Introduction
The diamondback moth, Plutella xylostella (Linnaeus, 1758) (Lepidoptera: Plutellidae) is the principal pest damaging crucifer crops in tropical areas throughout the world (Dickson et al. 1990), mainly due to its short cycle and high reproductive potential, which results is large annual generation numbers (Ulmer et al. 2002). In Brazil, it is found in the crucifer producing regions throughout the year (Castelo Branco and Guimaraes 1989; Barros et al. 1993; Melo et al. 1994). Besides the high biotic potential, the emergence of insecticide-resistant populations has contributed to hampering the management of this insect pest. Georghiou and Lagunes-Tejada (1991) have stated that, in 1989, P. xylostella showed resistance against 51 chemical insecticides, which have also been reported in Brazil by Castelo Branco and Gatehouse (1997) for different active ingredients.
The use of resistant cultivars is an alternative to insecticides that has assumed an important role in the management of diamondback moth (Lin et al. 1983; Lin et al. 1984; Eigenbrode et al. 1990; Ulmer et al. 2002). The plant resistance against P. xylostella has been investigated based on two major characteristics: the presence of alkanes in the foliar wax, and sinigrin content in leaves (Lin et al. 1983; Lin et al. 1984; Eigenbrode et al. 1990, 1991; Spencer 1996; Spencer et al. 1999; Ulmer et al. 2002). The resistance characteristics previously described are related to constitutive plant responses, which have high metabolic costs. In the other hand, mechanical injuries caused by insects together with the microorganisms’ inoculation may trigger the production and/or accumulation of compounds such as chitinases and glucanases, among others, involved in the induced resistance (IR) against pests and pathogens (Fidantsef et al. 1999; Stout et al. 1999; Bostock 1999; Felton and Korth 2000; Ramamoorthy et al. 2001; Samiyappan 2003).
The induced resistance processes activated by both biotic and abiotic agents are characterized by lower metabolic cost, without great physiological changes in the plant, being often highly effective (Vallad and Goodman 2004). Microorganisms such as fungi, bacteria, and viruses are related to the induced systemic resistance in plants, and can be epiphytic (which externally colonize plants) (Hallmann et al. 1997; Azevedo et al. 2000) or endophytic (part of their life cycle invade tissues of living plants without causing disease) (Hallmann et al. 1997). Most IR reports mediated by microorganisms are related to epiphytic or endophytic rhizobacteria colonizing any part of the plant, promoting beneficial effects such as plant development, resistance to diseases and arthropods, and adaptation to environmental stresses (Hallmann et al. 1997; Mariano and Palmer 2000; Tomczyck 1999; Palmer 2000; Sturz and Nowak 2000; Medeiros et al. 2001). Recent studies on these bacteria have shown that they may also exhibit entomopathogenic effects when used directly on insects (Thuler 2003).
The oviposition of many lepidopterous insects is generally mediated by several factors, including sensory mechanisms, mechano-receptors, and chemotherapy, which can be stimulated or inhibited depending upon the chemical and physical characteristics of the substrate used for this purpose. Thus, various Brassica species are related to some stimuli for feeding and oviposition substrate selection by P. xylostella. Whereas rhizobacteria act on biochemical pathways related to secondary metabolites, they can change the levels of these compounds when used in processes that culminate in the internal colonization of the plant.Spencer (1996) studied oviposition on sinigrin-treated substrates with and without alkane and found that the increase in sinigrin content led to an increase in the number of P. xylostella eggs, which was higher in the treatment containing high sinigrin contents associated with alkanes. In the choice preference tests, there was also a preference for the treatment using both metabolites. Important IR results mediated by rhizobacteria to insects have been observed in some studies, representing a small part of the potential use of this technique.
In Brazil, Silva and Mariano (1999) conducted preliminary studies on the action of rhizobacteria against the diamondback moth and observed greater effectiveness for the strains ENF14 - Enterobacter cloacae (Jordan, 1890), EN 4 - Kluyvera ascorbata (Farmer et al., 1981), EN5 - Alcaligenes piechaudii (Kiredjian et al., 1986), and E8 - Bacillus cereus (Frankland and Frankland, 1887). Subsequently, laboratory studies have shown that the bacterization of cabbage seeds, Brassica oleracea var. Capitata, with suspensions of strains EN5 -A. piechaudii, ENF14 -E. cloacae, and EN4 -K. ascorbata reduced up to 35 % the viability of P. xylostella caterpillars fed cabbage leaves after 45 days of seed treatment (Medeiros et al. 2001). Based on information from literature on rhizobacteria, this study aimed to evaluate the effects of some rhizobacteria strains on the biology and behavior of the diamondback moth in cabbage plants.
