabditis genus were more virulent to the insect, showing mortality of up to 90%.
Higher susceptibility to Heterorhabditidae can be partially explained by their small size, since Steinernematidae are bigger, and may present difficulty in the penetration of smaller insects by natural openings, such as cassava root mealybugs (Stuart et al. 1997; Lewis et al. 2006; Alves et al. 2009a). Geden et al. (1985) also emphasize that, besides Heterorhabditidae being smaller, they have small cephalic appendages, which allow the nematodes to penetrate in the insect by breaking its tegument. Besides, according to Grewal et al. (2001), the behavioral characteristics of both nematodes and host insect can influence the nematodes efficiency.
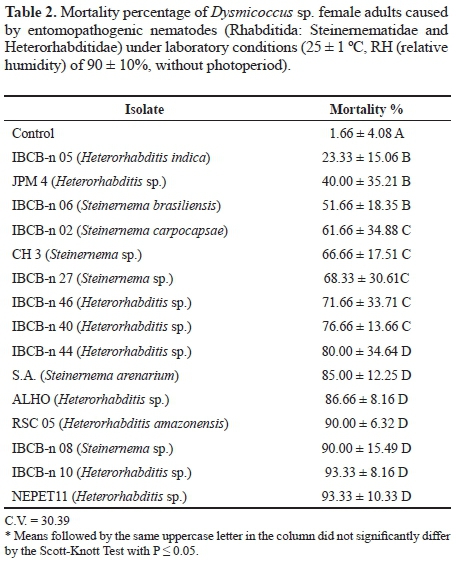
Concentrations test. Two isolates were selected for concentrations test, and lethal concentration (LC95) was approximately 10 IJs/cm2 for NEPET11, and 50 IJs/cm2, for RSC05, demonstrating that the former shows greater virulence to cassava root mealybug (Fig. 1). Alves et al. (2009a) also observed that the isolates chosen for the concentration test, although they had similar results in the selection test, they showed different CL95 values. Moreover, according to Lewis et al. (2006), each isolate has different specificity depending on the host. This specificity is related to several factors, such as the nematode's efficiency to reach the host, to penetrate it and cause infection; and the capacity to dribble the immunological system of the insect, so that the immunological system is unable to fight the nematode, which can justify the different virulence values observed in the present study.
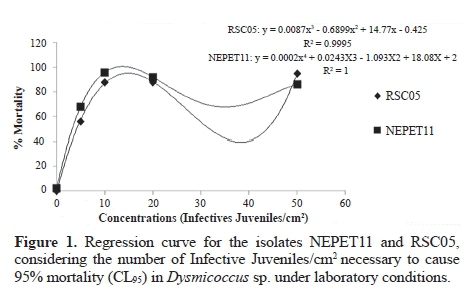
Vertical displacement in column. In sand column displacement test, it was observed that the two tested isolates differed from the control, causing 100% mortality for both, with no differences between the isolates (Table 3). Alves and Moino Jr. (2009) also carried out sand column displacement test aiming to control Dysmicoccus texensis with the isolates CCA and JPM3. The authors observed difference regarding the tested concentrations, but not between the evaluated isolates. In this work, it was also evident that cannot be a requirement for the choice of the isolate for cochineal control, since, apparentlly, both showed cruiser" habit, and both displaced easily in the sand column. Moreover, according to Lewis et al. (2006), each isolate has different specificity depending on the host. This specificity is related to several factors, such as the nematode's efficiency to reach the host, to penetrate it and cause infection; and the capacity to dribble the immunological system of the insect, so that the immunological system is unable to fight the nematode, which can justify the different virulence values observed in the present study.
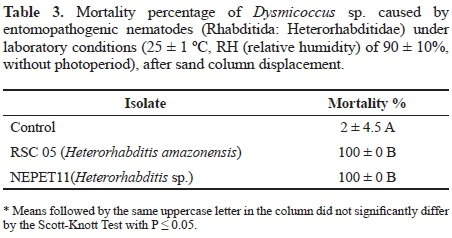
Production in vivo of Heterorhabditis amazonensis (RSC05) and Heterorhabditis sp. (NEPET11) isolates in Galleria mellonella larvae. At the end of the production trial of the isolates NEPET11 and RSC05 in G. mellonella larvae, the final production was approximately 7 x 10-4 and 7.2 x 10-4 IJs/g larvae, respectively. Therefore, there was no significant difference between treatments (Table 4). On the other hand, it was possible to observe that isolate NEPET11 showed significant difference in relation to the production period, reaching maximum value at the 5th day of evaluation, while isolate RSC05 reached production peak at the 7th day (Fig. 2). Barbosa (2005), when evaluating different production systems of the isolate Heterorhabditis bacteriophora in G. mellonella larvae, observed production of 4 x 10-5 IJs/g larvae in the method of the White trap method. Also, Bortoluzzi et al. (2013), when evaluating the production of isolates CB24 and CB40 in G. mellonella, verified production of 2.2 x 106 and 2.2 x 106 IJs/g larvae, respectively, by the White trap method.
