Introduction
Understanding of species distribution patterns is a key question in ecology (Sutherland et al. 2013). In a broad way, niche theory (NT), as proposed by Hutchinson (1959), has been widely applied for this purpose. NT predicts that environments with similar conditions will harbor similar species pool by establishing a relation between species distribution and habitat heterogeneity and availability (Chase 2003; Popielarz and Neal 2007). In aquatic environments, species relation with niche and environmental heterogeneity over different spatial and temporal scales are discussed by the hierarchical patch dynamics (HPD) (Thorp et al. 2006). HPD proposes that the zoning of geomorphic and hydrological characteristics is responsible for the formation of hydro-geo-morphological patches, which, in turn, are responsible for species distribution (Brasil et al. 2014a).
Environmental heterogeneity of the river channel, caused by spatial or temporal variation of water physicochemical features and substrate complexity, defines environmental patches that affect species distribution due to a set of features and resources favorable to their functional, physiological and life history traits (Poff and Ward 1990). Therefore, the substrate plays a major role in species distribution, as it provides conditions, resources, shelter and feeding for stream fauna (Resh and Rosenberg 1984; Shimano et al. 2012; Dias-Silva et al. 2013). The expression of refugees, habitats or factors that provide resistance and/or resilience to biotic communities occur at different spatial scales, such as micro-habitats and zones (Sedell et al. 1990) within the channel of the creeks, with the substrates: sand, root, gravel and litter composing the physical heterogeneity of the riverbed (García-Roger et al. 2011). Each one of these substrates have different characteristics and, therefore, different thresholds concerning its capacity for shelter and maintenance of the local fauna.
Among aquatic organisms, insects stand out in ecological analysis due to their high diversity (Dijkstra and Clausnitzer 2006), broad distribution and key roles in trophic webs (Tomanova et al. 2006, Ramírez and Gutiérrez-Fonseca 2014). Additionally, insects are essential for the energy flux between aquatic and terrestrial ecosystems, as their life cycle comprises a juvenile aquatic stage and an adult terrestrial stage (Wesner 2010). Other than that, specimens of the order Ephemeroptera, Plecoptera and Trichoptera (EPT) are widely used as biological indicators in ecological studies that evaluate the effect of biotic and abiotic processes in streams (Heino et al. 2007; Heino 2011; Couceiro et al. 2012; Heino and Peckarsky 2014; Nogueira et al. 2016). Other than EPT, orders Odonata (O) (Mendes et al. 2015; De Marco et al. 2015; Juen et al. 2016) and Heteroptera (H) (Dias-Silva et al. 2010; Giehl et al. 2014; Giehl et al. 2015; Cunha et al. 2015) were also indicated as relevant for understanding the ecological processes of aquatic insect communities in tropical streams, what reinforces the need to consider the whole EPTOH in order to understand ecological patterns of the Cerrado’s aquatic insect communities.
Previous studies indicate that, for Cerrado streams, organic substrates, such as leaf packs and roots, have a higher abundance and diversity of Heteroptera from both suborders Nepomorpha (Dias-Silva et al. 2013) and Ephemeroptera (Shimano et al. 2012), when compared with inorganic substrates, such as sand and gravel. However, there are no evaluations about preference and diversity patterns in organic and inorganic substrates when several orders are taken into account. In our study we tried to address this issue and better represent the entire community of aquatic insects by considering juveniles of Ephemeroptera, Plecoptera, Trichoptera and Odonata and adults of aquatic and subaquatic Heteroptera (EPTOH).
We verified the community structure in Cerrado streams with both organic and inorganic substrate and tested for possible differences in richness (i), abundance (ii) and genera composition. Further, (iv) we looked for genera that may act as biological indicators, due to its specificity and fidelity to organic or inorganic habitats.
