Introduction
Acca sellowiana (Berg) Burret (Myrtaceae) (feijoa) is a native fruit species found in the highland regions of the States of Rio Grande do Sul and Santa Catarina in Brazil as well as in the northern part of Uruguay and Argentina (Thorp and Bieleski 2002). Its fruits have an exotic flavor and aroma. The fruit presents great economic potential as a result of the presence of antimicrobial agents and its bioactive, anti-inflammatory, and antioxidant properties (Weston 2010) as well as its antidepressant properties (Mahmoudi et al. 2015). Although feijoa is native to southern Brazil, countries such as Uruguay and Argentina, Colombia, the United States, France, Italy, New Zealand (Thorp and Bieleski 2002) and recently China (Zhang et al. 2010) commercially produce the fruit and its derivatives. In Brazil, new perspectives to increase production are being outlined (Ducruquet et al. 2008) with the launch of four commercial feijoa cultivars in the State of Santa Catarina.
Feijoa orchards are damaged by different insect pests, especially the South American fruit fly, Anastrepha fraterculus (Wiedemann, 1830) (Rosa et al. 2013). This species has a Neotropical origin and can be found from the Southern United States to northern Argentina (Malavasi et al. 2000). Anastrepha fraterculus has a widespread presence in the commercial crops of feijoa as well as in natural forests. Moreover, it is considered to be the main pest of feijoa and it can infest up to 100% of the fruit in orchards without control (Luckmann et al. 2009; Rosa et al. 2013).
A key strategy for fruit fly management programs is to monitor the pest at the orchard level. In this sense, the availability of lures and traps is essential for defining the right moment for the control of the fruit flies in orchards. Several lures used for monitoring fruit flies are currently available, varying in type depending on fruit fly species, country, and location (Botton et al. 2012). Throughout the years, efforts have been directed toward identifying other readily available, low cost materials, which could be used locally by farmers (Epsky et al. 2014). In the South region of Brazil, 25 % grape juice is the lure recommended as the standard for capturing fruit flies in apple orchards (Kovaleski and Ribeiro 2002; Kovaleski 2004), and this information has been expanded to other crops such as grape, peach, plum and feijoa.
According to Kovaleski and Ribeiro (2002), Nora and Hickel (2002) and Muller et al. (2013), fruit fly control should start when a level of 0.5 flies per trap per day is reached. However, in recent years, there have been significant failures in the control of A. fraterculus in several orchards, even with the use of monitoring (Botton et al. 2012). One of the main reasons for this problem is connected to the inefficiency of some lures that are not capable of detecting the presence of the pest in orchards (Botton et al. 2013). This fact results in damage to production, whereby the fruit fly is present in the orchard, but not captured in the traps, as it is baited with inefficient lures.
During the decomposition of organic substances such as fruit juice, the release of secondary compounds is necessary. According to Heath et al. (1993), Salles (1999) and Nascimento et al. (2014), this fact can positively influence the capturing of fruit flies of the genus Anastrepha. Odor perception of insects, including fruit flies, was elucidated by Visser (1986), who demonstrated that most adult insects are attracted by specific (groups of) odors, which can be released during decomposition.
Despite the availability of various hydrolyzed proteins with proven efficiency for monitoring A. fraterculus (Scoz et al. 2006; Teixeira et al. 2010; Botton et al. 2012; Lasa et al. 2015), many fruit growers still choose to use 25 % grape juice. This is due, hypothetically, to old recommendations, because grape juice is easily available in the market and has a lower cost compared to hydrolyzed proteins.
The hydrolyzed protein of animal origin (Cera Trap®) was recently introduced to the Brazilian market for monitoring and controlling fruit flies. Cera Trap® is produced through the enzymatic hydrolysis of the intestinal mucosa of swine (Sierras et al. 2006) and causes the emission of volatiles, especially amines and organic acids, which are highly attractive primarily to tephritid females (Marín et al. 2006). One of the major advantages of this new hydrolyzed protein is its stability, as the attractiveness to adults is maintained over a period of 60 days without the need to exchange more than the evaporated content (Machota-Junior et al. 2013). Lasa and Cruz (2014), Lasa et al. (2015) emphasize the practicality of its use and the selectivity on other insects as well as the economic viability of this lure.
