Introduction
Soybean, Glycine max (L.) Merril, is an important legume in worldwide agricultural scenario, being one of the most important commodities in Brazil (Sturmer 2014). The estimated production during 2015/16 crop season was of 95.6 millions of tons, and yields reached 2882 kg.ha-1 (CONAB 2016). Several factors may negatively affect soybean yields reducing farmer profitability, among them, insect pests that attack soybeans throughout its life cycle. One of the main entomological problems found in soybean crops is the stink bugs complex, because they damage the marketed product (Rocha et al. 2015). These insects cause injuries produced by digestive enzymes injected during the insect feeding, which can cause grain abortion, low levels of seed germination and vigor, and also leaf retention (Oliveira 2010; Silva et al. 2013). Such losses may range from 49 to 125 kg.ha-1 at an insect density of one stink bugs.m-1 (Guedes et al. 2012).
Pesticides are the most commonly used management tactic to control these pests; however, it often fails to respect the recommended action thresholds for each crop, increasing thus the number of sprayings (Bueno et al. 2013). Conversely, an excessive use of insecticides might increase population resistance; besides of an augmented use of broad-spectrum insecticides that can reduce natural enemies, increase environmental contamination and risks to human health (Sosa-Gómez and Silva 2010; Bueno et al. 2011; Belo et al. 2012). Furthermore, potential prohibitions of certain insecticides, without market introduction of new molecules may lead to reduction on the number of active ingredients used to control stink bugs in soybeans (Guedes et al. 2012).
Natural enemies may be used to rationalize the use of insecticides; among them, the stink bug egg parasitoids, which are important agents to control these pests (Lopes et al. 2012). For that, the population of stink bugs have to be estimated through field sampling, which it can help in management decisions (Silva et al. 2014). In Brazil, an action threshold of two insects.m-1 is recommended for stink bugs in grain production and of one stink bugs.m-1 for seed production (Bueno et al. 2013).
The beat cloth is the sampling method recommended to estimate stink bug population levels and insecticide application (Bueno et al. 2013). With this estimation, it is possible to reduce the number of sprayings without compromising pest management. Therefore, this study aimed to evaluate different action thresholds for stink bugs management on soybean and their influence on the phytotechnical and physiological parameters as well as on stink bugs eggs parasitism.
Materials and methods
Experimental design
Field experiment was carried out at the Mato Grosso do Sul State University (Universidade Estadual de Mato Grosso do Sul) - Campus of Cassilândia (19º05’S latitude, 51º56’W longitude, and altitude of 471 m) in the crop season of 2010-2011, in a 2,000 m2 area. The soybean variety used was ‘Anta 82 RR’ (120 days cycle) with spacing 0.45m and no surrounding crops. Treatments were established of five action thresholds of phytophagous stink bugs: half, one, two, four insects per meter, and a control treatment (without insecticide spraying), with four replications each treatment, totaling 20 plots. Once stink bugs (> 0.5 cm) population achieved 90 % infestation for each action thresholds, the systemic imidacloprid (neonicotinoid) + beta-cyfluthrin (pyrethroid) 75.0 + 9.38 g active ingredient per hectare, respectively, was sprayed using a compressed CO2 sprayer equipped with six AXI11002 nozzles spaced in 0.5 m, at a working pressure of 2.5 bar.
Crop practices consisted of seed treatment, fertilization with 300 kg.ha-1 ordinary superphosphate and 100 kg.ha-1 potassium chloride at sowing. In addition, weed control was conducted spraying (glyphosate) at a dose of 1.59 kg ai.ha-1 and spray volume of 150 L.ha-1. Preventive fungicide spraying was carried out with azoxystrobin + cyproconazole at 60 and 24 g ai.ha-1, respectively, using a spray volume 150 L.ha-1 + 0.5 % mineral oil paraffin, applications started at the beginning of the flowering, and were sprayed again every 20 days. It noteworthy mention that no control measure was taken against defoliator pests, because they did not reach the action threshold during sampling.
