Introduction
Larvae of Diabrotica speciosa (Germar) (Coleoptera: Chrysomelidae) are considered to be one of the major subterranean pests of maize, Zea mays L. Losses of maize plants caused by this pest have been expensive in southern Brazil, as well as in some areas of the southeast and midwest regions of the country (Viana 2010). Injury imposed by D. speciosa larvae reduces plant ability to absorb water and nutrients; thereby, the plant produces fewer grains and becomes more susceptible to lodging, leading to increased losses in cases where maize is mechanically harvested. There are reports of maize yield reductions ranging from 10 to 13 % under high D. speciosa larvae infestations (Viana 2010).
The species Diabrotica speciosa is currently established in several states of Brazil and in some countries of South America. Because it is a polyphagous pest, D. speciosa causes damage to many plant species in addition to maize, such as soybeans (Glycine max [L.] Merril), common beans (Phaseolus vulgaris L.), peanuts (Arachis hypogaea L.), and potatoes (Solanum tuberosum L.), which demonstrates its economic expression. Also, D. speciosa is a vector of plant pathogens, primarily viruses (Laumann et al. 2003). In South America the most harmful chrysomelid species for agriculture belong to the Subfamily Diabroticinae, Tribe Galerucini, especially Diabrotica speciosa (Cabrera Walsh 2003). Ávila and Parra (2002) evaluated D. speciosa development on different host plants, and concluded that maize and potato roots are suitable food sources for the larvae. In addition, these authors reported that total survival, duration of larva-to-adult period, and weight of newly emerged adults were significantly influenced by the host species used as larval food source.
Control of D. speciosa in maize is done almost exclusively using synthetic insecticides (Arruda-Gatti and Ventura 2003). For larvae, difficulties in the control are increased to a larger extent in comparison to adults because of the subterranean habit, and is usually performed using seed treatment and in-furrow applications of granules and liquid insecticides at sowing (Viana et al. 2002). However, chemical control many times has been inefficient, expensive, and soil-polluting (Viana et al. 2002).
Due to the need of adopting less impactful control tactics to human and to the environment, plant extracts have been studied as an alternative to the use of synthetic insecticides. Characteristics of plant extracts such as low toxicity and persistence make them less harmful to the environment (Costa et al. 2004). Over centuries, the neem plant, Azadirachta indica A. Juss. (Meliaceae) has been used in India to control insect pests. Currently, active compounds have been extracted from neem and used against over than 200 insect species worldwide (Viegas Junior 2003). Because the active compounds are of plant origin, neem-based products are completely biodegradable and less toxic than other plant extracts that have been used in organic agriculture, as extracts from Nicotiana tabacum L. (Solanaceae) and Derris spp. (Fabaceae) (Martinez 2002).
Given the growing demands for alternative and sustainable methods of crop pest control, this study aimed at assessing the effects of application of neem formulations (oil-formulated and lignin-microencapsulated) to control of D. speciosa larvae. In addition, the effects of application of the neem formulations in reductions of plant growth were correlated.
Material and methods
The study was carried out at the Laboratório de Resistência de Plantas a Insetos, of Departmento de Fitossanidade, of Universidade Estadual Paulista, Jaboticabal, state of São Paulo, Brazil, located at geographical coordinates 21º15’S, 48º18’W, and 595 m altitude (Almeida et al. 2011). Assays were carried out at controlled conditions of 25 ± 2 ºC temperature, 70 ± 10 % relative humidity, and 12:12 (light:dark) h photoperiod.
In all assays, 25 g of sieved soil was oven-sterilized (model AS200S, Quimis, Diadema, state of São Paulo, Brazil) at 110 ºC for 48 hours and placed in 100 mL plastic containers. Three maize seeds (variety AL-Piratininga) were sown in each container, and 15 mL of deionized water was added. Deionized water (10-15 mL) was applied on the containers with a syringe on a daily basis to keep the plants turgid over the experiment conduction. Nine-day-old larvae that were collected from the laboratory colony were used in the assays. The larvae were reared on maize plants (variety AL-Piratininga) prior to the assays, following methodology of Ávila et al. (2000). All assays were arranged in a completely randomized design with 10 replicates, in which one plastic container harboring one maize plant plus two D. speciosa larvae was considered one replicate.
