Introduction
The Neotropical predator Chrysoperla externa Hagen (Neuroptera: Chrysopidae) is associated with several agricultural crops. This species exhibits a high degree of adaptability to different environmental conditions as well as a robust reproductive potential, an efficient predatory capability, and a ubiquitous distribution from the Antilles in the Caribbean down to the north of the Patagonia in Argentina (Adams and Penny 1987). Larvae of C. externa are effective natural enemies of aphids (Hemiptera: Aphididae), white flies (Hemiptera: Aleyrodidae), thrips (Thysanoptera: Thripidae), various families of lepidopterans (Lepidoptera), and acari (Arachnida: Acari) (Carvalho et al. 2002; Oliveira et al. 2009). Therefore, C. externa has been identified as a good candidate for being an effective biological control agent. During recent years the massive rearing of this species has been promoted along with its release in the field in South-American countries (Carvalho et al. 2002; Pappas et al. 2011).
Biological control and selective pesticides have proved to be compatible with Integrated Pest Management (IPM) (Kogan 1998; Galvan et al. 2005). Although chemical control should be the final option in an IPM program, several agroecosystems depend on pesticide applications, and then their lethal and sublethal effects against beneficial organisms must be known (Shinde et al. 2009). Moreover, conventional insecticides in Argentina continue to be the first choice due to the low cost and, sometimes, the lack of information about alternative measures (Villaamil Lepori et al. 2013; Pórfido et al. 2014). In order to maximize the compatibility of natural enemies with chemicals, studies about effects of pesticides on non-target insects are necessary (Medina et al. 2003; Desneux et al. 2007).
Studies of pesticides on different stages of C. externa have been evaluated in the last decades, most of all on larvae, the predator stage. Eggs are not mobile and could be laid on unprotected spaces and therefore be exposed to pesticides (Soares et al. 2002). The importance of the toxicity assessment on beneficial insects’ eggs resides in the fact that the establishment of the population depends on them and could be affected by pesticides applications (Castilhos et al. 2014). Among the groups of chemicals that have been analyzed on C. externa eggs are pyrethroids, neoniconidoids, IGR’s, botanicals, organophosphates, organochlorines (Iannacone and Lamas 2003; Bueno and Freitas 2004; Godoy et al. 2004), and chrysopids have shown a differential susceptibility against them.
The pyrethroid cypermethrin is a broad-spectrum insecticide, with a long-term residual effect. It has been used indiscriminately since its synthesis in the 60‘s, with negative effects as the development of insecticide resistance (Zhong et al. 2013). In the 90‘s, neonicotinoids like acetamiprid started to be commercialized, classified as biorational insecticides by the United States-Environmental Protection Agency (US-EPA) due to their low toxicity in non-target organisms. However, subsequent studies have demonstrated a high toxicity to pollinators (Laycock et al. 2012; Whitehorn et al. 2012; Rundlöf et al. 2015), and nowadays the classification is being reconsidered (United States-Environmental Protection Agency 2016). The botanical insecticide azadirachtin has been commonly reported as a biorational insecticide with low toxicity to vertebrates, high selectivity and short-term residuality; however, its mode of action and biosynthesis are not well known (Mordue 2004). Pyriproxyfen is an insecticide commonly used in Argentina, classified as an insect growth regulator (IGR) that mimics the action of the juvenile hormone (Pórfido et al. 2014) and whose insecticidal activity occurs upon either contact or ingestion.
The aim of this study was to assess both lethal and sublethal effects of the conventional and biorational insecticides cypermethrin, acetamiprid, azadirachtin, and pyriproxyfen after application on eggs of C. externa under laboratory conditions.
Materials and methods
Insects
Chrysoperla externa colony was maintained in a rearing chamber under controlled conditions (25 °C ± 2; 70 % ± 5 RH; 16:8 L:D). Adults were fed on artificial diet according to Vogt et al. (2000) and reared in plastic containers ventilated in the upper side with a fine mesh. Containers were covered inside with black cardboard as oviposition substrate. Eggs were removed from the container and larvae were fed on nymphs and adults of Rhopalosiphum padi L. (Hemiptera: Aphididae) and a dietary supplement (Haramboure et al. 2016).