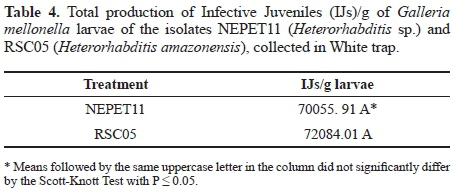
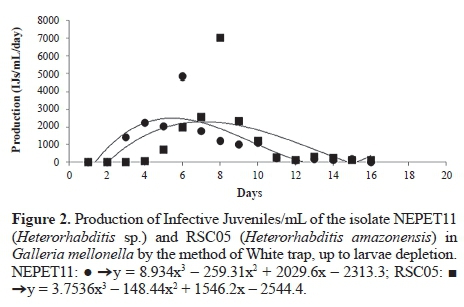
G. mellonella larvae are considered susceptible host to entomopathogenic nematodes, and can offer production above 1,0 x 105 IJs/g (Gaugler and Han 2002). Nevertheless, differences of infectivity and multiplication between ne-matodes species can be higher or lower, even for a susceptible host, as in the case of G. mellonella (Ozer and Unlu 2003). Thus, it is possible to explain the differences of the results of this study with those obtained by Barbosa (2005) and Bortoluzzi et al. (2013). Furthermore, Molina et al. (2004), when testing the production of different isolates in different hosts, such as G. mellonella, observed that the greatest production of IJs was at the first three days. Between the 7th and 8th day, it was observed decrease, until it reached depletion. In this work, IJs production peaks were observed between the 5th and 7th day of evaluation and decrease/depletion was observed between the 10th and 16th day. According to Ehlers (2001), the availability of food may influence the permanence of the nematodes inside the host and the formation of new generations. This fact can explain the difference of the results obtained by Molina et al. (2004) with the results of the present study. In this sense, when using EPNs as agents to control pests under field conditions, factors such as high infectivity, IJs emergency speed, and greater productivity in a shorter period of time are fundamental, since the success of the use of EPNs in IPM's programs is related to the possibility of its production in large scale (Barbosa 2005). Thus, in this work, NEPET11 is the most recommended isolate, since it presents production peak faster than RSC05. Besides, collections of infective juveniles must be carried out at the first days of emergency, since IJs collected at the last days may present low quality and low virulence to insects for being a product of hosts that have already nutritionally depleted (Molina et al. 2004).
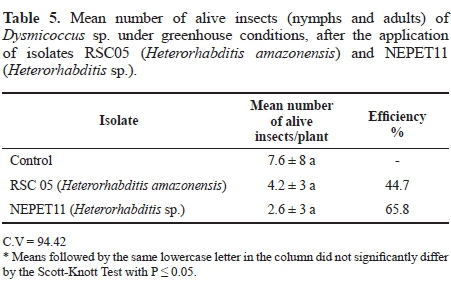
Greenhouse test. In the greehhouse test, it was possible to observe that in the treatments in which RSC05 and NEPET11 isolates were applied, the mean number of alive insects per plant was 4.2 and 2.6, respectively. In the treatment which received only distilled water (control), the mean number of alive insects per plant was 7.6. Although the number of alive insects in the control was higher when compared with the other treatments, there was no significant result (Table 5). However, taking into account the efficiency percentage, NEPET11 had 65,8%, and RSC05 had 44.7%, indicating that there was indeed a reduction of the cochineals populations in the soil. Although the number of insects was low, in the treatments in which it nematodes were applied, dead cochineals with symptoms of nematode infection were collected. When they were dissecated under stereoscope microscope, they showed nematodes inside them. Alves et al. (2009b) also carried out pathogenicity tests of the isolates CCA and JPM3 in Dysmicoccus texensis, in plots in greenhouse, applied by the method of aqueous suspension, and obtained values of 28% and 68%, respectively, which were higher when compared to those obtained in this work. According to Alves et al. (2009b), several isolates considered to be efficient in pests control under laboratory conditions, when evaluated under field conditions, they might not present the same results, since environmental facts, such as temperature, air and soil humidity, and luminosity, can influence efficiency of the pathogen, as well as the aspects of the host and the isolate (Dowds and Peters 2002). Moreover, when it is thought about the use of EPNs in program of pests control, these factors must be taken into account, since their evaluation over the efficiency of the nematodes under laboratory conditions is not always possible (Alves et al. 2009b).
Regarding the collected soil samples, it was possible to observe that all of them were positive for the presence of entomopathogenic nematodes, indicating that nematodes remained in the soil during the trial, and that they could still be acting on the remnant insect population. Moreover, Alves et al. (2009b), in field study, obtained indices of 83 to 100% recovery for entomopathogenic nematodes isolates, even after 30 days of application.