Oil-formulated neem extract
This assay evaluated the effects of five rates of oil-formulated neem extract on D. speciosa larvae and on maize plants growth, comparing these rates with two control treatments. The control treatments consisted of (1) deionized water (negative control) and (2) a synthetic insecticide (positive control), fipronil, Regent 800 WG®, at the rate of 80 g commercial product (0.032 %) (MAPA 2012).
To prepare the oil-formulated neem extract, the following ingredients were used: 0.6 g fraction-ethyl acetate obtained by liquid-liquid partition from neem crude ethanolic extract (Costa et al. 2013), 2 mL ethanol, 4.4 g commercial neem oil (Baraúna, Catanduva, SP, Brazil), and 0.5 g Renex 40. Once the emulsified oil was prepared, 6 g of oil was homogenized in water using a vortex, obtaining a final volume of 25 mL. After formulated, the total amount of azadirachtin in emulsion was determined by HPLC according to Forim et al. (2013). For the neem-oil treatment, the rates used were 0.25, 0.5, 1.0, 2.0, and 4.0 mL of emulsion (5 mg L-1) per 25 g of soil, corresponding respectively to the quantities of 1.25, 2.5, 5.0, 10.0 and 20.0 µg of azadirachtin. Thinning of maize plants was performed when they were 4-day old, keeping only one plant per container. The rates of oil-formulated neem extract were applied on the roots using a 1.0-mL syringe, simulating a drench application. Thereafter, the roots were covered by soil that had been previously removed. Finally, two 9-d-old D. speciosa larvae were released onto the soil of each container using a soft bristle paintbrush.
Lignin-microencapsulated neem extract
Lignin used in this assay was obtained from sugarcane bagasse by soda-pulping process as previously described by Costa et al. (2017). To prepare the formulation lignin-microencapsulated neem extract the following components first were added in separate beakers: 0.6 g fractions of ethyl acetate from neem crude ethanolic extract, 0.67 g Tween® 80, 3.5 g commercial neem oil (Baraúna, Catanduva, SP, Brazil), and 50 mL distilled water. The dispersion was homogenized by Ultra Turrax® (IKA T-10Basic) until an emulsion was formed. Thereafter, 3 % (m/v) micronized lignin was added and the emulsion was homogenized again. The colloidal suspension was dried using Spray-drying (Büchi B-290), obtaining powder microparticles (Costa et al. 2017).
After formulated the total amount of azadirachtin powder was determined by HPLC according to Forim et al. (2013). Five different quantities of azadirachtin in lignin-microencapsulated neem extract were assessed, comparing them with the control treatments mentioned previously. The rates used in this assay were 160, 80, 40, 20, and 10 mg of powder (microparticles, 1,100 mg kg-1) per 25 g of soil, corresponding respectively to the quantities of 176, 88, 44, 22 and 11 µg of azadirachtin. Prior to treatment application, the rates were quantified using an analytical scale (model AS200S, Florham Park, NJ), and the soil that had covered the plant roots was removed using a spatula. After application, the roots were covered with that same soil, and two 9-d-old D. speciosa larvae were released on the base of each plant using a soft bristle paintbrush.
Assessments of application of neem formulations on Diabrotica speciosa development and survival
Ten days after infesting the maize plants with D. speciosa larvae, we assessed the number of pupae, number of leaves per plant, and the plant height. The D. speciosa pupae were placed into Petri dishes (9 cm diameter) for posterior evaluation of adult emergence. Petri dishes were lined with 4 g fine-grained vermiculite, which was moistened with 1 mL deionized water. After adult emergence, 24 h-old adults were weighed using an analytical scale (model AS200S, Florham Park, NJ), and the proportion of males and females recorded.