Materials and methods
The experiments were conducted at the Entomology Laboratory of the Federal Institute of Triangulo Mineiro (LE-IFTM), Campus Uberaba-MG; Laboratory of Biology and Insects Creation (LBCI), Laboratory of Bacteria Genetics and Applied Biotechnology (LGBBA), Laboratory of Olericulture and Greenhouse of Vegetable Crops, Faculty of Agricultural and Veterinary Sciences at the Universidade Estadual Paulista (FCAV-UNESP) in Jaboticabal- SP. The strains used in this study are listed in table 1. Larvae of P. xylostella and adults used in the tests were collected from the population at LBCI, maintained according to the methodology of Thuler (2009).
Table 1 Strains used, host plants and isolation location of PGPR (Plant Growth Promoting Rhizobacteria), obtained from the Laboratory of Phytobacteriology at UFRPE.
| Species | Strains | Host plants | Location |
|---|---|---|---|
| Bacillus cereus | C210 | Brassica oleracea var. Acephala (leaves) | epiphytic |
| B. megatherium pv. Cerealis | RAB7 | Raphanus sativus (leaves) | epiphytic |
| Enterobacter cloacae | ENF14 | Phaseolus vulgaris (seeds) | endophytic |
| Kluyvera ascorbata | EN4 | B. oleracea var. Capitata (leaves) | endophytic |
| B. thuringiensis kurstakii | HPF14 | Heliconia sp. (leaves) | epiphytic |
| E. cloacae | PEP91 | Cucumis sativus (seeds) | epiphytic |
| B. subtilis | R14 | B. oleracea var. Capitata (leaves) | epiphytic |
| B. amyloliquefaciens | PEP81 | C. sativus (leaves) | epiphytic |
| B. pumilus | C116 | B. oleracea var. Acephala (leaves) | epiphytic |
| B. cereus | C240 | B. oleracea var. Acephala (leaves) | epiphytic |
| Alcaligenes piechaudii | EN5 | B. oleracea var. Capitata (leaves) | endophytic |
| B. thuringiensis kenyae | C25 | B. oleracea var. Acephala (leaves) | epiphytic |
Seeds of green cabbage cultivar Midori were bacterized by dipping into the strain suspensions of Table 1, at a concentration of 9 x1010 UFC.mL-1 and sterile distilled water (SDW, control treatment), for 30 minutes. The bacterized seeds were dried at room temperature for 12h overnight. The seeds were sown in polystyrene trays containing commercial substrate, and maintained in a greenhouse, and treated as the standard vegetable seeds in the Agro Monte Nursery in the city of Monte Alto-SP. The plants were later transferred to a greenhouse following the necessary cultural practices, and corrective fertilization at planting, with three fertilization coverages at 15, 30, and 45 days of planting. In the greenhouse, the plants were arranged in five blocks, divided into plots formed by 7 plants of each treatment. Twelve rhizobacteria strains and one control were tested, totaling 13 treatments, with 91 plants per block and a total of 455 plants in the five blocks.
Bioassay 1. Laboratory confinement and feed leaves suffering injuries in a greenhouse
A previous infestation with P. xylostella caterpillars was carried out in the greenhouse in the treated plants, in order to have injured leaves for the assays carried out in the laboratory. The infestation was performed 60 days after transplantation when the plants had sufficient size to support such stress. For this purpose, within each block, a treated plant received an infestation with 10 second instar caterpillars in plastic cages of 12 cm in diameter, attached to leaves without damage, comprising 50 larvae per treatment (five cages). These cages contained a circular hole covered with “voil” fabric to allow the gas exchange of the insects and leaves and were secured by interposing a leaf on both sides of the cage, pressed by elastic bands attached to rods of fine wood.
In the laboratory, ten second-instar caterpillars were housed in plastic Petri dishes (9 cm in diameter) and fed with circles (8 cm in diameter) taken from healthy leaves of cabbage plants of each treatment that have suffered injuries. The collection of leaves was performed at 75 days after transplantation. The larvae were maintained on plates until pupae formation to determine the larval duration and viability. The pupae were place in ELISA® plates with one pupa in each well, until the emergence of adults and the pupal duration and viability, pupal weight, and sex ratio of the emerged adults were determined. The initial area of leaf disks (8 cm in diameter) was 48.90 cm2, which was considered the total foliar area (TFA), and then the leaf consumption by caterpillars was calculated by measuring the leaf area after the infestation.