Material and methods
Study area
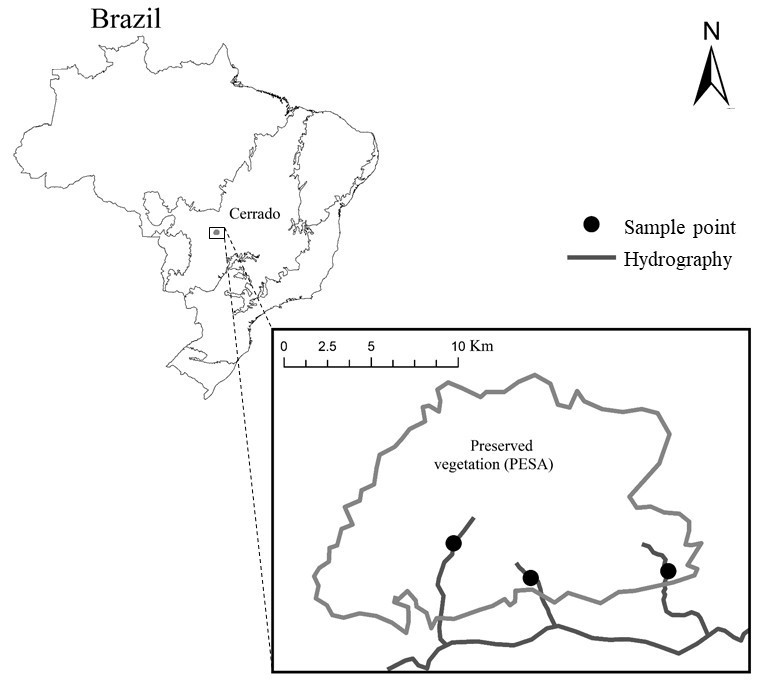
Samples were collected in three streams of the Cerrado Biome (coordinates: 15°50’19,9”S e 052°14’47,6”W; 15°51’11,5”S e 52°08’08,3”W; 15°51’24,4”S e 052°12’24,3”W) (Fig. 1). All streams are in the River Garças basin, affluent of Araguaia River. The region climate is Tropical Savanah Aw (Peel et al. 2007), characterized by two well-defined seasons, a rainy season from October to April and a dry season from May to September. All the streams are in the Parque Estadual da Serra Azul and its environmental protected area (APA), which is part of the Complexo Serra do Roncador, MT.
Taxon sampling and identification
We sampled immature aquatic insects of the orders Ephemeroptera, Plecoptera, Trichoptera, Odonata and adults aquatic and semi-aquatics Heteroptera in two different seasons: rainy (December 2012) and dry (August 2013). There are several benefits for collecting on two different seasons, such as:(i) account for possible sampling bias related to the seasonal variation of Cerrado’s streams, (ii) increase the number of captured organisms and (iii) reduce possible biases caused by the lower detectability of rare organisms. We also joined temporal replicates in a way that the combination of both represents a sampling unit to account for temporal dependence.
On each stream we defined 50 meter linear transects and further divided those transects to create five 10 meter sections. For each section we sampled twice with a Surber, one for organic substrate (litter) and the other for inorganic substrate (sand and gravel), in that way we had fifteen sampling units for each substrate type, five in each stream (three streams). According to Costa and Melo (2008), aquatic insect fauna has a greater difference in different habitats within a stream when compared with similar habitats on different streams. Therefore, it is reasonable to assume a certain degree of independence among different habitats within the same stream, which, in its turn, allows for the use of those locations as replicates, since there are little effects regarding possible bias related to spatial dependence among samples.
We identified EPTOH specimens by recurring to generic taxonomic keys (Nieser and Melo 1997; Costa et al. 2004; Salles 2006; Domínguez et al. 2006; Mugnai et al. 2010; Hamada et al. 2014; Neiss and Hamada 2014) and stored our samples on the Zoobotanical Collection “James Alexander Ratter”, UNEMAT, Nova Xavantina Campus.