Assessment of application of neem formulations on maize growth
To evaluate the effects of neem formulations on maize growth, the plants were oven-dried (model AS200S, Quimis, Diadema, state of São Paulo) at 60 °C for 48 hours, right after removal of pupae. Dried matter of the aerial part (aboveground plant tissue) and root system (belowground plant tissue) of plants were measured using an analytical scale. Reduction percentages of plant height, dry matter of plant aerial part and root system, and number of leaves were calculated based on differences of plant growth in untreated and treated maize using the formula: X = (PC - PT)/PC * 100, where X = reduction percentage; PC = value of evaluated parameter in control (uninfested maize); PT = value of evaluated parameter in the treatments (infested maize).
Statistical analysis
Data were checked for normality of residuals (Kolmogorov-Smirnov’s test) and homogeneity of variances (Bartlet’s test). Data on D. speciosa biological parameters were transformed to square root (x + 0.5) and data on plant growth parameters to log (x+5) prior to statistical analysis. Data were analyzed with one-way ANOVA, and when significant means were compared by Tukey’s HSD test (α = 0.05), using the software Sisvar, version 5.3 (Ferreira 2010). Percentage of control efficiency of treatments was calculated using Abbott’s formula (Abbott 1925).
Results
Oil-formulated neem extract
There were significant differences in the number of survived pupae of D. speciosa among treatments after application of oil-formulated neem extract (Table 1). The highest number of survived pupae was observed in the deionized water control (negative control treatment), in which all insects were recovered. The insecticide fipronil WG (positive control) caused complete D. speciosa larval mortality (no survived pupae); however, this control treatment did not significantly differ from all rates of oil-formulated neem extract.
Adults of D. speciosa emerged only in the deionized water control (Table 1). Hence, only for this treatment were reported results of duration of larva-to-adult period, adult weight, sex ratio, and adult longevity (23.89 days, 105.11 mg, 0.47, and 5.26 days, respectively) (data not shown).
Table 1 Number of survived pupae and adults (mean ± standard error) of Diabrotica speciosa after application of doses of oil-formulated neem extract.
| Treatment | Survived insects | |
|---|---|---|
| Pupae a | Adults | |
| Oil-formulated neem extract | ||
| 0.25 mL/25 g soil | 0.30 ± 0.15 a | -b |
| 0.50 mL/25 g soil | 0.20 ± 0.20 a | - |
| 1.00 mL/25g soil | 0.30 ± 0.15 a | - |
| 1.00 mL/25 g soil | 0.10 ± 0.10 a | - |
| 4.00 mL/25 g soil | 0.10 ± 0.10 a | - |
| Control (deionized water) | 2.00 ± 0.00 b | 1.90 ± 0.10 |
| Fipronil WG (0.032 %) | 0.00 ± 0.00 a | - |
| F | 25.38*** | - |
| P | < 0.0001 | - |
Means followed by the same letter in column did not differ significantly by Tukey’s test at 5 % probability. For analysis, data were transformed in (x + 0.5)1/2. a Number of insects that passed to the pupa phase; b Insufficient data to perform statistical analyses. ***, significant at 0.1 % of probability by F test.
Results of percentage of control efficiency (Abbott 1925) of D. speciosa based on the number of survived pupae are illustrated in Figure 1. Values ranged from 85 to 100 % larval control, and even the lowest rate of oil-formulated neem extract (0.25 mL 25 g-1 soil) provided an efficient percentage (> 80 % mortality) of D. speciosa / control.