The test was carried out under controlled conditions in the laboratory, at 25 ± 1 °C, relative humidity of 70 ± 10 %, and 12h light period. For this bioassay, the reduction in leaf disk area caused by feeding the caterpillars, larval and pupal viability, duration of larval and pupal stages, sex ratio, and total mortality were determined.
Bioassay 2. Non-preference for oviposition and feeding of diamond back moth on cabbage plants inoculated with rhizobacteria
This experiment was performed in diamondback moth growth cages. Four cabbage quarter circles of 9 cm in diameter were used as a support for oviposition, being 2 quarters from leaves whose seeds were treated with one of the strains of Table 1 (treatment - T) and two other quarters were the untreated sample (control - C), equally distant. For the feeding assay, cabbage quarter circles of 2 cm were used. The leaves of the treated plants used in the test were collected at 100 days after planting.
The non-preference for feeding was carried out in plastic Petri dishes with 15 cm in diameter, with the bottom covered with a moistened filter paper of equal size. Within the plates, two rounds of cabbage leaves originating from plants whose seeds were treated with the strains of table 1 (T) were placed equidistantly with two equally sized disks from plants that suffered no treatment (C).
The non-preference for oviposition was evaluated by posture counts on each leaf disk quarter, and the non-preference for feeding was assessed by counting the number of larvae feeding in each leaf disk. These tests were carried out under the same conditions mentioned previously.
Statistical analysis
Data from bioassay 1 were subjected to analysis of variance and means compared by the Tukey's test (P = 0.05). The biological characteristics were analyzed by multivariate data exploratory analysis using the Hierarchy Cluster Analysis (HCA) (Sneath and Sokal 1973) based on Euclidean distance for classifying the bacteria according to the best findings since this analysis allows verifying the general tendency of the effect of the treatments on the biological characteristics.
For the non-preference tests, the repellency index (RI) was determined according to the following formula: RI = 2T / (T + C), where: T refers to the disks or leaf quarters - treatment and C refers to the disks or leaf quarters - control. Indices tending to 0 refer to the preference for the control, or repellency, while values tending to 1 indicate neutrality, and values tending to 2 indicate a preference for the treatment, i.e. non-repellency. Standard error values (SE) were added or subtracted from the value obtained for each pair of samples (T x C) to ensure data safety. Therefore, only RI values located outside the range of 0.5 to 1.5, with their respective SEs were considered a repellency condition.