Data analysis
For the statistical analysis it was considered 30 sampling units, of these, 15 were from organic substrates and 15 inorganic substrates. The experimental design and the number of insects collected per sample are detailed in Supplementary 1. In order to test for possible differences for both richness and abundance among organic and inorganic substrates we performed one t-test for each, in which we used genera richness (i) and individual abundance (ii) as response variables, and the substrate type (organic or inorganic) as predictor variables. To test for possible differences in beta diversity among substrate types we first performed an ordination while testing for divergences in multivariate homogeneity, as recommended by Anderson et al. (2006), on the specimens matrix using the Bray-Curtis distance. We then tested cluster significance with a PERMANOVA by using the same genera abundance matrix and considering organic and inorganic microhabitats as a factor (iii). Finally, we verified taxon fidelity and specificity to a micro-habitat (organic or inorganic) by calculating its indicator value (IndVal) (iv) (Dufrene and Legendre 1997).We performed a Principal Coordinate Analysis (PCoA) using the array of species composition (abundance) transformed with the Bray-Curtis distance to demonstrate the relationship of selected taxa in IndVal with the sampling units (organic and inorganic) (Legendre and Legendre 2012). All analyses were performed in R software (R Core Development Team 2017).
Results
A total of 1,156 EPTOH specimens from 41 genera were collected, of those the most abundant were Ulmeritoides Traver, 1959 with 226 individuals (19.55 % of the total abundance) and Miroculis Edmunds, 1963 with 212 (18.34 %) (Ephemeroptera: Leptophlebiidae). The rarest genera were Baetodes Needham e Murphy, 1924 (Ephemeroptera: Baetidae), Elasmothemis Westfall, 1988 (Odonata: Libellulidae) and Lauromacromia Geijskes, 1970 (Odonata: Corduliidae) with only one sampled individual, which stands for 0.09 % of the total abundance (Supplementary).
Community structure differed in all aspects, when comparing organic and inorganic substrates. Organic substrates had an average of 6.6 genera and 60 individuals more than inorganic substrates (Tsep. var.= -3.46, df = 21.21, P = 0.002, Tsep. var.= -4.27, df = 14.41, P < 0.001, respectively).
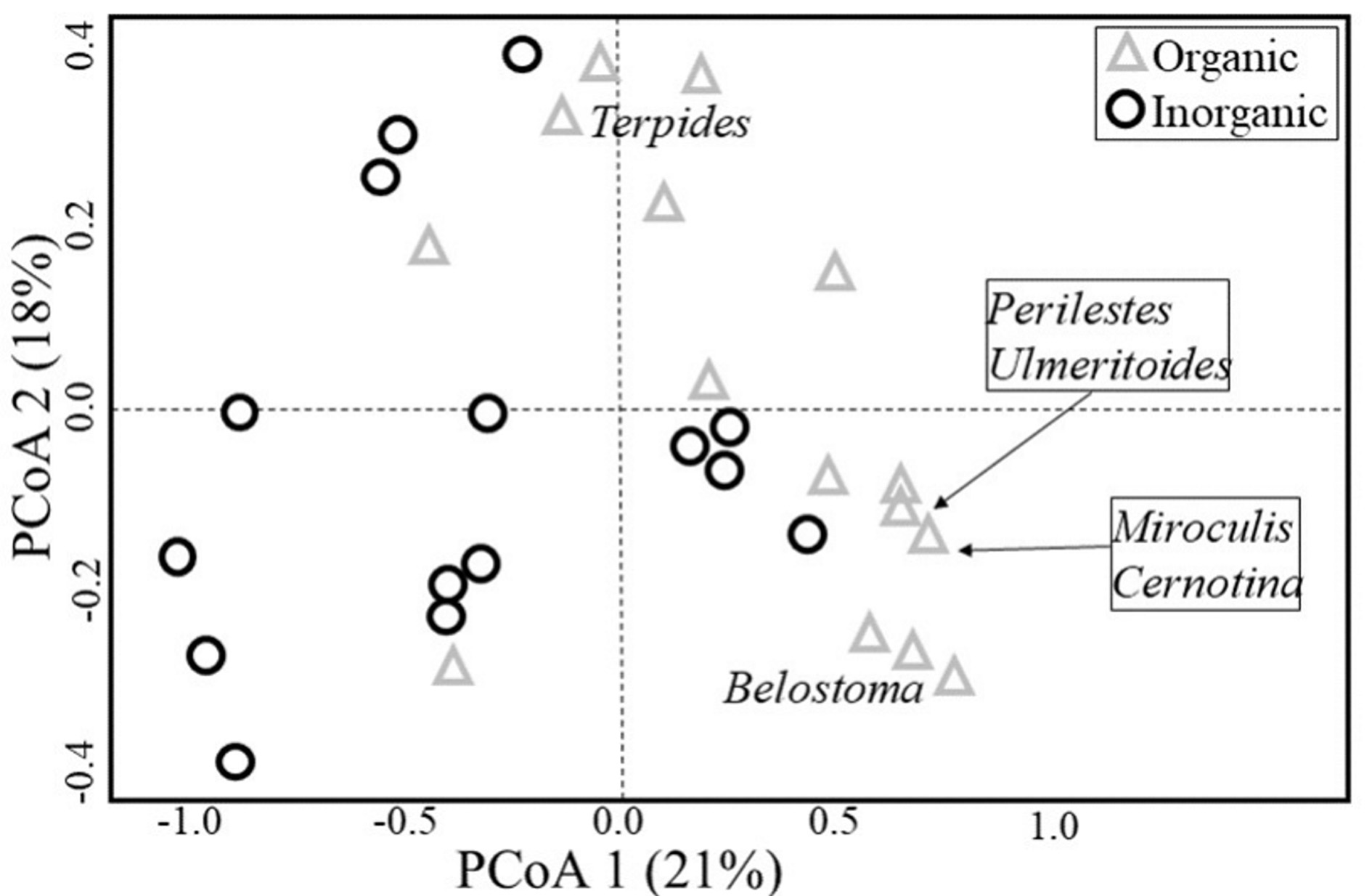
Species composition also differed among habitats (PERMANOVA, pseudo F = 3.407, P = 0.001) (Fig. 2), with six genera being characterised as indicators for organic habitats (Table 1 and Fig. 3). The distance between centroids in Figure 2 and the low overlap (or peripheral overlap) of the organic sampling units over the area defined by inorganic sampling units reinforces the difference between habitats and suggests a community associated with organic substrates which is different from another associated with inorganic ones, even if those locations occur on the same stream.
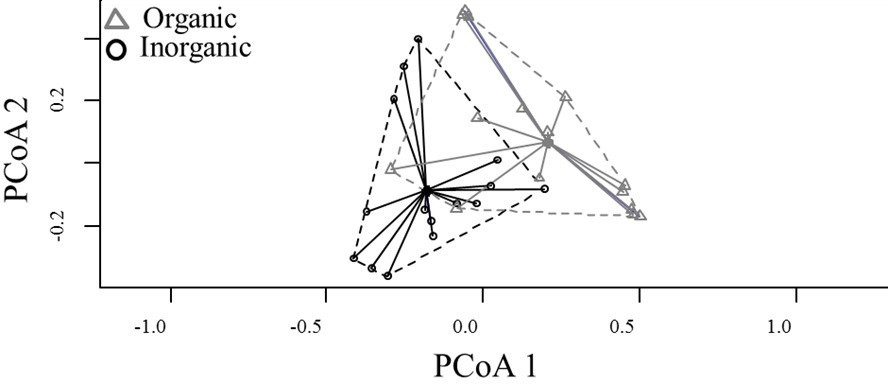
Figure 2 Ordination (Homogeneity of multivariate dispersions within groups) of the taxonomic composition of aquatic insects in organic and inorganic habitats within Cerrado streams of Central Brazil.