Stink bug sampling
Stink bugs were sampled by beat cloth at three spots by replication. Samplings were performed at different phenological stages: R2 (full flowering), R3 (end of flowering), R4 (early pod filling), R5.1 (early grain filling - 10 % full grains), R5.2 (11 to 25 % full grains), R5.3 (26 to 50 % full grains), R5.4 (51 to 75 % full grains), R5.5 (76 to 100 % full grains), R6 (100 % full grains), R7.1 (early leaf and pod yellowing), R7.2 (75 % leaf and pod yellowing), R7.3 (above 76 % leaf and pod yellowing) and R8.1 (50 % defoliation) (Borges and Anselmo 2011). In total 13 samplings.
Evaluation on soybean phytotechnical and physiological parameters
Soybean phytotechnical and physiological parameters were conducted after samplings of stink bugs and parasitoids. Yield assessment was obtained from 8-m plants within centerline of each replication which was manually threshed and weighted. A sample of 100 seeds of each repetition was selected randomly to determine the moisture content as follows: seeds were weighted and placed on Petri dishes and taken to closed circulation oven at 105 °C for 24 hours, being the moisture content estimated by the formula: MC = 100 x (W - w) / (W - t), where MC = moisture content, W = container weight + wet seed weight, w = container weight + dry seed weight, t = container weight. Subsequently, the weight of seeds were corrected to 13 % moisture using a correction factor: CW = MS x (100 - MC) / (100 - 13), where, CW = seed weight corrected to 13 % of moisture content and MS = moist seed weight and it was estimated the yield per hectare. Another sample of 100 seeds from each replicate was randomly selected to determine the 100-seeds mass. We emphasized that the moisture content of the 100-seeds mass was also corrected to 13 %.
Pod damage index - PDI (%) was determined in pods sampled from plants within two meters along centerline of each replication. Firstly, pods were classified in full, intermediary and empty pods according to method proposed by Nagai et al. (1987). PDI was calculated using the formula: PDI= ½ (% intermediary pods) + (% empty pods).
The ratios seeds per pod (seeds.pood-1), seeds per plant (seeds.plant.-1) and pods per plant (pods.plant-1) were obtained from 10 soybean plants randomly selected that were used in PDI quantifications. These plants were threshed and pods and seeds were subsequently quantified. Leaf retention was assessed in all plants on centerline of each replication, being individually evaluated and classified into plants with and without leaf retention. We assumed as leaf retention, those plants with at least one green leaf or stem.
Tetrazolium test was performed with the seeds harvested (5m) from plants of the centerline of each plot. Seeds from all plots for each treatment were homogenized and 100 seeds were randomly selected for each treatment. Tetrazolium test was performed with two replicates of 50 seeds, according to the methodology proposed by França Neto et al. (1998). This test was used to quantify seed vigor, viability and damage (%) in addition to their respective causes (stink bugs, moisture, and mechanical damage).
Parasitism evaluation
During sampling of stink bugs, eggs were collected to evaluate parasitism. All plants were sampled within one meter for each replication. Egg clusters were taken to the laboratory, individually and placed into Petri dishes on moistened filter paper. The containers were stored inside BOD type chambers at a temperature of 25 ± 2 °C, 70 ± 5 % relative humidity and 12 h photoperiod, until stink bug nymph hatching or parasitoids emergence. After emergence, parasitoids were placed in 70 % alcohol to compare with specimens identified by Dr. Valmir Antônio Costa, from the Instituto Biológico (Biology Institute). Specimens are kept in collections of the Entomological Museum from Mato Grosso do Sul State University, Cassilândia Campus, Cassilândia, MS, Brazil.