Results and discussion
Two treatments directly affected the biology of leaf-feeding P. xylostella caterpillars with rhizobacteria-treated leaves. According to the cluster analysis, although the treatment containing the strain EN4 was the farthest from the control, it was grouped with HPF14 belonging to the strains more active in the induction of resistance towards P. xylostella (Fig. 1).
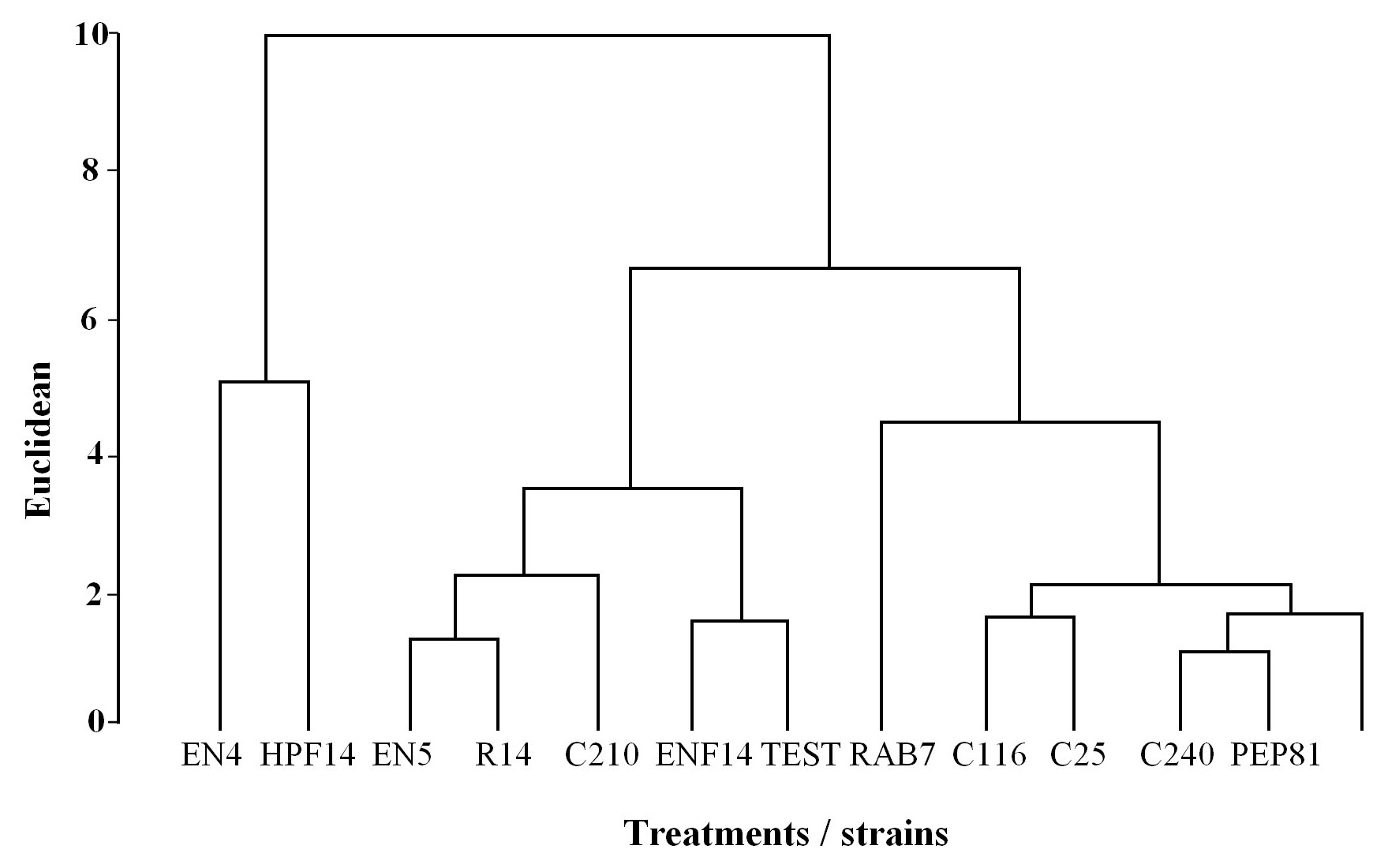
Figure 1 Dendrogram showing the structure of the groups formed by the treatments with different rhizobacteria strains and the control, in relation to the effects of the treatments on second instar Plutella xylostella larvae maintained in the laboratory on cabbage leaves from plants suffering injuries.
The strain EN4 stood out in the dendrogram, and tables 2 and 3 show the cumulative effects found in the cluster analysis.
Slight variations were observed in the duration of the larval stage, pupal weight, and sex ratio, thus indicating a greater effect of the treatment with the strain EN4, followed by the other treatments, with lower relevance. However, the mean comparison test (Tukey test) was not effective to indicate the best treatment once each treatment individually affected a different insect characteristic, with minimum statistical differences due to the great numerical variability, which affects the consistency of the analysis, once it does not reach the normality between data and homogeneity of variance.
Table 2 Viability and duration of larval and pupal stages (± SE) of Plutella xylostella, kept in the laboratory on cabbage leaves from rhizobacteria-treated seeds suffering injuries during cultivation.
| Strains | Larval stage | Pupal stage | ||
|---|---|---|---|---|
| Viability (%) | Duration (days) | Viability (%) | Duration (days) | |
| PEP91 | 78.0 ± 12.00 a* | 7.3 ±0.10 b | 85.7 ± 9.30 a | 3.9 ± 0.10 a** |
| C25 | 82.0 ± 5.80 a | 7.6 ±0.10 b | 75.9 ± 7.50 a | 3.7 ± 0.10 a |
| RAB7 | 88.0 ± 5.80 a | 7.6 ± 0.10 ab | 81.7 ± 2.70 a | 3.7 ± 0.20 a |
| C116 | 78.0 ± 5.80 a | 7.4 ± 0.10 ab | 78.0 ± 13.4 a | 3.8 ± 0.10 a |
| ENF14 | 70.0 ± 8.30 a | 8.0 ± 0.10 ab | 90.5 ± 6.60 a | 3.6 ± 0.10 a |
| PEP81 | 86.0 ± 6.00 a | 7.3 ± 0.20 b | 81.8 ± 5.50 a | 3.8 ± 0.00 a |
| C210 | 64.0 ± 8.12 a | 8.0 ± 0.40 ab | 87.1 ± 6.20 a | 3.4 ± 0.40 a |
| HPF14 | 54.0 ± 11.66 a | 8.3 ± 0.30 a | 68.8 ± 7.80 a | 3.7 ± 0.30 a |
| R14 | 70.0 ± 8.94 a | 7.6 ± 0.10 ab | 82.7 ± 5.10 a | 3.5 ± 0.20 a |
| EN5 | 72.0 ± 8.00 a | 7.3 ± 0.10 b | 79.8 ± 6.40 a | 3.4 ± 0.20 a |
| EN4 | 70.0 ± 5.47 a | 8.1 ± 0.10 ab | 71.2 ± 5.20 a | 3.3 ± 0.10 a |
| C240 | 80.0 ± 7.07 a | 7.6 ± 0.10 ab | 82.2 ± 3.10 a | 3.7 ± 0.10 a |
| TEST | 76.0 ± 7.40 a | 7.6 ± 0.10 ab | 89.1 ± 4.90 a | 3.6 ± 0.20 a |
| CV ( %) | 12.78 | 5.00 | 19.07 | 11.17 |
* Means followed by the same letter in columns do not differ by Tukey's test (P = 0.05).