According to Botton et al. (2012), monitoring should provide information that adequately represents the behavior of the A. fraterculus population in orchards. In addition, reduced lure longevity and low capture rates for adult fruit flies may compromise monitoring and thereby increase the costs of monitoring programs (Lasa et al. 2014).
Therefore, the search for better and more efficient lures for each crop and region should be performed constantly to ensure that the observed capture rates of the traps properly correspond to the actual populations of fruit flies present in orchards. In this study, we evaluate for the first time under field conditions, the commercial hydrolyzed protein Cera Trap® and the different aging combinations of grape juice for the capture of A. fraterculus adults in feijoa orchard.
Materials and methods
Description of the study area
The work was performed in an 18-year old feijoa orchard located at the Experimental Station for Agricultural Research and Rural Extension of Santa Catarina (EPAGRI), São Joaquim, SC, Brazil, at 28°17’39” S and 49°55’56” W, at an altitude of 1,415 m (Fig. 1). The local climate is Cfb, mesothermal, according to Köppen’s classification, which means it is moist without a dry season, with fresh summers (22 °C) and an annual average temperature of 13.5 °C. The experiment was performed during the 2014 production cycle, comprising the fruiting period (January to May). The orchard had a rectangular form measuring 1.5 ha spaced with three meters between the plants and five meters between the rows. The orchard is surrounded by native fields, urban areas, Pinus reforestation areas, and natural forest remnants (Araucaria Forest).
Lures and traps
Four food baits with Mcphail traps were compared in the field: a) Hydrolyzed protein Cera Trap®, Bioiberica (Barcelona, Spain), undiluted, replacing only the volume lost through evaporation; b) Fresh grape juice (Embrapa Uva e Vinho, Bento Gonçalves, RS); c) Grape juice aged for 7 days; d) Grape juice aged for 14 days. Distilled water was used as a control. Treatments with grape juice were diluted in the recommended dose of 25 % and aged in the recipients covered by a voile type fabric, packed under field environmental conditions, but protected from the sun and rain during the aging period.
For all lures, we used a commercial Mcphail Trap (Isca Technologies, Iují, RS) baited with 300 mL of each lure. The traps were hung in trees at a distance of 15 m apart and were rotated within the block every week. The number of fruit flies captured was counted every 7 days for 14 consecutive weeks. The collection of flies, cleaning of the traps, and rotation within the block were performed every 7 days, along with the changing of the bait (grape juice lures) and the replacement of the evaporated volume in the Cera Trap® treatment.
Insects
The fruit flies caught in the trap were separated from the bait solution through a sieve and placed in 80 mL plastic vials containing 70% ethanol. Then, the samples were screened, sex was determined, and samples were identified and counted in the laboratory. The identification of the genus Anastrepha was based on morphological characters; observation of standard wings, thorax, and female aculeus morphology (Alberti et al. 2012) as well as on the taxonomic keys developed by Steyskal (1977) and Zucchi (2000).
Statistical analysis
The experiment was carried out in a randomized complete block design. For the evaluation of the lures, the number of captured A. fraterculus was transformed to the average number of flies per trap per day (FTD). The FTD values and the percentage of females per trap session were transformed into √(x + 1.0) to stabilize variance. The data were analyzed using ANOVA, and the means were compared by Tukey's test (P < 0.05) using the software R (R Development Core Team 2012).
Results and discussion
During the production cycle, a total of 210 Tephritoidea individuals were captured, of which 209 (99.9 %) belonged to the species A. fraterculus; 57.4 % were females and 42.6 % males, thereby confirming the previous results that showed this species as the dominant one in the region (Teixeira et al. 2010; Rosa et al. (2013). In the neighboring State of Rio Grande do Sul, Kovaleski et al. (2000), Scoz et al. (2006) and Nunes et al. (2013) also found similar percentages of prevalence of the same species. The other captured species was Anastrepha dissimilis Stone, a species associated with passiflora fruits (Zucchi 2007). The capture of this species in feijoa orchards is possibly due to the presence of native passion fruits (Passiflora spp.) near the area of study.
The hydrolyzed protein Cera Trap® presented the highest number of A. fraterculus captured during the evaluation period (59.8 %). The fresh grape juice had the second highest capture rate (20.6 %), while grape juice with aged 7 and 14 days showed 13.9 % and 5.7 %, respectively. These results are similar to those of Herrera et al. (2016), who showed that Cera Trap® was the most efficient lure for capturing Anastrepha flies when compared to different commercial grape products in several experiments in fruit orchards in Mexico.
