Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Entomología
Print version ISSN 0120-0488
Rev. Colomb. Entomol. v.42 n.2 Bogotá 2016
Notas científicas / Scientific notes
Facilitated harvesting of eggs from laboratory-reared Chrysoperla externa (Neuroptera: Chrysopidae)
Facilidad de cosecha de huevos de Chrysoperla externa (Neuroptera: Chrysopidae) bajo condiciones de laboratorio
ANA LUIZA VIANA SOUSA1,4, BRÍGIDA SOUZA2,4, CARLOS EDUARDO SOUZA BEZERRA3,4 and BRUNO BARBOSA AMARAL3,4
1 Biologist, Post-Graduate student, sousa.alvs@gmail.com.
2 Agronomist, Professor, brgsouza@den.ufla.br, corresponding author.
3 Agronomist, Ph. D.,carlos.esb@gmail.com; bamaral82@yahoo.com.br.
4 Entomology Department, Federal University of Lavras, 37200-000, Lavras, MG, Brazil.
ABSTRACT
Chrysoperla externa (Neuroptera: Chrysopidae) eggs are attached to the oviposition substrate by long silk stalks. The complete removal of these stalks is crucial for efficient egg release in biological control programs. The present study aimed at establishing an appropriate oviposition substrate and determining the best embryonic stage for submission of C. externa eggs to manual destalking and harvesting. Eggs oviposited on bond or chamois paper substrates were transferred from rearing cages and incubated in a growth chamber under controlled conditions for 24, 48, 72 or 96 hours according to the embryonic stage development required. Substrates were positioned in an inclined tray and softly brushed with a folded rectangle of soft muslin cloth. Destalked eggs were placed individually in microtiter plates and incubated in a growth chamber until hatching. Egg destruction at all embryonic stages and oviposition on the chamois substrate were considerably higher as compared to those from bond paper. Young eggs harvested from chamois paper were particularly susceptible and exhibited 88 % destruction, whereas eggs aged 48, 72 or 96 hours showed < 10 % destruction on both substrates. Viability of eggs collected at 24 hours for both substrates was significantly different from the observed for the other embryonic stages. The method described will contribute to improve the efficiency of manual harvesting of C. externa eggs and can be employed as an alternative to chemical techniques of destalking in mass rearing.
Key words: Destalking. Egg mass production. Manual harvesting. Oviposition substrate. Viability.
RESUMEN
Los huevos de Chrysoperla externa (Neuroptera: Chrysopidae) están unidos al sustrato de oviposición por largos pedicelos de seda, cuya eliminación total es esencial para la liberación eficiente de los huevos en programas de control biológico. El presente estudio tuvo como objetivo el establecimiento de un sustrato de oviposición apropiado y establecer la etapa embrionaria de los huevos de C. externa para retirar en forma manual los pedicelos y proceder a la cosecha de huevos. Los huevos depositados sobre papel sulfito o sobre papel gamuza fueron trasladados de las jaulas de cría e incubados en una cámara de crecimiento, bajo condiciones controladas por 24, 48, 72 ó 96 horas, dependiendo del desarrollo embrionario requerido. En seguida, se colocó el sustrato en una bandeja inclinada y los huevos fueron rozados suavemente con un paño rectangular de muselina doblada. Los huevos sin pedicelo fueron colocados individualmente en placas de microtitulación e incubados en la cámara de crecimiento hasta su eclosión. Los niveles de destrucción de los huevos, en todos los estados embrionarios mantenidos en papel gamuza, fueron considerablemente superiores en comparación con los de papel sulfito. Los huevos jóvenes (24 horas) colectados del papel gamuza fueron particularmente susceptibles y exhibieron 88 % de destrucción, mientras que aquellos de 48, 72 ó 96 horas mostraron, en ambos sustratos, menos de 10 % de destrucción. La viabilidad de los huevos fue afectada significativamente por la fase embrionaria al momento de retirar el pedicelo para los huevos cosechados a las 24 horas, en los dos sustratos. Se espera que el método descrito contribuya a la mejora de la eficiencia en la cosecha manual de huevos de C. externa y que sea utilizado como una alternativa a la técnica química de despedicelamiento, en la cría masiva de esta especie.
Palabras clave: Despedicelamiento. Producción masiva de huevos. Colecta manual. Sustrato de oviposición. Viabilidad.