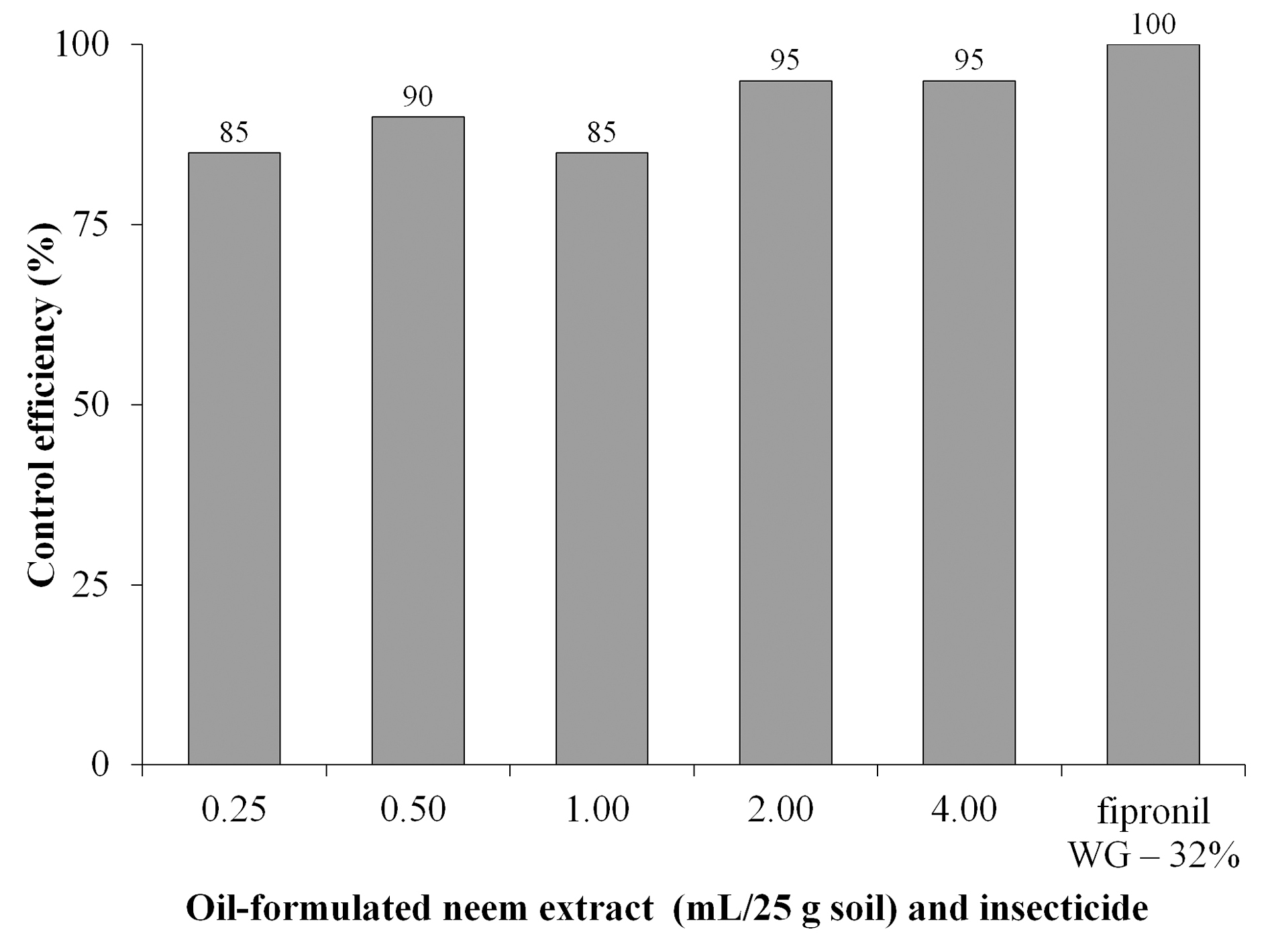
Figure 1 Percentage of control efficiency of Diabrotica speciosa (based on pupae number) by doses of oil-formulated neem extract and insecticide fipronil, calculated by Abbott’s formula (1925).