Insecticides
The following insecticides were used: Glextrin 25® (cypermethrin 25 % w/v, EC, Gleba S.A.), Mospilan® (acetamiprid 20 % w/w, WP, Summit-Agro S.A.), Neem-Azal® (azadirachtin 1.2 % w/v, EC, Agristar S.A.) and Epingle® (pyriproxyfen 10 % w/v, EC, Summit-Agro S.A.). Maximum field recommended concentrations (MFRC) were evaluated for each treatment: 25 mg a.i./L cypermethrin, 200 mg a.i./L acetamiprid, 40 mg a.i./L azadirachtin and 75 mg a.i./L pyriproxyfen. Dilutions were made using distilled water and controls were treated with distilled water alone. The term conventional insecticide will be used for cypermethrin, and biorational insecticide for acetamiprid, azadirachtin and pyriproxyfen, throughout the text.
Bioassays
Eggs of the same cohort of less than 24 h laid were treated. Little pieces of cardboard with eggs were cut and immerse for 5 s in a 10 mL glass beaker with the dilutions. Three replicates of 20 eggs were treated for each chemical and fallowed during the life cycle until the adult stage. After that, eggs were maintained in Petri dishes under the controlled conditions before mentioned; recently hatched larvae were kept individually in 2 cm diameter x 1 cm height plastic vials until pupation. Individuals that survived to adults were identified by gender through the external genitalia, then paired in five couples and kept in 4 cm diameter x 6 cm height cylindrical vials.
Eggs viability and survival of their offspring were evaluated, as well as the developmental time, preoviposition period (time from adult emergence to first oviposition), and five-days-accumulated fecundity (total number of eggs laid during five days) and fertility (total number of hatched larvae from those eggs).
Statistics
Kaplan-Meier curves were calculated to compare probability of death between the treatments, from egg to adult stage. Survival curves were compared through long-rank tests with χ². Those individuals who reached the adult stage were censured data.
Egg viability, developmental time of eggs, larvae, and pupa, fecundity and fertility were analyzed by ANOVA. When normality and homoscedasticity assumptions were not accomplished they were analyzed by the non-parametric Kruskal-Wallis test. Means or medians, respectively, were compared using LSD or Box and Whisker Plot tests. P ≤ 0.05 was considered significant. SPSS Software was used.
Results and discussion
Viability and survival curves
The viability of eggs treated with the four insecticides did not differ from the control, which was of 100 % (F = 2.83; df = 4, 10; P = 0.082). The chorion of chrysopids eggs has a proteic, crystalline component arranged in layers (Furneaux and Mackay 1972); this natural barrier which encloses the embryo could lend to mechanical resistance, being this stage one of the most tolerant to insecticides (Silva et al. 2012; Rugno et al. 2015). Similar results were obtained after exposition of eggs from the same species to different insecticides (Rimoldi et al. 2008; Silva et al. 2012). On the other hand, Rugno et al. (2015) recorded a high mortality of the chrysopid Ceraeochrysa cubana Hagen (Neuroptera: Chrysopidae) treated with the neonicotinoid imidacloprid. Even though they belong to the same family, chorion of C. cubana eggs could be formed by a different structure from those of C. externa, conferring a higher susceptibility. As Castilhos et al. (2014) have hypothesized, oil-based formulations could form a layer around the egg, blocking the micropile not allowing air interchange between the embryo and the environment causing asphyxia and dead. In the present study, oil solvent based insecticides were cypermethrin, azadirachtin and pyriproxyfen, and the wettable powder one was acetamiprid. Even so, the eclosion was of 100 % in all treatments and the developmental period between eggs to larvae was the same, showing that asphyxia did not happen and neither did the penetration/absorption of the insecticides.