Based on the presented results, it is possible to infer that entomopathogenic nematodes have potential to control cassava root mealybug, Dysmicoccus sp. However, in field tests need to be carried out in order to prove the nematodes' efficiency in these conditions. Also, further studies are necessary regarding the proper epoch for carrying out control. Technologies of nematodes application in field are also necessary, since cassava is a culture which lacks technologies applied to its production process, and these are challenges for further researches.
Conclusion
All the tested isolates showed pathogenicity to Dysmicoccus sp. The isolates NEPET11 and RSC05 were the ones with the greatest virulence, and differed neither concerning the capacity of sand column displacement, or concerning IJs production in G. mellonella larvae. NEPET11 caused greater mortality in smaller concentrations, and was faster in relation to the emergency of the host cadaver. It is therefore, the most recommended isolate for subsequent tests under field conditions.
Literature cited
ALVES, V. S.; MOINO, J. A.; SANTA-CECILIA, L. V. C.; ANDALÓ, V.; SOUZA, G. C. 2009a. Patogenicidade de nematoides entomopatogênicos a cochonilha-da-raiz-do-cafeeiro Dysmicoccus texensis (Tinsley) (Hemiptera: Pseudococcidae) em laboratório. Arquivos do Instituto Biológico 76: 67-73. [ Links ]
ALVES, V. S.; MOINO JR., A.; SANTA-CECILIA, L. V. C.; ROHDE, C.; SILVA, M. A. T. da. 2009b. Testes em condições para o controle de Dysmicoccus texensis (Tinsley) (Hemiptera: Pseudococcidae) em cafeeiro com nematoides entomopatogênicos do gênero Heterorhabditis (Rhabditida: Heterorhabditidae). Revista Brasileira de Entomologia 53: 139-143. [ Links ]
ALVES, V. S.; MOINO JR., A. 2009. Deslocamento vertical de nematoides entomopatogenicos (Rhabditida: Heterorhabditidae) na busca por Dysmicoccus texensis (Tinsley) (Hemiptera: Pseudococcidae) em laboratório e casa-de-vegetação. Ciência Agrotécnica 33: 971-976. [ Links ]
ANDALÓ, V.; MOINO JR., A.; SANTA-CECILIA, L. V. C.; SOUZA, G. C. 2004. Seleção de isolados de fungos e nematoides entomopatogênicos para a cochonilha-da-raiz-do-cafeeiro Dysmicoccus texensis (Tinsley). Arquivos do Instituto Biológico 71: 181-187. [ Links ]
BARBOSA, C. R. C. 2005. Técnicas de produção in vivo de nematoides entomopatogênicos (Rhabditida: Heterorhabditidae) em Galleria mellonela (L.) (Lepidoptera: Pyralidae) e hospedeiros alternativos. Mestrado em Entomologia. Universidade Federal de Lavras. Lavras, Brazil. 91 p. [ Links ]
BEDDING, R. A.; AKHURST, R. J. A. 1975. Simple technique for the detection of insect parasitic rhabditid nematodes in soil. Nematologica 21: 109-110. [ Links ]
BORTOLUZZI, L.; ALVES, L. F. A.; ALVES, V. S.; HOLZ, N. 2013. Entomopathogenic nematodes and their interaction with chemical insecticide aiming at the control of banana weevil borer, Cosmopolites Sordidus Germar (Coleoptera: Curculionidae). Arquivos do Instituto Biológico 80: 183-192. [ Links ]
COCK, J. H. 1982. Cassava: A basic energy source in the tropics. Science 218: 755-762. [ Links ]
DOWDS, B. C. A.; PETERS, A. 2002. Virulence Mechanisms. pp. 79-93. In: Gaugler, R. Entomopathogenic nematology. CABI, Wallingford, 400 p. [ Links ]
EHLERS, R. 2001. Mass production of entomopathogenic nematodes for plant protection. Applied Microbiology and Biotechnology 56: 623-633. [ Links ]
FERREIRA, D. F. 2011. SISVAR: A computer statistical analysis system. Ciência e Agrotecnologia 35: 1039-1042. [ Links ]
FOOD AND AGRICULTURE ORGANIZATION (FAO). 2013. Save and grow: Cassava. A guide to sustainable production intensification. Available in: http://www.fao.org/docrep/018/i3278e/i3278e.pdf. [Review date: 15 June 2014]. [ Links ]