** Tukey's test was used for generalized models. Deviance analysis in gamma distribution was used for the residues.
It can be stated that the caterpillars kept in EN4-treated leaves fed very little, despite a longer period in the larval stage (Fig. 2), or had low feed conversion rate, because the weight of the formed pupae was lower (Table 3), resulting in less damage to the plant.
Table 3 Pupal weight and sex ratio (mean ± SE) of Plutella xylostella maintained in the laboratory on cabbage leaves from rhizobacteria-treated seeds suffering injuries during cultivation.
| Strains | Pupal weight (mg) | Sex ratio | Mortality (%) |
|---|---|---|---|
| PEP91 | 5.0 ± 0.20 a* | 0.4 ± 0.10 b | 32.0 ± 13.90 a |
| C25 | 5.2 ± 0.10 a | 0.4 ± 0.10 b | 38.0 ± 7.30 a |
| RAB7 | 5.2 ± 0.20 a | 0.7 ± 0.10 a | 28.0 ± 5.80 a |
| C116 | 5.0 ± 0.20 a | 0.2 ± 0.10 b | 38.0 ± 12.4 a |
| ENF14 | 5.0 ± 0.10 a | 0.4 ± 0.00 b | 38.0 ± 6.60 a |
| PEP81 | 5.0 ± 0.10 a | 0.4 ± 0.10 b | 30.0 ± 5.50 a |
| C210 | 5.3 ± 0.10 a | 0.3 ± 0.10 b | 46.0 ± 4.00 a |
| HPF14 | 4.7 ± 0.10 ab | 0.6 ± 0.10 ab | 60.0 ±11.00 a |
| R14 | 4.8 ± 0.20 ab | 0.3 ± 0.10 b | 42.0 ± 8.00 a |
| EN5 | 5.1 ± 0.20 a | 0.4 ± 0.10 b | 44.0 ± 4.00 a |
| EN4 | 4.1 ± 0.20 b | 0.4 ± 0.10 b | 50.0 ± 5.50 a |
| C240 | 4.8 ± 0.10 ab | 0.4 ± 0.10 b | 34.0 ± 6.80 a |
| TEST | 4.9 ± 0.10 a | 0.6 ± 0.10 ab | 32.0 ± 8.6 a |
| CV (%) | 6.81 | 4.85 | 46.58 |
* Means followed by the same letter in columns do not differ by Tukey's test (P = 0.05).
The reduction of leaf area measured according to the total leaf area (48.90 cm2) during larvae feeding was lower than 20 %. A greater reduction in leaf area was observed in both the control and the treatment with the strain C240, which were significantly different only from the treatment C210, with the lowest reduction, followed by the treatments EN4 and HPF14, with no significant differences from the control (Fig. 2). The results of the reduction of foliar area have a different tendency compared to the results of the other assays, such as the morality indices in table 3, for instance. As observed in the structure of the groups, the treatment C210 was different from the other strains with better responses, including EN4 and HPF14 (Fig. 1). However, the strain C210 should be further investigated, once the responses were very interesting, reflecting an overall mortality of 46.0 %, with a reduction of leaf area below 5.0 %.