Introduction
Green lacewings of the genus Chrysoperla (Neuroptera: Chrysopidae) are predatory insects that can play an important role in natura in regulating the populations of various arthropods. The biological control of agricultural pests mediated by such natural enemies has been amply demonstrated in laboratory and other sheltered environments (Carvalho and Souza 2009; Pappas et al. 2011), while beneficial insects, including those of the genus Chrysoperla, are produced and marketed in a number of countries (Tauber et al. 2000). However, the success of a program for the biological control of aphids, thrips and other soft-bodied arthropods depends on the augmentative release of natural enemies and this is only possible when the beneficial insects are available on a large-scale (Van Lenteren 2009).
The mass rearing of insects under laboratory conditions is typically time consuming and labor intensive, especially for insects that exhibit unusual behavior patterns. In the case of green lacewings, the females lay their eggs at the end of ~10 mm long silk stalks that are connected to the oviposition substrate, thereby rendering the removal of eggs one of the most difficult steps in large-scale production (Pinto and Parra 2002; Carvalho and Souza 2009).
For small-scale rearing operations, eggs can be harvested by cutting the stalks with a sharp blade or thin long pointed scissors, but this method is not practical for medium- or largescale production since it requires considerable time and effort. Furthermore, pedicels cannot be completely removed using blades or scissors, and the remaining parts may tangle during storage and transportation thereby inducing aggregation of the eggs. Such egg clusters cannot be distributed uniformly and this may hinder or block the release process. Complete destalking is, therefore, a crucial step in freeing eggs in a mass rearing operation (Finney 1948; Finney 1950; Carvalho and Souza 2009).
Destruction of the stalk may be achieved by treating the eggs with hypochlorite solution containing an appropriate concentration of the sodium (Krishnamoorthy and Nagarkatti 1981; Nordlund and Correa 1995; Ferreira 1997; Amaral 2011), potassium (Nasreen et al. 2004) or calcium (Bezerra et al. 2014) salt. However, this technique needs careful monitoring of the time of exposure of the egg to hypochlorite ion in order to prevent loss of viability of the embryo (Morrison 1977). An alternative method developed for the rapid and efficient collection of Chrysoperla carnea (Stephens, 1836) eggs involves the gentle rubbing of a ball of nylon netting across the oviposition substrate (Ridgway et al. 1970).
While these techniques have proven to be useful for removing the stalks from Chrysopidae eggs, they are known to exert negative effects on viability egg (Nasreen et al. 2004; Carvalho et al. 1998). Thus, there remains a need for developing new methodologies, or improving existing ones, in order to facilitate the mass rearing of green lacewings through increasing the efficiency and decreasing the costs associated with the freeing of eggs. Within this context, the present study aimed at establishing an appropriate oviposition substrate and the best embryonic stage for the submission of C. externa (Hagen, 1861) eggs to manual destalking and harvesting.
Materials and methods
Based on results obtained previously by Amaral (2011), the papers chosen as oviposition substrates for C. externa were bond (216 x 330 mm; 75 g/m2; International Paper, Mogi Guaçu, SP, Brazil) and chamois (60 x 40 cm; 60 g/m2; Art Floc, São Paulo, SP, Brazil). Substrate papers were cut (33 x 21 cm) to fit inside polyvinyl chloride (PVC) cages (21 cm height x 10 cm diameter), and used to line the internal walls of these cages. These substrates were maintained in position for approximately 12 hours, after this time sufficient numbers of eggs had been laid to be used in the experiment. Substrates were removed from the cages and transferred to a growth chamber at 25 ± 1 °C, 70 ± 10 % R.H., and 12L:12D photoperiod, where they remained for 24, 48, 72 or 96 hours according to the embryonic stage development required.
Following incubation, substrates were placed individually in a white tray (30 x 40 cm) maintained at a slight angle to the horizontal, and the eggs were removed manually by brushing the substrates gently with a rectangle of soft muslin cloth folded so as to resemble a sponge. The pressure applied to the cloth was the minimum required to separate the eggs from their stalks. Eggs were collected and placed individually in microtiter plates (Sigma-Aldrich Ltda., SP, Brazil) with 96 wells with volume 400 μL, which were subsequently covered with polypropylene film and incubated in the growth chamber under the described conditions until hatching. The mechanical damage caused to the chorion was verified by examining the eggs under a stereomicroscope.