Larvae from eggs treated with cypermethrin died within the first 48 h, whilst those treated with acetamiprid, azadirachtin and pyriproxyfen survived to the adult stage in percentages of 75 %, 70.6 % and 84.6 %, respectively (Fig. 1 and Table 1). During the eclosion neonates had to contact the insecticides in the chorion surface, exposing themselves to their toxicity. The deleterious effect of cypermethrin agrees with that of lambda-cyalothrin on C. cubana (Rugno et al. 2015). On the contrary, results obtained by Silva et al. (2012) have suggested that the viability of eggs of C. externa would not vary after exposition to betacyfluthrin. Although both insecticides belong to the same chemical group, pyrethroids are formed by mixtures of cis and trans isomers, with more or less insecticide effect, respectively. Depending on the percentage of the isomers in the given pyrethroid, the insecticidal effect is different (Aldridge 2013; Casida 2013). In this case, cypermethrin has 48-50 % of cis isomers and betacyfluthrin 30-40 % (Vodeb and Petanovska-Ilievska 2006); consequently, cypermethrin must be more toxic. It is also important to take into account the exposition method; in Silva et al. (2012) the eggs were sprayed by a Potter’s tower Residual tests on Chrysoperla carnea Stephens (Neuroptera: Chrysopidae) eggs with different groups of pesticides have shown a differential viability according with the treatment, being acetamiprid and imidacloprid highly toxic compared to IGR’s, which did not differ to the control (Nasreen et al. 2005). Medina et al. (2003) have hypothesized that C. carnea performs a rapid excretion of pyriproxifen once inside the body, so it could explain the low toxicity. Neonates mortality within the first 24 h of treated was related to the insecticide residual in the chorion when the egg hatches, in the same line as the present study. Also, Cloyd and Bethke (2011) concluded that acetamiprid residues were toxic to newly hatched nymphs of the plant bug Deraeocoris brevis Uhler (Hemiptera: Miridae), but did not affect the egg hatch.
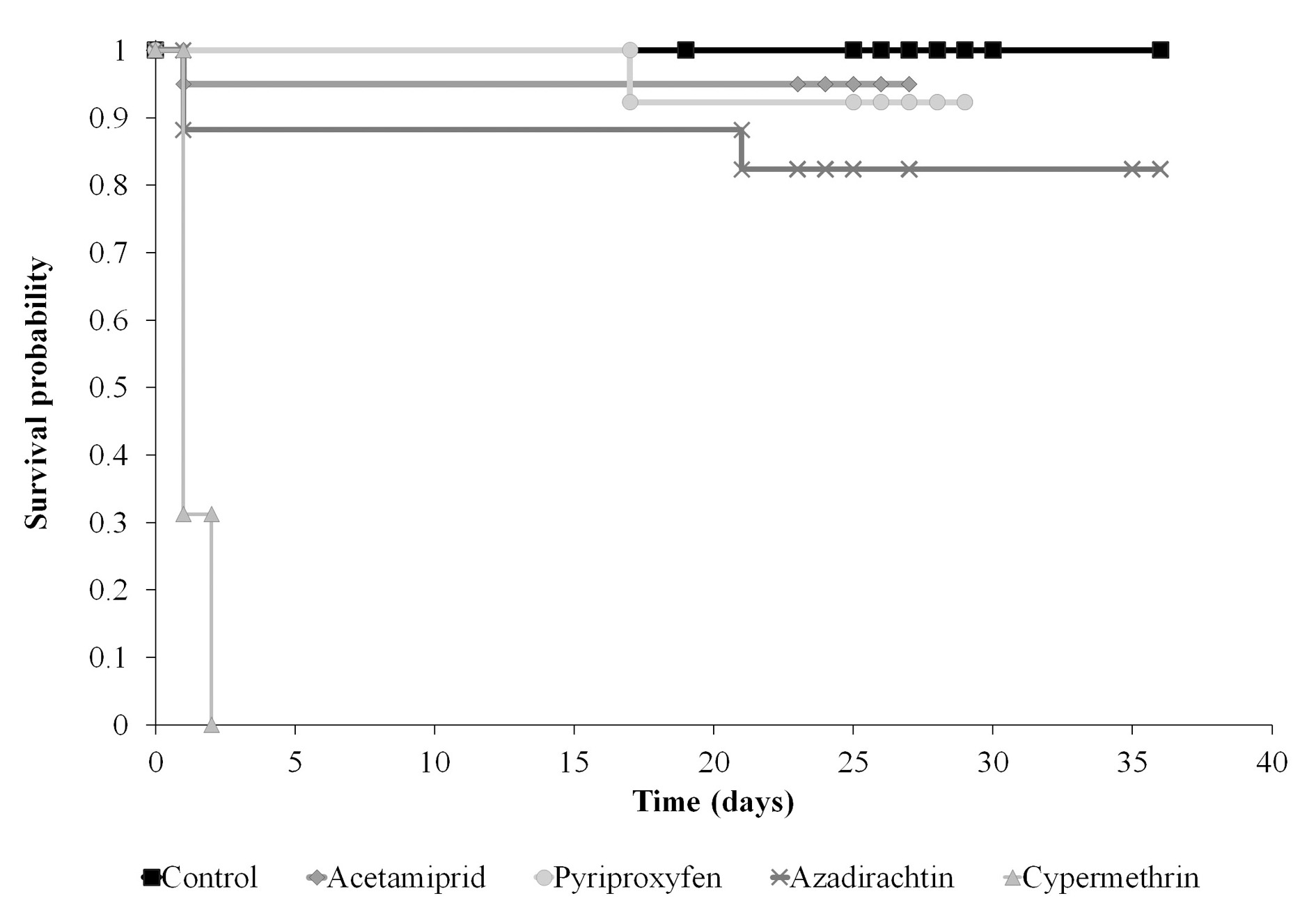
Figure 1 Insecticide effect on the survival of Chrysoperla externa offspring. Kaplan-Meier, χ2, log rank test, P ≤ 0.05.
Table 1 Effect of cypermethrin, acetamiprid, azadirachtin and pyriproxyfen on the survival of Chrysoperla externa offspring. Kaplan-Meier, χ2 log rank test, P ≤ 0.05.