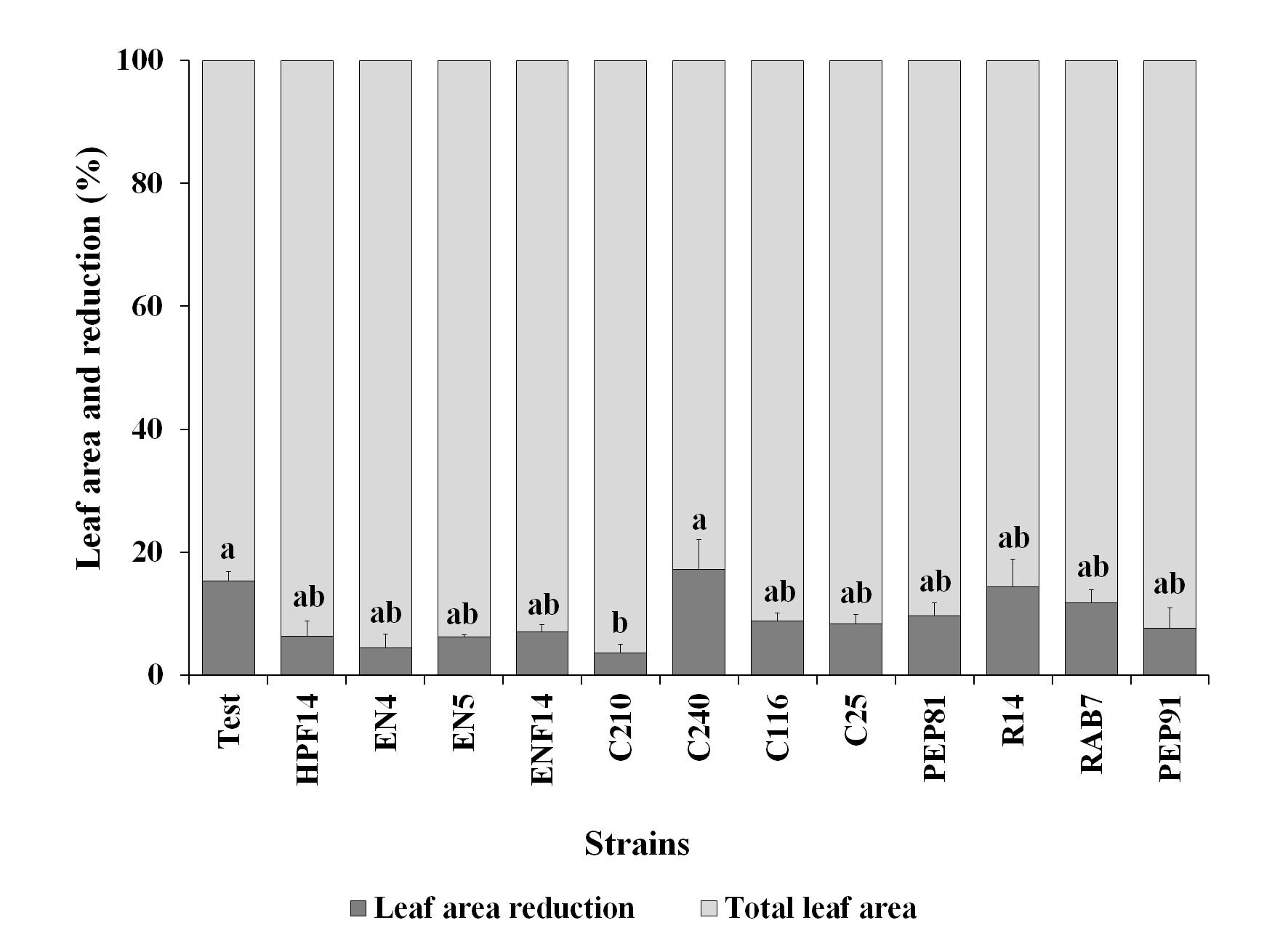
Figure 2 Leaf area (%) (± SE) of cabbage consumed by second instar Plutella xylostella larvae. Leaves from plant seeds previously treated with rhizobacteria strains suffering injuries in the greenhouse.
Regarding the bioassay for the assessment of non-preference for oviposition and feeding of diamondback moth on cabbage plants inoculated with rhizobacteria, there was no repellency for oviposition (Fig. 3) or feed supply (Fig. 4). However, among the strains, the C25 stood out for having apparently exerted attraction as oviposition site for the moths (Fig. 4).