Concerning the reduction percentages of plant growth (Table 2), there were significant differences only for dry matter of root system. Higher reduction percentages of dry matter of plant root system were found for the rates of 1.0 mL 25 g-1 soil (11.93 % reduction) and 2.0 mL 25 g-1 soil (11.58 % reduction), differing from fipronil WG (no reduction). However, these rates did not differ from the other rates of oil-formulated neem extract. Also, the rates 0.25, 0.5, and 4.0 mL 25 g-1 soil did not differ from fipronil WG (9.14, 9.09, and 8.85 % reduction of dry matter of root system, respectively).
Table 2 Reduction percentages (mean ± standard error) of height, dry matter of the aerial part and root system, and number of leaves of maize plants after application of doses of oil-formulated neem extract and insecticide fipronil.
| Treatment | Height (cm) | Dry matter (mg) | Leaves (No.) | |
|---|---|---|---|---|
| Aerial part | Root system | |||
| Oil-formulated neem extract | ||||
| 0.25 mL/25 g soil | 31.83 ± 8.97 a | 35.52 ± 11.73 a | 9.14 ± 4.75 ab | 25.83 ± 7.90 a |
| 0.50 mL/25 g soil | 11.38 ± 6.57 a | 11.07 ± 5.26 a | 9.09 ± 2.62 ab | 13.33 ± 4.51 a |
| 1.00 mL/25g soil | 12.77 ± 7.01 a | 6.15 ± 4.19 a | 11.93 ± 3.49 b | 7.50 ± 3.82 a |
| 1.00 mL/25 g soil | 13.31 ± 7.13 a | 10.69 ± 5.53 a | 11.58 ± 3.45 b | 19.17 ± 7.56 a |
| 4.00 mL/25 g soil | 22.55 ± 6.98 a | 9.08 ± 6.22 a | 8.85 ± 3.57 ab | 19.17 ± 5.70 a |
| Fipronil WG (0.032 %) | 30.80 ± 10.06 a | 18.56 ± 7.61 a | 0.00 ± 0.00 a | 25.00 ± 6.92 a |
| F | 1.30 NS | 1.31 NS | 2.80 * | 0.95 NS |
| P | 0.3000 | 0.2736 | 0.0257 | 0.4562 |
Means followed by the same letter in column did not differ significantly by Tukey’s test at 5 % probability. For analysis, data were transformed in log (x + 5). *, significant at 5 % probability by F test. NS, non- significant at 5 % probability by F test.
Lignin-microencapsulated neem extract
The powder of lignin microparticles loaded with ethyl acetate fraction from neem ethanolic extracts showed clusters of microparticles, which had an average size around 1.0 µm (Fig. 2). Once the colloidal suspension dried by Spray-drying, all azadirachtin was incorporated in the lignin microparticles.
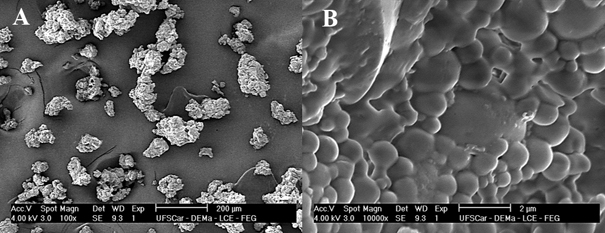
Figure 2 Scanning electron micrographs of lignin microparticles with amplification of (A) 100× and (B) 10,000×.
There were significant differences in the number of survived pupae of D. speciosa after application of rates of lignin-microencapsulated neem extract (Table 3). For the other biological parameters of insect development, however, there were not enough data to perform statistical analysis due to high larval mortality. Significantly higher D. speciosa survivorship was observed in the deionized water control, whereas the other treatments provided similar results for insect survival. For the rates of 160, 40, and 20 mg 25 g-1 soil of lignin-microencapsulated neem extract, no survived pupae was recovered, as well as in fipronil-treated plants.