Statistical analyses. The experiment was performed in a completely randomized blocks design, with five treatments and four replications. Data from yield, 100-seed mass and PDI were analyzed with Levene’s homoscedasticity test and Cramer Von Misses normality test. If their assumptions were not met, data was transformed according to the Box-Cox test and analyzed again to homoscedasticity and normality test, data with normal distribution and variance homogeneity (yield) were analyzed using PROC GLM (ANOVA) and their means were compared by Tukey’s test by SAS 9.3 software (SAS Institute 2011).
Data exhibiting non-homogeneous variance and/or non-normal distribution (100-seed mass and PDI) were analyzed with the Friedman’s test, and the means were compared by Dunns’ test with the aid of Prism 4 software (Graphpad 2003).
Data for pods.plant-1, seeds.pods-1, seeds.plant-1 and leaf retention were analyzed by Poisson distribution using PROC GENMOD and the means of these variables were compared by Tukey’s test by SAS 9.3 software. All statistical results were considered significant at P < 0.05. Data for stink bugs eggs parasitism were not analyzed statistically because at the action threshold of half stink bugs per meter, not eggs were found in some replicates.
A multivariate analysis was performed to discriminate more easily the action threshold for soybean stink bugs with similar characteristics, through a joint analysis of the evaluated variables. Therefore, each variable with means calculated were subjected to hierarchical cluster analysis (HCA) through Ward’s method and based on Euclidean distance as dissimilarity measure. Additionally, a principal component analysis (PCA) was carried out to identify most influencing factors on group separation. The multivariate analyses were performed using the Statistica 7 software (Statsoft 2004). Results from HCA and PCA analyses were used to determine group separations that better represented the action thresholds, being “cut off” in the Euclidean distance from linkage group 1.9 to 3.1.
Results
Population of stink bugs reached the action threshold twice for four stink bugs.m-1, five times for two stink bugs.m-1, six times for one stink bug.m-1, and seven times for half stink bug.m-1; therefore, insecticide spraying was carried out every time (Table 1). Average yields were significantly higher for action thresholds of half and one stink bug.m-1 when compared to the others treatments. Control and four stink bugs.m-1 had significantly lower yields, also differing from two stink bugs.m-1 (F4,12 = 47.82, P < 0.01). The largest 100-seed mass was observed in the action threshold of one stink bug.m-1, reaching twice as many weight as found in control (χ2 4,12 = 15.40, P < 0.01). The PDI was significantly lower in the actions threshold of half and one stink bug.m-1, differing from control (χ2 4,12 = 15.40, P < 0.01) (Table 1).
Table 1 Number of insecticide sprayings, means and standard errors of the yield Kg.ha-1, 100-seed mass (g) and pod damage index (PDI) (%) of soybeans managed according to five action thresholds for stink bugs.
| Action thresholds | Insecticide sprayings (number) | Yield (Kg.ha -1 ) -2 | 100-seed mass (g) -3 | PDI (%) -3 |
|---|---|---|---|---|
| Control | - | 347.9 ± 86.6 a | 8.4 ± 0.32 a | 99.0 ± 0.64 b |
| Four stink bugs.m-1 | 2 | 565.7 ± 125.59 a | 9.6 ± 0.45 ab | 89.8 ± 3.41 ab |
| Two stink bugs.m-1 | 5 | 2466.3 ± 331.38 b | 16.8 ± 0.29 ab | 65.7 ± 8.07 ab |
| One stink bug.m-1 | 6 | 4726.4 ± 158.25 c | 17.4 ± 0.18 b | 29.9 ± 0.62 a |
| Half stink bug.m-1 | 7 | 4303.3 ± 478.61 c | 16.0 ± 0.58 ab | 29.8 ± 0.52 a |
-2 Means with different letters in the column differ from each other by the Tukey’s test (P < 0.05). -3 Means with different letters in the column differ from each other by Dunn’s test (P < 0.05).