| χ 2 | df | P | |
|---|---|---|---|
| Global comparison | 85.08 | 4 | < 0.001 |
| Control - Cypermethrin | 39.22 | 1 | < 0.001 |
| Control - Acetamiprid | 5.75 | 1 | 0.016 |
| Control - Azadirachtin | 5.86 | 1 | 0.015 |
| Control - Pyriproxyfen | 5.92 | 1 | 0.015 |
Developmental time
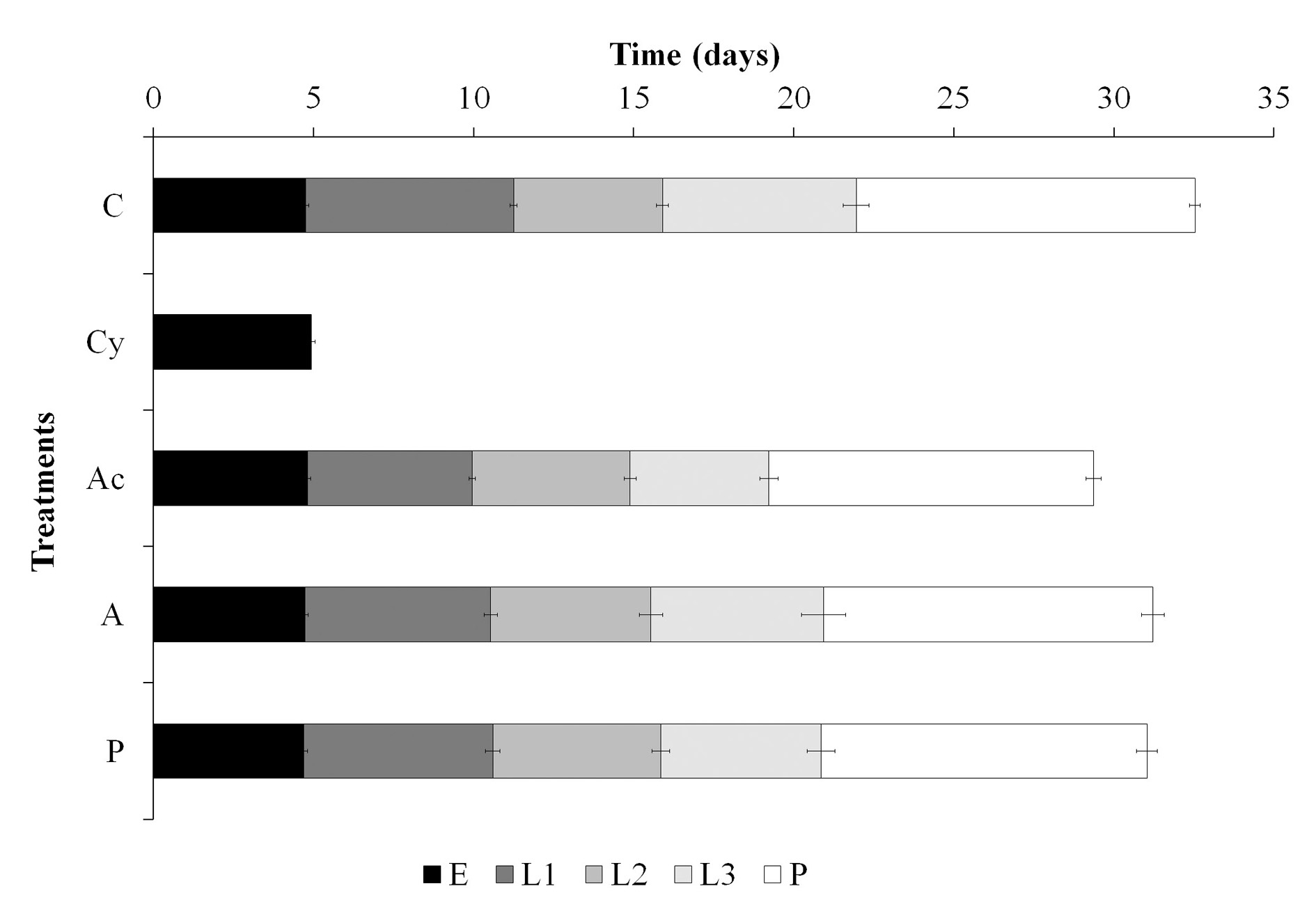
Developmental time from eggs to neonates did not differ between all treatments (F = 0.85; df = 4, 84; P = 0.49) and was of 4.78 (± 0.1) days on average. However, developmental time of larvae was shortened to approximately 3 days with acetamiprid, in first and third larval stages (Fig. 2) (L1-L2: H = 26.56; df = 3; P ≤ 0.001. L2-L3: F = 0.96; df = 3, 58; P = 0.418. L3-P: F = 3.14; df = 3, 58; P = 0.032). Azadirachtin and pyriproxyfen shortened the first stage as well, but did not affect the total larval stage. Pupal stage lasted for approximately 10 days in all treatments (H = 1.57; df = 3; P = 0.66). Studies with different insecticides on C. externa eggs and the Palearctic C. carnea did not reveal differences in the developmental time of eggs and pupae as our results, but neither in the larval stage (Silva et al. 2012; Rugno et al. 2015).