The FTD values in grape juice treatments were significantly lower compared to Cera Trap® during the season (F = 7.83; df = 4, 20; P > 0.0001) (Table 1) and (Fig. 2). The percentage of females caught in the McPhail traps was significant (F = 15.38; df = 4, 20; P > 0.0001). Grape juice tested with 14 days of aging showed the higher capture of females (66.6 %) 1 ♂: 2 ♀. However, this lure presented a small number of total flies captured during the season (5.7 %) and a low FTD index (0.12 ± 0.04), and it did not differ from the control treatment (Table 1). Although there was no significant difference for fresh juice (48.8 %) and juice aged for 7 days (44.8 %), Cera Trap® also had a high percentage of captured females (61.6 %) (Table 1), with a sex ratio of 1: 1.6 ♀. Similarly, Herrera et al. (2016) and Lasa et al. (2015), respectively, captured 77.06 % and 76.1 % of Anastrepha ludens (Loew) females in traps baited with Cera Trap® in citrus orchards.
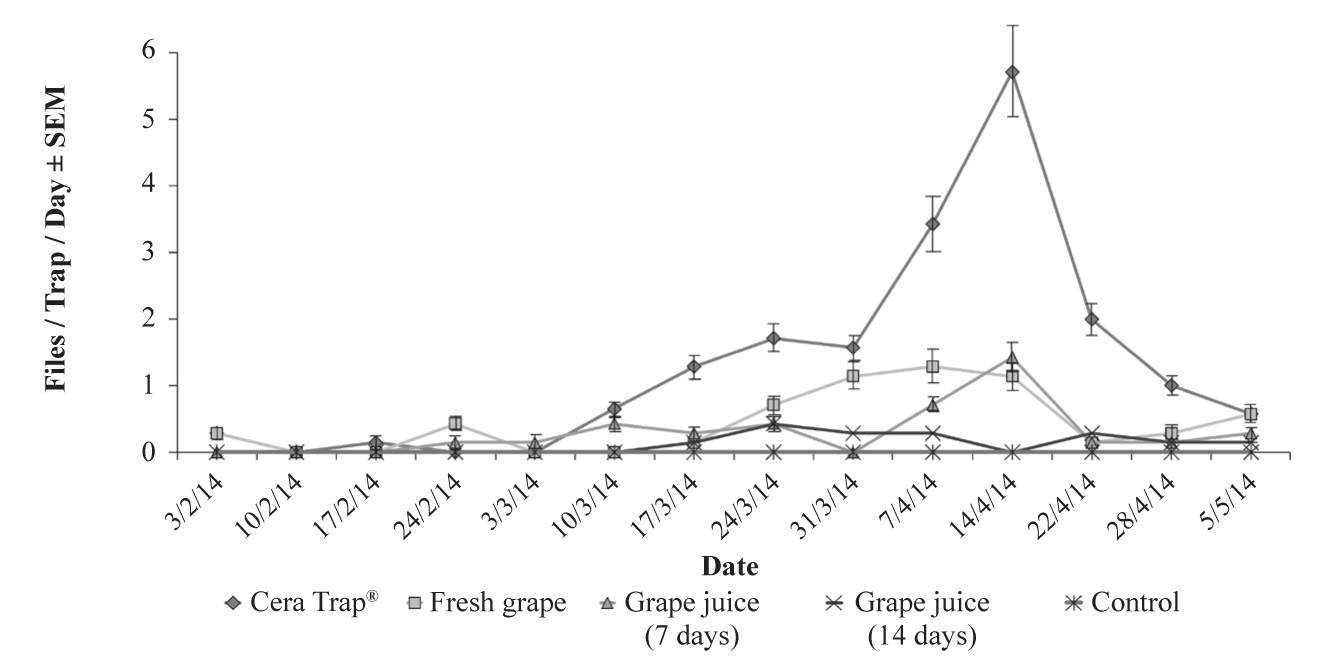
Figure 2 Weekly evaluation of Anastrepha fraterculus (FTD ± SEM) in a feijoa orchard monitored with McPhail traps baited with Cera Trap® and grape juice at different ages. A.t: Action threshold.