The percentages of eggs destroyed during harvesting and egg viabilities were evaluated for all treatments using a completely randomized experimental design comprising three repetitions of 40 eggs at the four different embryonic stages from both oviposition substrates, consisting on a 4 x 2 factorial design. Eggs destroyed were not used for the analysis of viability. Data were analyzed using R® software (R Development Core Team 2015) and the level of significance in all tests was established at P ≤ 0.05. All the data were transformed to arcsine in order to meet the assumptions of normality and homogeneity of variances, and then submitted to an analysis of variance (ANOVA) with mean values being compared by the Scott-Knott test.
Results and discussion
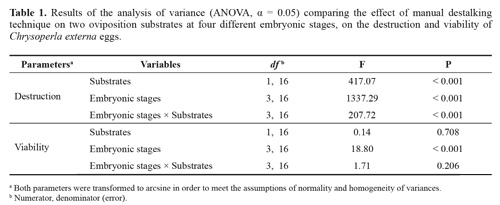
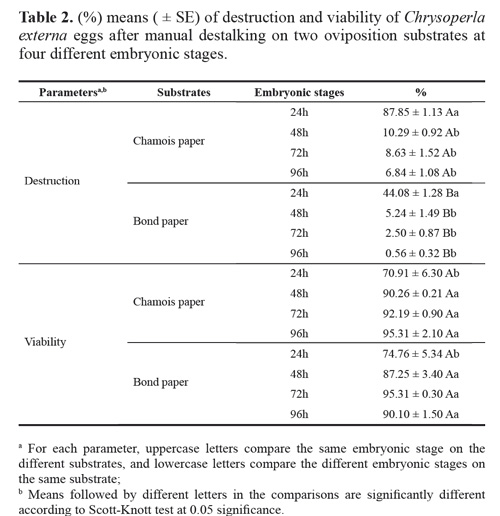
Eggs harvested on the chamois substrate suffered significantly more damage during stalk removal as compared to those deposited on bond paper (F = 417.07, df = 1, 16, P < 0.001) (Tables 1 and 2). This effect was observed at all four embryonic stages and was attributed to the cotton-like texture of the chamois substrate. This type of paper does not allow the eggs to slide over the substrate, requiring greater pressure on the eggs to be destalked, and consequently, causing higher destruction. For chamois and bond paper, the significantly different percentages of destroyed eggs (87.8 and 44.1 %, respectively) were recorded when harvesting was performed at 24 hours (F = 1337.29, df = 3, 16, P < 0.001), indicating that immature eggs were more vulnerable to mechanical manipulation. Eggs aged 48, 72 or 96 hours suffered less damage during destalking, since the percentage of destruction was lower than 10 % in such treatments, although harvesting on the chamois paper significantly destroyed more eggs. These findings are in agreement with those of Carvalho and Souza (2009) who reported that C. externa young eggs are more susceptible to stalk removal because of the delicate and less resistant chorion.
Eggs viability was significantly affected by the embryonic stages at the time of harvesting (F = 18.80, df = 3, 16, P < 0.001) (Tables 1 and 2), but not by the type of paper used as substrate (F = 0.14, df = 1, 16, P > 0.05). The eggs viability collected at 24 hours for both substrates was significantly different from the observed for the other embryonic stages (Table 2). These results suggest that the embryonic stages of the eggs at the time of destalking is of critical importance since those aged < 48 hours are more likely to be damaged during the process because the external membrane is more susceptible to abrasion.
Recent studies have focused on the destalking and harvesting from Chrysopidae eggs through immersion of the oviposition substrate in dilute hypochlorite solutions, although Morrison (1977) has long warned of problems arising from the use of this method in the mass production of natural predators. More than 40 years ago, Ridgway et al. (1970) reported the efficient use of a ball of nylon netting for scraping C. carnea eggs from substrates while simultaneously removing the stalks and recommended that care should be taken with regard to the age of eggs in order to preserve viability. We have revived this idea through the development of a modified method involving the manual brushing of substrates with muslin cloth, a technique that permitted effective destalking while, at the same time, preserving the integrity of the eggs. Application of this method to a laboratory-reared population of C. externa resulted in the successful harvesting of viable stalkless eggs. Based on the results obtained in the present study, scale-up of the method would appear to be perfectly feasible.
Conclusions
Bond paper is a better substrate than chamois paper for the production and subsequent harvesting of C. externa eggs using a technique involving the manual removal of stalks. This methodology can be recommended for destalking eggs aged ≥ 48 hours, and can be employed as an alternative to the chemical method of destalking in mass production of Chrysopidae species.
Acknowledgements
We thank to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for financial support of the study and for MSc scholarship and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for DSc scholarship conceded to ALVS.