Insecticide sprayings were carried out at different times for each treatment. The first spraying was performed at R4 for half and one stink bugs.m-1, at R5.2 for two stink bugs.m-1, and at R5.4 for four stink bugs.m-1. Stink bug population reached peaks at R5.3 and at R5.4, for all treatments, with exception in the control treatment, where the population remained increasing until reach a peak at R7.2, reducing markedly in subsequent samplings. Sprayings have reduced the amounts of stink bugs, however, the population of stink bugs remained above the action thresholds stipulated for treatment half, one and two stink bugs.m-1 between R5.2 to R5.5 phenological stages (Fig. 1).
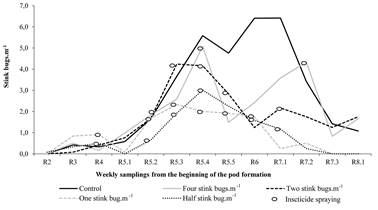
Figure 1 Average number of stink bugs.m-1 and the insecticide sprayings in soybeans managed according to five action thresholds for stink bugs.
There was no difference in the average number of pods.plant-1 among the action thresholds (χ2 4 = 9.96, P = 0.04). However, the average number of seeds.pod-1 was significantly higher in the action thresholds of two, one and half stink bug.m-1, differing from four stink bugs.m-1 and from control, showing the lowest numbers of seed.pod-1 (χ2 4 = 90.30, P < 0.01). The average number of seeds.plant-1 differed between all the action thresholds, with the highest (1 stink bugs.m-1) to the lowest (control) average number of seeds per plant (χ2 4 = 4461.30, P < 0.01). Action thresholds for leaf retention achieved 100 % in control, four and two stink bugs.m-1, differing for thresholds of one and half stink bug.m-1 (χ2 4 = 250,47, P = < 0.01) such index values were 34.6 and 43.6, respectively (Table 2).
Table 2 Means and standard errors of the average number of pods.plant-1, seeds.pod-1, seeds.plant-1 and leaf retention (%) in soybeans, managed according to five action thresholds for stink bugs.
| Action thresholds | Pods.plant -1 | Seeds.pod -1 | Seeds.plant -1 | Leaf retention -2 |
|---|---|---|---|---|
| Control | 35.2 ± 2.59 a | 0.2 ± 0.08 a | 73.3 ± 33.37 a | 100.0 ± 0.00 b |
| Four stink bugs.m-1 | 36.6 ± 4.32 a | 0.6 ± 0.15 a | 190.0 ± 32.05 b | 100.0 ± 0.00 b |
| Two stink bugs.m-1 | 36.2 ± 3.13 a | 1.2 ± 0.86 b | 415.3 ± 36.68 c | 100.0 ± 0.00 b |
| One stink bug.m-1 | 47.5 ± 4.56 a | 1.8 ± 0.03 b | 852.0 ± 74.19 e | 34.6 ± 4.56 a |
| Half stink bug.m-1 | 39.6 ± 4.30 a | 1.9 ± 0.03 b | 732.8 ± 80.07 d | 46.3 ± 9.14 a |
-1Means with different letters in the column differ from each other by Tukey’s test (P < 0.05).
Seed vigor and viability in the action thresholds of one and half stink bug.m-1 were twice greater than the others treatments, highlighting stink bug damages as the major cause of seed infeasibility. Interestingly, mechanical and moisture-related damages were less intense compared to insect injuries (Table 3).