GAUGLER, R.; HAN, R. 2002. Production technology. p. 289-310. In: GAUGLER, R. ED. Entomopathogenic nematology. Wallingford: CABI Publishing. 400 p. [ Links ]
GREWAL, P. S.; NARDO, E. A. B. DE; AGUILLERA, M. 2001. Entomopathogenic nematodes: Potential for exploration and use in South America. Neotropical Entomology 30 (2): 191-205. [ Links ]
GEDEN, C. J.; AXTELL, R. C.; BROOKS, W. M. 1985. Susceptibility of the lesser mealworm, Alphitobius diaperinus (Coleoptera: Tenebrionidae) to the entomogenous nematodes Steinernema feltiae, S. glasseri (Steinernematidae) and Heterorhabditis heliothidis (Heterorhabditidae). Journal of Entomological Science 20: 331-339. [ Links ]
GUERREIRO, J. C.; BUSOLI, A. C.; BERTI FILHO, E. 2003. Oviposition and predation of Pentilia egena Mulsant (Coleoptera: Coccinelidae) in response to temperature. Scientia Agricola 60 (3): 587-589. [ Links ]
LEWIS, E. D.; CAMPBELL, J.; GRIFFIN, C.; KAYA, H.; PETERS, A. 2006. Behavioral ecology of entomopathogenic nematodes. Biological Control 38 (1): 66-79. [ Links ]
MOLINA, J. P.; LÓPEZ, N. J. C. 2001. Producción in vivo de três entomonematodos con dos sistemas de infección en dos hospedantes. Revista Colombiana de Entomología 27 (1-2): 73-78. [ Links ]
MOLINA, J. P. A.; MOINO JR, A.; CAVALCANTI, R. S. 2004. Produção in vivo de nematoides entomopatogênicos em diferentes insetos hospedeiros. Arquivos do Instituto Biológico 71: 347-354. [ Links ]
OLIVEIRA, C. M.; FIALHO, J. F.; FONTES, J. R. A. 2005. Bioecologia, disseminação e danos da cochonilha-das-raízes da mandioca Prototonia navesi Fonseca (Hemiptera: Margarodidae). Available in: www.cpac.embrapa.br/download/355/t. [Review date: 15 June 2014]. [ Links ]
OLIVEIRA, C. M.; PAULA-MORAES, S. V. 2011. Principais pragas da mandioca no Cerrado. In: Fialho, J. F.; Vieira, E. A. Mandioca no Cerrado: orientações técnicas. Available in: http://www.infoteca.cnptia.embrapa.br/handle/doc/247449. [Review date: 15 June 2014]. [ Links ]
OTSUBO A. A.; MERCANTE F. M.; MARTINS C. S. 2002. Aspectos do Cultivo da Mandioca em Mato Grosso do Sul. Embrapa Agropecuária Oeste, Campo Grande, UNIDERP. 219 p. [ Links ]
OZER, N.; UNLU, I. O. 2003. Evaluation of the reproductive potential and competition between two entomopathogenic nematodes, Steinernema feltiae Filipjev, 1934 (Rhabditida: Steinernematidae) and Heterorhabditis bacteriophora, Poinar 1976 (Rhabditida: Heterorhabditidae). Turkish Journal of Biology 27: 149-155. [ Links ]
PARRA, J. R. P. 1998. Criação de insetos para estudos com patógenos. p. 1015-1037. In: ALVES, S.B. Controle microbiano de insetos. Fealq, Piracicaba, Brazil. 1163p. [ Links ]
PIETROWSKI, V.; RINGENBERGER, R.; RHEINHEIMER, A. R.; BELLON, P. P.; GAZOLA, D.; MIRANDA, A. M. 2010. Insetos praga da cultura da mandioca na Região Centro-Sul do Brasil. 2010. Marechal Cândido Rondon, PR. 42 p. [ Links ]
SECRETARIA DA AGRICULTURA E DO ABASTECIMENTO DO PARANÁ (SEAB). 2012. Cotações mensais de produtos agropecuários. Available in: http://www.seab.pr.gov.br. [Review date: 18 September 2013]. [ Links ]
SOUZA, J. C.; RIBEIRO, J. A. 2003. Cochonilha-da-raiz: cafeicultor conheça e saiba como controlar esta praga com inseticidas neonicotinóides. EPAMIG, Circular Técnica 162. [ Links ]
STUART, R. J.; POLAVARAPU, S.; LEWIS, E. E.; GAUGLER, R. 1997. Differential susceptibility of Dysmicoccus vacinni (Homoptera: Pseudococcidae) to entomopathogenic nematodes (Rhabdita: Heterorhabditidae and Steinernematidae). Journal of Economic Entomology 90 (4): 925-932. [ Links ]
TAKAHASHI, M.; GONÇALO, S. 2005. A cultura da mandioca. Olímpica, Paranavaí, Brasil. 116p. [ Links ]
WHITE, G. F. 1927. A method for obtaining infective nematodes larvae from cultures. Science 66: 302-303. [ Links ]
Received: 26-Oct-2014
Accepted: 22-May-2016