Table 1 Average (± SEM) number of Anastrepha fraterculus adults captured per trap per day (FTD), percentage of females, and number of action thresholds in the feijoa orchard.
| Lures | Fly / Trap / Day | Action thresholds 1 | |
|---|---|---|---|
| FTD ± SEM | % Females ± SEM | ||
| CeraTrap® | 1.28 ± 0.40 a | 61.6 ± 5.6 ab | 9 |
| Fresh grape juice | 0.44 ± 0.10 b | 48.8 ± 4.7 b | 4 |
| Grape Juice (7 days) | 0.30 ± 0.10 bc | 44.8 ± 4.3 b | 2 |
| Grape Juice (14 days) | 0.12 ± 0.04 c | 66.6 ± 4.2 a | 0 |
| Control | 0.00 ± 0.00 c | 0.0 ± 0.0 c | 0 |
¹ Threshold for fruit flies in Brazil. Above 0.5 FTD, the control of fruit flies is recommended.
Means followed by different letters in the same column differed significantly (randomized block ANOVA, Tukey’s test, P < 0.05, non-transformed means presented).
In general, after emergence, A. fraterculus adults, especially females, need protein sources to ensure their fertility (Heath, 1993). Furthermore, the high attractiveness of protein derivatives for A. fraterculus females may be associated with the need for the intake of amino acids preceding egg production and the generation of fertile offspring (Zucoloto 2000; Nunes et al. 2013). According to Cangussu and Zucoloto (1997), fruit fly females that feed with high protein levels are more receptive to copulation compared to females that receive a diet with a lower content of these substances. Therefore, there is generally a larger number of female captures in traps baited with protein attractants.
Despite the fact that the sex ratio is not considered for decision-making about control in Brazil, the large number of trapped females is a positive factor, as they are responsible for oviposition and puncture which cause damage to fruits. In addition, higher percentages of females trapped are desirable in the development of mass trapping programs.
According to Monteiro et al. (2007), the attraction exerted by proteins is higher when compared to juice attractants in McPhail traps. Contrarily to the results found by Salles (1999), our study did not show a better effect for the capture of A. fraterculus with the aging grape juice (Table 1). As reported previously by Salles (1999), the aging and decomposition of food attractants such as red wine vinegar (25 %) and peach juice (10 %) showed a direct relationship with an increasing number of captured flies belonging to the Anastrepha genus (especially A. fraterculus) in peach orchards (Prunus persica L.). However, this relation was not found in the feijoa orchard.
For the majority of temperate climate fruits grown in the South region of Brazil, fruit fly control should start when a level of 0.5 flies per trap per day is reached (Kovaleski and Ribeiro 2002; Nora and Hickel 2002; Muller et al. 2013). In this aspect, Cera Trap® was the most efficient, presenting a greater number of action threshold (> 0.5 FTD), even in periods with a lower density of the A. fraterculus population compared to fresh or aged grape juice (Table 1).
The greatest occurrence period of A. fraterculus, measured in the orchards, begins when the feijoa fruit reaches the critical size to receive the postures, approximately 30 mm (Ducroquet et al. 2000). However, periodic monitoring of the population should not be dismissed because the fruit flies have no pre-established distribution pattern because of the presence of alternative hosts and different climatic conditions over the years (Aluja 1994). This is especially true for southern Brazil, with its large amounts of native fruits near commercial orchards.
A. fraterculus is the species with the highest distribution, abundance, and dominance in the temperate fruit producing regions in the States of southern Brazil (Garcia et al. 2011). Both feijoa orchards as well as temperate fruits are geographically distributed in the same regions and have similar reproductive periods, facilitating the multiplication of the fruit fly in these hosts.
Nora et al. (2000) recommend 25 % grape juice for monitoring A. fraterculus in the State of Santa Catarina, Brazil. These authors reported that this bait stands out compared to other attractants because of the fermentation process. However, in our study, both the fresh grape juice as well as the aged grape juice did not provide effective results for capturing and monitoring A. fraterculus in the feijoa orchard (Table 1). Furthermore, Mangan and Thomas (2014) indicate that the rapid fermentation of grape juice results in unpleasant odors. This process possibly facilitates the capture of non-target insects such as Lepidoptera, common flies, wasps and bees. Although this data was not quantified in our work, we observed, in the field, that traps containing grape juice attractant captured large amounts of unwanted insects. This assertion is supported by Herrera et al. (2016), who found a major amount of unwanted insects in traps baited with grape juice in citrus orchards.