Table 3 Means and standard errors of soybean seeds vigor (%), viability (%) and damage types (%), determined by the tetrazolium test in soybeans managed according to five action thresholds for stink bugs.
| Action thresholds | Vigor | Viability | Damage (%) | ||
|---|---|---|---|---|---|
| Stink bugs | Moisture | Mechanic | |||
| Control | 0.0 ± 0.00 | 3.0 ± 1.00 | 69.0 ± 1.00 | 27.0 ± 3.00 | 0.0 ± 0.00 |
| Four stink bugs.m-1 | 2.0 ± 2.00 | 9.0 ± 1.00 | 58.0 ± 4.00 | 29.0 ± 1.00 | 2.0 ± 2.00 |
| Two stink bugs.m-1 | 9.0 ± 3.00 | 28.0 ± 1.00 | 49.0 ± 1.00 | 21.0 ± 1.00 | 0.0 ± 0.00 |
| One stink bug.m-1 | 35.0 ± 3.00 | 59.0 ± 1.00 | 32.0 ± 2.00 | 4.0 ± 4.00 | 2.0 ± 1.00 |
| Half stink bug.m-1 | 44.0 ± 2.00 | 65.0 ± 1.00 | 31.0 ± 1.00 | 3.0 ± 1.00 | 0.0 ± 0.00 |
We established the “cut off” between the Euclidean distances from 1.9 to 3.1 (dotted lines), based on results of the HCA and PCA analyses, in which we could observe that the group separation within this “cut off” represented better the results. Thus, thresholds were divided into three groups, depending on the degree of similarity. The first group (G1) consisted of half and one stink bug.m-1. The second (G2) consisted of two stink bugs.m-1. Finally, the third group (G3) included four stink bugs.m-1 and control (Fig. 2).
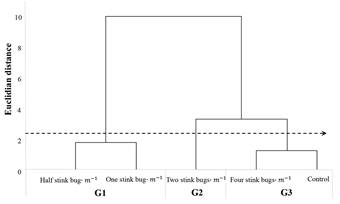
Figure 2 Dendrogram based on soybean phytotechnical and physiological parameters when managed according to five action thresholds for stink bugs. The arrow indicates the Euclidian distance used for group separation. First group (G1), second group (G2) and third group (G3).
In the PCA (Fig. 3), it was observed that the action thresholds half and one stink bugs.m-1 were to the left of third quadrant. These treatments were mostly influenced by higher numbers of pods.plant-1, higher germination rates and seeds vigor, aside from smaller leaf retention. The action thresholds of four stink bugs.m-1 and control were found in the fourth quadrant, being highly influenced by higher average number of stink bugs.m-1, higher DPI, smaller numbers of seeds.pod-1, seeds.plant-1, yields and 100 seed mass. The action threshold of two stink bugs.m-1 was isolated at the top of the first quadrant, near the center of PC 1, indicating thus little influence from a determined parameter.
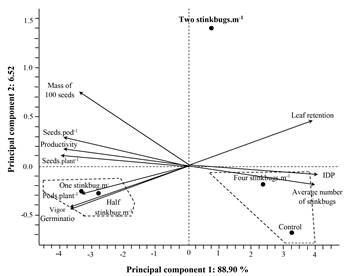
Figura 3 Principal component analysis (PCA) for the average number of stink bugs, and soybean phytotechnical and physiological parameters, when managed according to five action thresholds for stink bugs.
Eggs masses of Euschistus heros (Fabricius, 1798) (Hemiptera: Pentatomidae) and Piezodorus guildinii (Westwood, 1837) (Hemiptera: Pentatomidae) were found in all actions thresholds. The highest number of eggs was found in the control and the smallest in the area where sprayings were most frequent (half stink bug.m-1). Two parasitoids species were found, Telenomus podisi (Ashmead, 1983) (Hymenoptera: Scelionidae) in eggs of E. heros and P. guildinii and Ooencyrtus submetallicus (Howard, 1987) (Hymenoptera: Encyrtidae) in eggs of E. heros. The parasitoid, T. podisi, was responsible for 99.1 % parasitism, showing higher rates in four stink bugs.m-1 and lower rates in half stink bug.m-1 (Table 4).