Literature cited
AMARAL, B. B. 2011. Otimização da criação de Chrysoperla externa (Hagen, 1861) (Neuroptera: Chrysopidae) visando sua produção em escala comercial [MSc dissertation]. Universidade Federal de Lavras. Lavras. 65 p. [ Links ]
BEZERRA, C. E. S.; NOGUEIRA, C. H. F.; SOUSA, M. M.; SOUZA, B.; ARAUJO, E. L. 2014. Calcium hypochlorite for removing stalks on eggs of the green lacewing Chrysoperla genanigra (Neuroptera: Chrysopidae). Applied Entomology and Zoology 49 (3): 483-486. [ Links ]
CARVALHO, C. F.; CANARD, M.; ALAUZET, C. 1998. Destruction of egg pedicels by sodium hypochlorite and its effects on the hatching of eggs of Chrysoperla mediterranea (Hözel) (Neuroptera: Chrysopidae). Acta Zoologica Fennica 209: 75-77. [ Links ]
CARVALHO, C. F.; SOUZA, B. 2009. Métodos de criação e produção de crisopídeos. pp. 77-115. In: Bueno, V. H. P. (Ed.). Controle biológico de pragas: Produção massal e controle de qualidade, 2nd ed. Editora UFLA. Lavras. 429 p. [ Links ]
FERREIRA, R. J. 1997. Técnicas para a produção massal de crisopídeos (Neuroptera: Chrysopidae) [MSc Dissertation]. Universidade Estadual Paulista, Jaboticabal. 115 p. [ Links ]
FINNEY, G. L. 1948. Culturing Chrysopa californica and obtaining eggs for field distribution. Journal of Economic Entomology 45 (5): 719-721. [ Links ]
FINNEY, G. L. 1950. Mass-culturing Chrysopa californica to obtain eggs for field distribution. Journal of Economic Entomology 43 (1): 97-100. [ Links ]
KRISHNAMOORTHY, A.; NAGARKATTI, S. 1981. A mass rearing technique for Chrysopa scelestes Bank (Neuroptera: Chrysopidae). Journal of Entomological Research 5 (1): 93-97. [ Links ]
MORRISON, R. K. 1977. Developments in mass production of Trichogramma and Chrysopa spp. p. 149-151. In: Proceedings of the Beltwide Cotton Production Research Conference. National Cotton Council. Memphis. 400 p. [ Links ]
NASREEN, A.; MUSTAFA, G.; IQBAL, M.; ASHFAQ, M. 2004. Viability of eggs of green lacewing harvested by AM-Tech and other methods. Pakistan Journal of Biological Sciences 7 (1): 126-127. [ Links ]
NORDLUND, D. A.; CORREA, J. A. 1995. Description of green lacewing adult feeding and oviposition units and a sodium hypochlorite-based egg harvesting system. Southwestern Entomologist 20 (3): 293-301. [ Links ]
PAPPAS, M. L.; BROUFAS, G. D.; KOVEOS, D. S. 2011. Chrysopid predators and their role in biological control. Journal of Entomology 8 (3): 301-326. [ Links ]
PINTO, A. S.; PARRA, J. R. P. 2002. Liberação de inimigos naturais. pp. 191-208. In: Parra, J. R. P.; Botelho, P. S. M.; Corrêa- Ferreira, B. S.; Bento, J. M. S. (Eds). Controle Biológico no Brasil: Parasitoides e predadores, 1st ed. Manole. Barueri. 609 p. [ Links ]
R DEVELOPMENT CORE TEAM. 2015. R: a language and environment for statistical computing. R Foundation for Statistical Computing. Viena. Available at: http://www.Rproject.org [Review date: 10 June 2015] [ Links ].
RIDGWAY, R. L.; MORRISON, R. K.; BADGLEY, M. M. 1970. Mass rearing a green lacewing. Journal of Economic Entomology 63 (3): 834-836. [ Links ]
TAUBER, M. J.; TAUBER, C. A.; DAANE, K. M.; HAGEN, K. S. 2000. Commercialization of predators: Recent lessons from green lacewings (Neuroptera: Chrysopidae: Chrysoperla). American Entomologist 47 (1): 24-50. [ Links ]
VAN LENTEREN, J. C. 2009. Critérios de seleção de inimigos naturais. pp. 11-32. In: Bueno, V. H. P. (Ed.). Controle biológico de pragas: Produção massal e controle de qualidade, 2nd ed. Editora UFLA. Lavras. 429 p. [ Links ]
Received: 25-Jun-2014
Accepted: 14-Nov-2016