Hüntermann et al. (2013) found that hydrolyzed proteins used for the capture of A. fraterculus have a higher concentration of phenol after nine days of aging compared to grape juice. According to the authors, these compounds volatilize more quickly in grape juice, which can directly influence the loss of attractiveness and capture efficiency. Moreover, the fermented grape juice attractant may, from time to time, change its chemical odor and potentially change its attractiveness (Epsky et al. 2015).
The monitoring and quantification of specific pests in a culture is a necessary instrument for implementing any control strategy involving an integrated pest management approach (Aluja et al. 2012). Therefore, the monitoring and knowledge about the number of captured fruit flies serve as a basis for decision making about the control of these insects (Hickel 2008; Rosa et al. 2013). According to Nava and Botton (2010), a major problem in fruit production losses is the fruit fly attacks where there is no detection by a pest monitoring system. In this case, an inadequate lure for capturing and monitoring populations of A. fraterculus can compromise the decision-making time, reflecting negatively on the production with the highest percentage of damaged fruits.
The detection of the action level for the control of A. fraterculus (> 0.5 FTD) in traps baited with Cera Trap® was observed 14 days before it was observed in the traps with fresh grape juice and 28 days before the observation in the traps with the grape juice with 7-day aging (Fig. 2). In addition to the reduced number of A. fraterculus captured, the 14-day aged grape juice did not provide an action threshold (> 0.5 FTD) and there was no significant difference compared to the control during the production cycle. Similarly, Epsky et al. 2015 found no difference in the number of A. suspensa adults captured in traps baited with grape juice aged in the laboratory for 3, 6, and 9 days. These authors did not observe significant differences in the percentages of females captured for the treatments with grape juice at different ages.
Fruit juice is used in Brazil because of its low price and the fact that it is easily obtainable in the market. However, when comparing food baits, the hydrolyzed proteins showed more durability in their attractiveness in the field (Nunes et al. 2013). The percentage of fruit flies captured in fruit juice may be associated with the sugar concentration, which is directly related to the origin of the harvest and storage conditions of the juices (Santos et al. 2009).
The feijoa has a long harvest period, with both ripe fruits and fruits in development observed in the same tree. In hypothesis, fresh or aged grape juice does not present sufficient attractiveness because of high concentrations of volatiles emitted by mature feijoa fruits in harvesting periods or by decomposing fruits in the orchard, thereby reducing the effectiveness of the monitoring. The presence of different odors and mature fruit volatiles within the orchard is considered as a preponderant factor for the masking of lures baited with fruit juice (Cornelius et al. 2000; López-Guillén et al. 2010), which can become imperceptible to tephritids. In addition, the chemotactic behavior of fruit flies can be compromised in the presence of ripening fruit volatiles (Cornelius et al. 2000). Another hypothesis may be related to maturation anticipation or premature drop of fruits that are attacked by other insects, such as Conotrachelus psidii (Coleoptera: Curculionidae), with high incidence in the feijoa orchards in this region (Rosa et al. 2015). Decomposing feijoa fruits may also present high sugar concentrations which may be satisfying the food needs of A. fraterculus adults, thus justifying the low attraction and capture rates in traps baited with grape juice. On the other hand, fruit flies need to consume protein as adults for sexual maturation and ovarian development (Drew and Yuval 2000). Based on this, we may also explain the higher catch rate of A. fraterculus in traps baited with Cera Trap® in our study.
Management of fruit flies (Tephritidae) in Brazilian fruit farming is an increasing challenge. Effective lures for pest monitoring associated with specific tools for reducing the adult population, e.g. toxic baits, mass trapping, and biological control, can be used in combination for adequate control within the integrated management strategy, considering the required standards of the current market. The South Region of Brazil also distinguishes itself by the production of a wide variety of temperate fruit, such as apples, grapes, peaches, and plums. We recommend further studies to assess the validation of the Cera Trap® lure for capturing A. fraterculus in different fruits species.
In conclusion, fresh grape juice or grape juice at different aging stages showed deficiencies in monitoring and detecting the A. fraterculus population in feijoa orchards. Cera Trap® was a better lure for detecting A. fraterculus even in periods with low population densities. For this reason, Cera Trap® can be considered as an alternative for improving monitoring systems, thereby enabling greater accuracy for detecting A. fraterculus in orchards.