Table 4 Means number of stink bug eggs, stink bug eggs parasitism (%), and parasitoids species founded in soybeans managed according to five action thresholds for stink bugs.
| Action thresholds | Stink bugs species | Stink bugs eggs (mean number) | Parasitism (%) | Parasitoids species |
|---|---|---|---|---|
| Control | Euschistus heros | 73.0 | 28.8 | Telenomus podisi |
| Piezodorus guildinii | 209.0 | 26.3 | Telenomus podisi | |
| Total | 282.0 | 27.0 | Telenomus podisi | |
| Four stink bugs.m-1 | Euschistus heros | 16.0 | 0.0 | - |
| Piezodorus guildinii | 231.0 | 55.8 | Telenomus podisi | |
| Total | 247.0 | 52.2 | Telenomus podisi | |
| Two stink bugs.m-1 | Euschistus heros | 93.0 | 47.3 | Telenomus podisi |
| 9.7 | Ooencyrtus submetallicus | |||
| Piezodorus guildinii | 191.0 | 33.5 | Telenomus podisi | |
| Total | 284.0 | 38.0 | Telenomus podisi | |
| 3.2 | Ooencyrtus submetallicus | |||
| One stink bug.m-1 | Euschistus heros | 41.0 | 29.3 | Telenomus podisi |
| Piezodorus guildinii | 83.0 | 20.5 | Telenomus podisi | |
| Total | 124.0 | 23.4 | Telenomus podisi | |
| Half stink bug.m-1 | Euschistus heros | 12.0 | 0.0 | - |
| Piezodorus guildinii | 30.0 | 26.7 | Telenomus podisi | |
| Total | 42.0 | 19.1 | Telenomus podisi |
Discussion
The action thresholds for controlling soybean stink bugs had influence on egg parasitism as well as on phytotechnical and physiological crop parameters. The number of sampled stink bugs overtook the stipulated action thresholds, which is an important fact, since low populations could harm the differences among the treatments.
The average number of stink bug decreased as action threshold were intensified, unlike yield data, which increased at higher action thresholds. Based on this, we may infer that actions thresholds have influenced in insect populations as well as increased crop yield. Gazzoni (1998), Côrrea-Ferreira and Azevedo (2002) and Bueno et al. (2015) found different results. These authors reported that different action thresholds for stink bug have no effect on crop yield.
Treatments with one and half stink bugs.m-1 had higher yields than the other three action thresholds; in both thresholds, first insecticide spraying was required at R4, which reduced insect number in the following sampling. On the other hand, sprayings for the action thresholds of two and four stink bugs.m-1 were only held at R5.2 and R5.4, respectively, when population were most stable, what have increased the time to reduce them. We have already highlighted that when the first insecticide spraying is carried for populations of one stink bug.m-1, insect population reductions become more difficult and may affect yields. For the action thresholds of two and four stink bugs.m-1 as well as control, stink bugs damage were responsible for decreasing seed weight, number seeds.pod-1 and seeds.plant-1, besides causing indirect effects as leaf retention, promoting humidity damages by growth of microorganisms.
The highest number of sprayings and time when carried might have also had a slightly positive effect on yields because some pesticides can change plant metabolism and morphology (Pereira 2007). There are systemic insecticides of neonicotinoid group that may have effect on plant bioactivity, increasing production of plant hormones and enhancing vigor, germination and root development (Castro 2006). An example of this is thiamethoxam, as reported by Tavares et al. (2007), which has a bio-activating effect on soybean plants, increasing root system and dry matter production. Thereby, the insecticide used for stink bugs control here, besides reducing pest population, may have enhanced plant development, especially for action thresholds of half and one stink bug.m-1, in which sprayings started before seed formation.
Regarding to phytotechnical parameters, we noted that the number of pods.plant-1 was similar between all actions thresholds. Nevertheless, the number of seeds.plant-1 and seeds.plant-1 were higher in action thresholds that tolerate a smaller number of stink bugs. It has been evidenced that all treatments had a regular pod formation; however, at phenological stages of seed formation and filling, there was a large infestation of stink bugs at the action thresholds of four stink bugs.m-1 and control. Samplings carried at control and four stink bugs.m-1 treatment have reached over five and six stink bugs.m-1, respectively. These high populations might have impaired plant development or even raised grain abortion, which is a phenomenon known as empty pods, thus corroborating with Corrêa-Ferreira (2005), who highlights increasing number of empty pods for higher population of stink bugs.