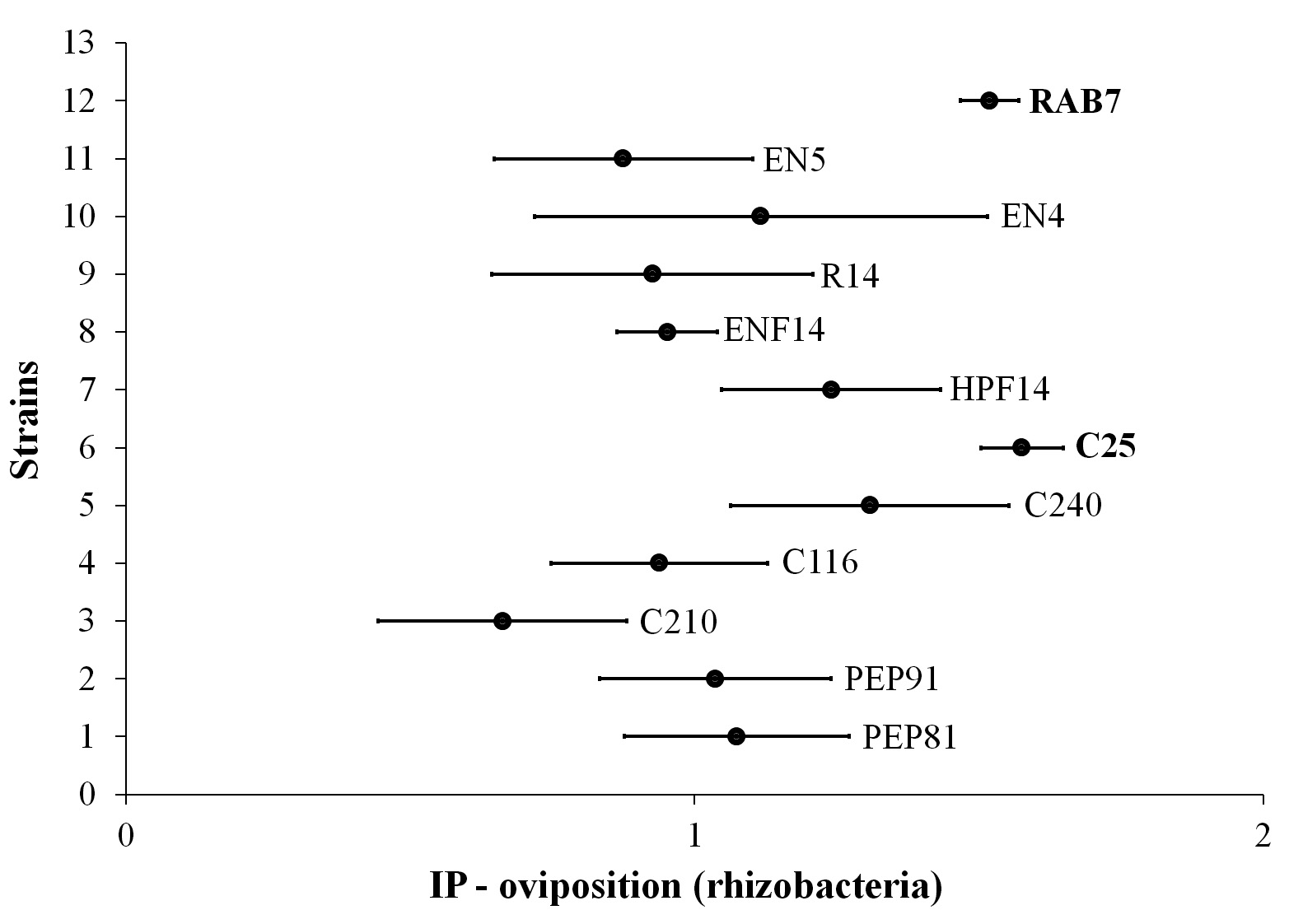
Figure 3 Repellency indices for oviposition of Plutella xylostella on cabbage leaves from plant whose seeds were previously treated with rhizobacteria strains (bold: strain out of the void interval).