Since all treatments caused high D. speciosa larval mortality, adult emergence was observed only in the deionized water control (Table 3). Values of duration of larva-to-adult period, adult weight, sex ratio, and adult longevity were 20.64 days, 8,47 mg, 0.64, and 5.67 days, respectively (data not shown).
Table 3 Number of survived pupae and adults (mean ± standard error) of Diabrotica speciosa after application of doses of lignin-microencapsulated neem extract and insecticide fipronil.
| Treatment | Survived insects | |
|---|---|---|
| Pupae a | Adults | |
| Lignin-microencapsulated neem extract | ||
| 160 mg/25 g soil | 0.00 ± 0.00 a | -b |
| 80 mg/25 g soil | 0.10 ± 0.00 a | - |
| 40 mg/25 g soil | 0.00 ± 0.00 a | - |
| 20 mg/25 g soil | 0.00 ± 0.00 a | - |
| 10 mg/25 g soil | 0.10 ± 0.10 a | - |
| Control (deionized water) | 1.40 ± 0.16 b | 1.20 ± 0.20 |
| Fipronil WG (0.032 %) | 0.00 ± 0.00 a | - |
| F | 40.36*** | - |
| P | < 0.0001 | - |
Means followed by the same letter in column did not differ significantly by Tukey’s test at 5 % probability. For analysis, data were transformed in (x + 0.5)1/2. a Number of insects that passed to pupa phase. b Insufficient data to perform statistical analysis. ***, significant at 0.1 % probability by F test.
The values of percentage of control efficiency of D. speciosa are shown in Figure 3, and ranged from 93 to 100 %. According to the results, even the lowest rate of lignin-microencapsulate neem extract (20 mg 25 g-1 soil) provided an efficient control (> 80 % mortality) of D. speciosa.

Figure 3 Percentage of control efficiency of Diabrotica speciosa (based on pupae number) calculated by Abbott (1925) formula, for doses of neem extract microencapsulated by lignins.
Considering the reduction percentages in maize growth recorded from untreated and treated plants (Table 4), significant differences were not found among treatments. Similar to the effects observed in maize growth following application of oil-formulated neem extract, lignin microencapsulated neem extract did not affect maize growth, and all rates of the neem extract were similar to fipronil WG.
Table 4 Reduction percentages (mean ± standard error) of height, dry matter of the aerial part and root system, and number of leaves of maize plants after application of doses of lignin-microencapsulated neem extract and insecticide fipronil
| Treatment | Height (cm) | Dry matter (mg) | Leaves (No.) | |
|---|---|---|---|---|
| Aerial part | Root system | |||
| Lignin-microencapsulated neem extract | ||||
| 160 mg/25 g soil | 27.74 ± 6.26 a | 17.75 ± 8.20 a | 3.43 ± 2.63 a | 30.00 ± 5.85 a |
| 80 mg/25 g soil | 29.64 ± 9.06 a | 24.44 ± 8.86 a | 0.00 ± 0.00 a | 25.83 ± 9.17 a |
| 40 mg/25 g soil | 31.51 ± 10.93 a | 27.58 ± 10.28 a | 1.46 ± 1.46 a | 27.50 ± 7.56 a |
| 20 mg/25 g soil | 49.73 ± 10.45 a | 46.29 ± 8.83 a | 0.00 ± 0.00 a | 53.33 ± 6.48 a |
| 10 mg/25 g soil | 39.43 ± 9.91 a | 30.76 ± 9.56 a | 0.61 ± 0.61 a | 30.00 ± 7.68 a |
| Fipronil WG (0.032 %) | 30.80 ± 10.06 a | 18.56 ± 7.61 a | 0.00 ± 0.00 a | 25.00 ± 6.92 a |
| F | 0.52 NS | 1.81 NS | 1.18 NS | 1.59 NS |
| P | 0.7623 | 0.1265 | 0.3324 | 0.1785 |
Means followed by the same letter in column did not differ significantly by Tukey’s test at 5 % probability. For analysis, data were transformed in log (x + 5). NS, non-significant at 5 % probability by F test.