PDI quantifies the number of “empty pods” arising, mainly, from stink bug attack, which reached 99.0 % in control, with malformed pods or undeveloped seeds, contributing thus to lower yields. Another factor that contributed to poor yields in these action thresholds was the lower seed weights. It can be highlighted that control seeds did not reach half weight of one stink bug.m-1.
Insect attack also affected plant physiology, which can be noted by the number of withheld leaves in plants with 100 % pods dry, as observed in the action thresholds of four and two stink bugs.m-1 as well as control. The rest of green material could have influenced the mechanical harvest. Silva et al. (2013) report that soybean under stink bug outbreaks during reproductive stage may lose grains because of physiological alterations as leaf retention. Leonard et al. (2011) mentions that soybean plants with grains injuries caused by stink-bug attack might have a delayed maturation.
Seed viability and vigor tested by the Tetrazolium test, were low for all treatments. This might happen because insect populations remained above the stipulated in some samplings, reaching three stink bugs.m-1, causing pod injuries. Corrêa-Ferreira (2005) emphasized significant losses in soybean seed quality and potential germination under stink bugs infestation. We also observed that seeds from plants under the action thresholds of one and half stink bug.m-1 showed approximately 30 % of their damages arising from insect attack, being this more or less, the percentage of unfeasible seeds.
Multivariate analysis was important to discriminate treatments with similar characteristics. Smaller Euclidean distance indicates higher similarity between treatments (Eduardo et al. 2016). Four stink bugs.m-1 and control, were the most similar action thresholds, this shows that higher action thresholds are related to areas without spraying. Moreover, the action thresholds of one and half stink bug.m-1 had similar results, being the most productive treatments. Based on these results, it was possible to differentiate treatments properly, grouping them according to their similarities related to analyzed parameters jointly. Additionally, by the PCA analysis, it was possible to observe the influence of each parameter on separation or grouping of the action thresholds, thus making it easier to visualize and understand which of them have similar or different results when used in a pest management program.
The fewer number of eggs with parasitoids in the half stink bug.m-1 treatment might have been due to the lowest number of stink bugs eggs found, restricting parasitism increase. However, insect populations remained higher for longer time in control, four and two stink bugs.m-1 treatments, which was the same for stink bugs eggs and, consequently, parasitism rate. This might have occurred due to the greater availability of food for parasitoids. Furthermore, in treatments half and one stink bug.m-1, there was a higher number of sprayings, which would cause death of these natural enemies.
Additionally, defense mechanisms of plants under attack by herbivores may have contributed to increase parasitism in treatments that enabled a larger number of stink bugs. According to Michereff et al. (2011), soybean plants damaged by stink bugs have a chemical profile with compounds that attract parasitoids of T. podisi. In this study, plants sprayed at higher action thresholds were mostly attacked by stink bugs, which might have unchained the production and release of these secondary compounds that attract T. podisi, which is responsible for 99.0 % of parasitism in this research.
Based on the results, the most severe action thresholds had positive influences on phytotechnical and physiological soybean parameters. Among all, the most appropriate action threshold was one stink bug.m-1 despite having less number of spraying than half stink bug.m-1, it had a similar yield, without decreasing seed quality, despite the lower level of egg parasitism for this threshold. We may also highlight that finding action thresholds have a major importance for decision-making on when to perform the first spraying, because delayed spraying may hamper stink bug control. However, further studies need to be performed associating other control tactics and different insecticide action modes, in different regions, taking into account not only the insect control but also costs and environmental risks to manage these pests sustainably.