Reproductive parameters
Preoviposition period lasted 7.55 ± 0.27 days (F = 1.69; df = 3, 16; P = 0.21). No differences have been found between controls and all treatments, as reported by Silva et al. (2012)) in C. externa eggs treated with several other pesticides, and by Gontijo et al. (2014) in larvae of C. carnea treated with chlorantraniliprole and thiamethoxam. Apparently, the development of the ovaries is not affected by these insecticides. Cumulative fecundity and fertility during the first five ovipositions was higher in the controls and azadirachtin treatment, whereas exposition to pyriproxyfen and acetamiprid reduced significantly these parameters (Fecundity: F = 36.35; df = 3, 16; P ≤ 0.001. Fertility: F = 25.29; df = 3, 16; P ≤ 0.001) (Fig. 3). These two insecticides reduced the reproductive capacity of the females, and this was in accordance with treatments on eggs from the same species with the IGR methoxyfenozide, although the lower fecundity was observed within the 24 h of the oviposition period, besides fertility was not affected (Rimoldi et al. 2008). Pyriproxyfen is a juvenile hormone analogue that inhibits the vitellogenesis and cause abnormal development of the ovary and subsequent sterility of the insect (Ohashi et al. 2012). The lower values of both fecundity and fertility provoked by a neonicotinoid were also observed in other beneficial organisms as pollinators (e.g. Bombus terrestris L., Osmia bicornis L., Apis mellifera L.) (Laycock et al. 2012; Whitehorn et al. 2012; Rundlöf et al. 2015) and coccinellids (e.g. Serangium japonicum Chapin, Harmonia axyridis Pallas, Coleomegilla maculate DeGeer, Hippodamia convergens Guérin-Méneville) (He et al. 2012; Awasthi et al. 2013; Moscardini et al. 2015). The physiology of an insect is affected by its nervous system; therefore, a neurotoxin should interfere in these processes. On the other hand, the innocuous effect of azadirachtin on reproductive parameters of C. externa was also recorded with the bioinsecticide spinosad (Rimoldi et al. 2008). This could be due to an enhanced metabolism of azadirachtin that was applied in eggs, through the life time of the insect, leading to adults with no effect of this insecticide. Moreover, Medina et al. (2004) tested azadirachtin on adults of C. carnea and found that they were not affected irrespective of the mode of exposure; authors stated that presence of this insecticide must be constant to produce any effect. This could explain that adults of C. externa from treated eggs did not show deleterious effects in reproductive parameters due to the absence of the insecticide in the insect body.