Table 1 Indicator Species Index (ISI) of those genera identified as indicators of organic habitats within Cerrado streams of Central Brazil.
| Order | Family | Genera | ISI | P | Microhabitat |
|---|---|---|---|---|---|
| Odonata | Coenagrionidae | Argia Rambur, 1842 | 0.632 | 0.045 | Organic |
| Perilestidae | Perilestes Hagen in Selys, 1862 | 0.577 | 0.040 | Organic | |
| Trichoptera | Polycentropodidae | Cernotina Ross, 1938 | 0.679 | 0.010 | Organic |
| Ephemeroptera | Leptophlebiidae | Miroculis Edmunds, 1963 | 0.906 | 0.045 | Organic |
| Terpides Demoulin, 1966 | 0.570 | 0.035 | Organic | ||
| Ulmeritoides Traver, 1959 | 0.816 | 0.020 | Organic |
Discussion
EPTOH’s community structure was different among habitats for all evaluated aspects. Organic substrate habitats had a greater diversity and a set of taxa that was specific and faithful to it. Our results indicating a higher diversity in organic substrates are in accordance with previous observed patterns for Ephemeroptera (Shimano et al. 2012), Heteroptera (Dias-Silva et al. 2013), Simuliidae (Hamada 1989) and for the entire aquatic insects’ community (Beisel et al. 1998).
Taxa distribution within a stream is strongly related to the availability of environmental resources (Allan and Castillo 2007), among which food supply stands out (Cummins et al. 2005). That becomes clear in environmental patches with abundant food supply for a specific group, which results in a high abundance of individuals for this group and affects patterns of taxa distribution within a stream (Brasil et al. 2014a ). As organisms preferentially colonize environmental patches with suitable conditions (Thorp et al. 2006). Environmental conditions at small spatial scale are what guide the distribution of aquatic insect communities (Godoy et al. 2016).
In our study six taxa had fidelity and specificity to organic substrates, among which three were dependant on the litter (organic substrate) for feeding: shredders genera Terpides Demoulin, 1966 (Ephemeroptera: Leptophlebiidae) and genera Ulmeritoides (Shimano 2012, Brasil et al. 2014b ) and the brusher genera Miroculis, that sweeps decanting particles (Baptista et al. 2006). The other three taxa, which were all predators (Corbet 1999, Spies et al. 2006), can also have its distribution being affected by the availability of food supply, since the higher values for richness and abundance of EPTOH were found in organic substrate habitats, which indicates a higher availability of potential prey. Such relation of the availability of prey affecting its predators’ distribution was already described for aquatic environments, especially in cases where there is a bottom-up relation between prey and predator (Angelini et al. 2013).
The lack of taxa with high specificity and fidelity to habitats with inorganic substrates could be explained by the low abundance of apparent potential taxa, such as Baetodes, Brechmorhoga Kirby, 1894 (Odonata: Libeluliidae), Elasmothemis e Macrostemum Kolenati, 1859 (Trichoptera: Hydropsychidae). Shimano et al. (2012) obtained indication Baetodes for stones and leaf litter of rapids, being of scraper MPOF weakly attached to substrates.
Other than food supply, another factor that may play a key role in the distribution of aquatic insects within a stream is the ability of the habitat to provide shelter. That is because most taxa do not have the specific morphology which would allow them to occupy locations with high currents, an example of a genera with such adaptations would be Spiritiops Lugo-Ortiz e McCafferty, 1998 (Ephemeroptera: Baetidae), found in the walls of waterfalls (Brasil et al. 2014a). Therefore, the shelter created by the litter in habitats with organic substrate may play a major role in reducing the odds of stream loading the processes of ecological drift, in which the water flow causes the passive dispersion of a subset of the community (Waters 1972; Brittain and Eikeland 1988).
Conclusion
There are differences in genera richness and composition and individual abundance between organic and inorganic substrates. It is possible to imply genera that have a high fidelity and specificity to organic habitats, of which two are shredders, one is a brusher and three are predators. Habitats created by litter (organic) are used by a broader diversity of aquatic insects, due to, according to several authors, its higher food supply and structure as shelter. As riparian forests are the main source of allochthones materials, which are responsible for the creation and maintenance of the litter inside streams, our results reinforce the need to preserve those forests. In addition, besides the advantages listed on this paper, riparian forests are the ones that makes it possible for the stream to sustain the diversity of aquatic insects as they are responsible for a high input of energy into the system, in the form of leaves.