Discussion
Overall, the botanical insecticide neem in both formulations, oil and microencapsulated by lignin, proved to be as efficient as the synthetic insecticide fipronil WG to control D. speciosa larvae under laboratory controlled conditions. In the assays, all doses of both neem formulations caused pest mortality rates similar to that provided by fipronil WG, preventing the emergence of adults. In addition, negative effects on maize growth were not found.
According to Isman (2000), botanical pesticides with highest efficacy for controlling insect pests may also be the most toxic to plants. However, in the present study all tested rates of both neem extract formulations did not affect maize growth parameters. Despite the fact that oil-formulated neem extract at 1.0 mL 25 g-1 soil and 2.0 mL 25 g-1 soil reduced the dry matter of plant root system, no interference on the other plant growth variables was observed. According to Corrêa and Salgado (2011), the occurrence of phytotoxic effects depends on how chemical compounds are applied and the doses that are used. In this context, our research addressed different neem formulations, application methods, and doses to control D. speciosa larvae, achieving successful results also for the performance of maize plants growth. Because of the high insect mortality rates and antifeedant activity caused by plants of the Meliaceae family (Carpinella et al. 2003), we can infer that the neem formulations at the conditions evaluated in our study do not cause phytotoxicity in maize.
In spite of the importance of studying botanical pesticides to develop new strategies of pest management, there is a huge limitation for the use of some botanical pesticides formulations under determined conditions. For example, it is not efficient to use plant aqueous extracts with insecticidal activity under intensive sunlight (Ventura and Ito 2000). The current research has shown the efficiency of different formulations of neem-based bioinsecticides in the laboratory, and the most efficient doses are promising to be further assessed under greenhouse and field conditions. It is noteworthy that lignin-microencapsulated neem extract formulation is protected by a lignin layer, which prevents the rapid photo and thermal degradation of the active compound azadirachtin (Costa et al. 2017); this is considered to be a limitation for the use of botanical insecticides in crop protection. Therefore, the lignin-microencapsulated formulation displayed 100 % control when used at some doses, which did not happen with the oil-formulated extract. In addition, the lowest mortality rates caused by the lignin-microencapsulated formulation reached 93 % control, which is above the minimum efficacy threshold required in Brazil to allow registration of a conventional insecticide (MAPA 1995; Guedes 2017).
Use of synthetic insecticides is currently regarded as the most efficient tactic for pest management; however, the evolution of insect resistance and other negative collateral effects in the environment have incited a growing body of research with the aim of developing new strategies of pest control. Wild plants with insecticidal activity, such as neem, may provide proper plant protection against the attack of herbivores due to their wide range of chemical compounds, which may be explored for protection of susceptible but high-yield cultivars of plants with economic importance to agriculture (Luo et al. 1995). In addition, they represent a base for effective botanical pesticides and environmental security (Carpinella et al. 2003). Finally, botanical pesticides slow the development of pest resistance as they usually have several active ingredients in their composition as compared to the majority of synthetic insecticides available that have only one active ingredient.
Botanical pesticides may eventually impose some risks, and complete safety cannot be assumed as long as all types of effects on different non-target organisms are tested. To date, most botanical pesticides are characterized by low toxicity to mammals, reduced effects on non-target organisms, and minimal environmental persistence (Isman 2006).
It is very important to develop other means of pest control, not relying just on a couple of them, because insects can become resistant if rational control methods are not adopted (Fabrick et al. 2014). From the results obtained in this preliminary study under laboratory conditions, neem microencapsulated formulations emerge as an alternative and promising method for controlling D. speciosa larvae in maize, benefiting a sustainable pest management. However, new studies are necessary to investigate the efficacy of lower doses of lignin-microencapsulated neem extract formulations, having as standard the lowest doses tested in this research. Thereafter, the lowest highlighted doses will be assessed in the greenhouse and field to test for their control efficiency and residual effect.