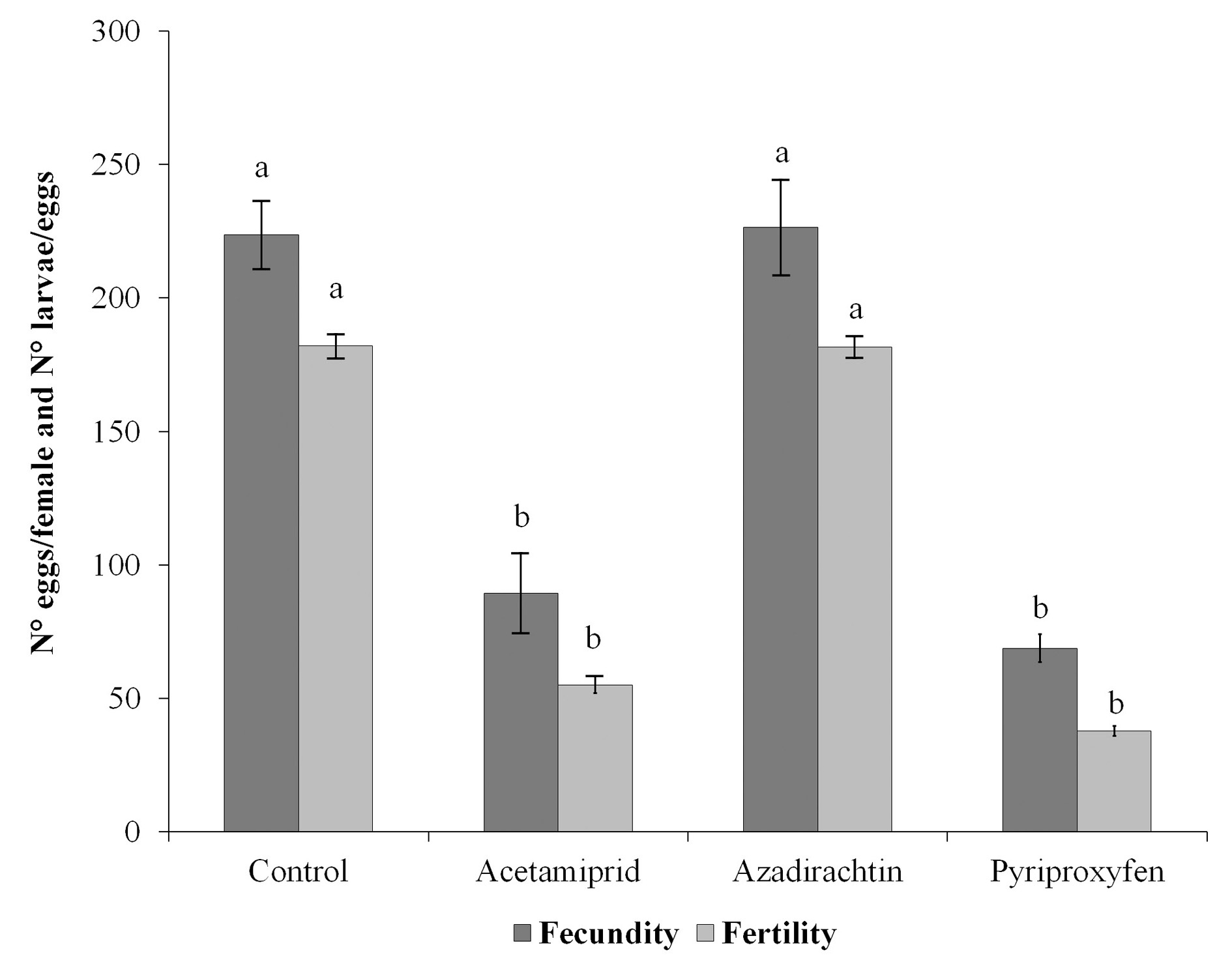
Figure 3 Fecundity (number of eggs/female) and fertility (number of larvae/eggs) of Chrysoperla externa with the different treatments. Bars are means ± SE. Different letters denote significant differences between treatments. ANOVA, Fisher LSD, P ≤ 0.05.
The importance of evaluating the effects of pesticides on natural enemies relies on the occurrence of these beneficial arthropods in the agroecosystems, and the potential exposure to chemical applications. Predators could be exposed to a given insecticide indirectly by feeding on contaminated prey, or directly by insecticide spray and by residual contact with treated plants (Medina et al. 2003). The present work has presented the effect of 25 ppm of cypermethrin in C. externa eggs; with this concentration and without any surfactant, the insecticide resulted highly toxic. There are no previous immersion tests in C. externa eggs with acetamiprid, pyriproxyfen or azadirachtin so far, in any concentration; the sublethal effects are variable among those products. It is remarkable that the eggs survived to all the insecticides applied, but the residual effect did last as long as to affect the neonates in contact with the chorion. Future studies on the ultrastructure of the chorion of C. externa eggs should be done in order to explain their tolerance to insecticides.
Conclusions
The viability of C. externa eggs treated by immersion with cypermethrin, acetamiprid, azadirachtin and pyriproxyfen were not affected, but neonates hatched from eggs treated with cypermethrin died after 48 h. On the other hand, reproductive parameters were affected by pyriproxyfen and acetamiprid which reduced the fecundity and fertility of adults from treated eggs. These results show the importance of evaluate the compatibility between insecticides and natural enemies, especially in situations where chemical control is predominant and conventional insecticides are still used. In an IMP program with C. externa acting as a bio-controller, cypermethrin should be avoided and pyriproxyfen and acetamiprid should be used with caution. Azadirachtin proved to be innocuous. Future studies on the other stages of this species treated with these insecticides should be done; also, the ultrastructure of the chorion should be characterized to explain the lack of penetration.