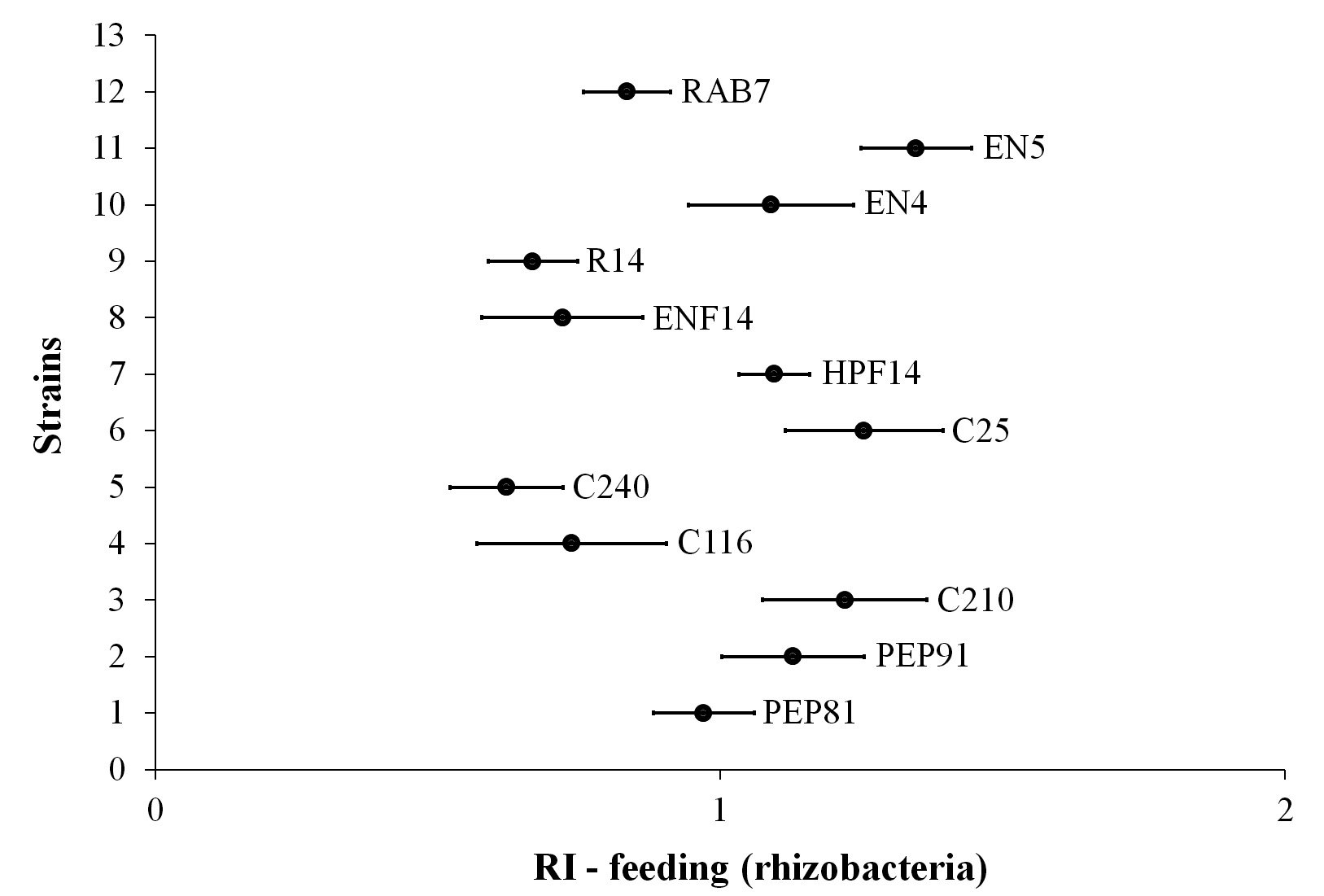
Figure 4 Repellency indices for the feeding of Plutella xylostella on cabbage leaves whose seeds were treated with rhizobacteria strains.
The condition of neutrality was even more evident in the treatments when the RI was determined (Fig. 4), once the values tended to 1, representing the neutral condition, for all treatments. Since the repellency for oviposition and feeding was not observed, it can be stated that despite the treatments have not prevented the infestation, the non-repellency indicates that the insects cannot identify the treatments, thus they may be more easily affected.
Regarding the preference for oviposition by P. xylostella or even in relation to food preference, several insect responses such as antenna rotation, positioning the ovipositor, and successive touches of the antenna on the posture substrate can demonstrate the behavior for choosing the place of oviposition, as reported by Justus and Mitchell (1996). These authors also reported that there is no oviposition in the simultaneous absence of olfactory and taste stimuli or only taste stimuli in adult insects.
Spencer (1996) showed that the oviposition is increased in waxy substrates and containing relatively high sinigrin (Brassica secondary metabolite) and alkane concentrations, proving the importance of internal and surface chemical substrate stimuli for location and oviposition by diamondback moth.
Similarly, Eigenbrode et al. (1991) demonstrated the need for surface physical stimuli on the substrate selection for P. xylostella oviposition when comparing glossy smooth and waxy surfaces of cabbage leaves, concerning the resistance of these plants towards the diamondback moth.
Conclusion
The EN4 strain of Kluyvera ascorbata caused deleterious effects on Plutella xylostella biology, which can probably be seen over generations, indicating that cabbage plants grown from seeds inoculated with this strain may reduce pest populations in the field. It can be suggested that the treatment of cabbage seeds with rhizobacteria strains was not effective to cause metabolic and surface changes capable of influencing the host preference by P. xylostella. It is worth noting that other strains of the present study exhibited good responses, including R14, HPF14 (Bacillus thuringiensis kurstakii) and C210 (Bacillus cereus), thus further studies using these strains and different cultures are required.














